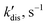Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards water-tolerant ansa-aminoboranes for parahydrogen-induced polarization and beyond†
Karolina
Konsewicz
a,
Timo
Repo
 b and
Vladimir V.
Zhivonitko
b and
Vladimir V.
Zhivonitko
 *a
*a
aNMR Research Unit, Faculty of Science, University of Oulu, P.O. Box 3000, Oulu, 90014, Finland. E-mail: vladimir.zhivonitko@oulu.fi
bDepartment of Chemistry, University of Helsinki, A. I. Virtasen aukio 1, Helsinki, 00014, Finland
First published on 5th March 2025
Abstract
Three orders of magnitude of 1H NMR signal enhancement were observed in the metal-free activation of parahydrogen using an ansa-aminoborane in the presence of a 100-fold water excess at 9.4 T. Kinetic studies corroborated the reversibility of water addition to the intramolecular N–B Lewis centers in both polar and nonpolar solvents. Furthermore, DFT modeling supported these results.
Allowing for metal-free activation of small molecules,1ansa-aminoboranes (AABs), as an example of intramolecular frustrated Lewis pairs (FLPs),2–5 show remarkable reactivity towards dihydrogen.6–11 This feature can open new horizons in catalysis based on more sustainable main group elements,3 which has already been demonstrated through AAB-catalyzed hydrogenation reactions of various substrates such as imines and alkynes under mild conditions.8,9 In addition to the catalytic applications, it has been documented that AABs show prominent nuclear spin hyperpolarization in activations of parahydrogen (para-H2),12–15 the spin-0 nuclear spin isomer of H2, utilized for dramatic NMR sensitivity enhancement through the so-called parahydrogen-induced polarization (PHIP).16–18 Typically, precious metal complexes are employed to generate PHIP,19,20 whereas AABs can provide metal-free alternatives. Furthermore, the amine–borane functionality carried by these species can be adopted as a platform for the molecular design of organic functional groups that generate PHIP. However, AABs are very sensitive to traces of moisture and typically quench through the formation of inactive Lewis adducts with water.21,22
In general, the moisture sensitivity problem of FLPs has attracted attention since the transition from research to industrial settings requires certain stability levels of the catalysts.23 For instance, it has been demonstrated that variation of substituent bulkiness in the aromatic rings of tri(aryl)boranes can lead to suitable water tolerance of the Lewis acidic boron center to perform catalytic hydrogenations and reductive aminations of carbonyl compounds in water-containing solvents.24,25 AABs have not been reported yet to have any remarkable water tolerance. To the contrary, these species were considered water intolerant,21 which stimulated a search for other FLP systems with ansa scaffolds that could have more favorable properties.21,22 These activities led to the invention of an ansa-phosphinoborane with cyclohexyls at the phosphine site that demonstrated activation of dihydrogen in the presence of 6 equivalents of H2O.21 However, it was found that at the same time water reduction occurs as a side reaction leading to the formation of the corresponding phosphine oxide. The latter problem could be resolved if one could find a water-tolerant AAB, since the nitrogen center is not prone to oxidation.
Herein, we report that a previously known orthophenylene-bridged AAB with two mesityl substituents at the boryl site (1-(2-(dimesitylboraneyl)phenyl)-2,2,6,6-tetramethylpiperidine, MesCAT)12 demonstrates strong nuclear spin hyperpolarization effects upon activation of para-H2 in acetonitrile in the presence of more than two orders of magnitude excess of water (Fig. 1A). The observation of PHIP proved undoubtedly that MesCAT demonstrates water tolerance. The observed signal enhancements for the generated MesCAT-H2 adduct exceeded three orders of magnitude at 9.4 T. Interestingly, changing mesityls to phenyl substituents leads to completely irreversible quenching of the AAB active center (Fig. 1B), highlighting the importance of balance between steric effects and Lewis acidity. We envision that further rational design can lead to efficient water-tolerant AABs for para-H2 activation.
Initial hyperpolarization experiments were performed using a 1 mM solution of 15N-labelled MesCAT adduct with water, MesCAT-H2O, in water-containing acetonitrile-d3 with H2O concentration of 0.1 M, see ESI† for details. The MesCAT-H2O÷H2O ratio was 1÷100, so there was a significant excess of water present in the unbound form in solution. The presence of free H2O was evident from 1H NMR spectra shown in the ESI† (Fig. S1). The follow-up experiments with para-H2 were performed by charging 6 bars of para-H2 (ca. 92%) to this solution in a gas-tight NMR tube, shaking, and transferring the sample into the magnet for the acquisition of spectra; see ESI† for details. Already at room temperature, this procedure resulted in the observation of hyperpolarization effects in the 1H NMR spectra (Fig. 2A). The room temperature signal enhancement for the NH group proton in the MesCAT-H2 adduct was about 50. However, heating the sample to 308 K led to more pronounced signal enhancement of more than two orders of magnitude (ca. 300-fold), as shown in Fig. 2B. Heating to 318 K produced even higher enhancements (ca. 1000-fold, Fig. 2C). Further heating to 328 K resulted in the further increase of the enhancement factor to ca. 1500 (see Table 1). For all temperatures, 11B NMR revealed an enhanced antiphase signal corresponding to the hyperpolarized boron site in MesCAT-H2 (Fig. S4 in ESI†), a feature13,14 commonly present in the activation of para-H2 with various orthophenylene AABs. We also note that increasing the MesCAT-H2O÷H2O ratio is expected to raise the equilibrium concentration of MesCAT-H2, boosting the absolute amplitudes of both its thermal and hyperpolarized 1H signals. A general discussion of this effect can be found in the ESI† (Section S1.7). However, a detailed study is beyond the scope of this communication and will be addressed in future work.
 | ||
| Fig. 2 Thermal (TH, red) and hyperpolarized (HP, blue) 1H NMR spectra measured in the experiments with a 2.3 mM solution of 15N-labelled MesCAT-H2O charged with 6 bars of para-H2 in acetonitrile-d3 at (A) 297, (B) 308, and (C) 318 K. The NH group resonances corresponding to MesCAT-H2 are depicted in the figure. These species were generated in the solution due to the dynamic equilibria shown in Fig. 1A. Enhancement factors, ε, and multiplication factors used for signal amplitude scaling are indicated for the hyperpolarized spectra. The spectra were acquired using π/4-pulses to maximize the amplitudes of the hyperpolarized signals (PASADENA condition).18 The thermal spectra were measured after the complete relaxation of the samples to thermal equilibrium. | ||
Interestingly, similar experiments with PhCAT-H2O, the phenyl analogue of MesCAT-H2O, and para-H2 in acetonitrile-d3 showed no hyperpolarization effects in 1H NMR spectra in the temperature range from 297–328 K (Fig. S23–S27 in ESI†). At the same time, it was demonstrated previously that PhCAT-H2 provides similar 1H NMR signal enhancements to MesCAT-H2.15 The enhanced boron resonances for hyperpolarized PhCAT-H2 were also not visible in 11B NMR. Generally, measured 1H and 11B NMR spectra did not reveal any changes after the addition of para-H2 to the reaction solution, implying that the dissociation of PhCAT-H2O and adduct and the subsequent formation of the hyperpolarized PhCAT-H2 did not take place.
To discuss these results, it is convenient to consider the sequence of events leading to the hyperpolarized MesCAT-H2 in Fig. 1A. Being inactive towards H2 activation as such, MesCAT-H2O must dissociate to release a sensible amount of MesCAT species that in turn can activate para-H2. Therefore, an important prerequisite for the hyperpolarization is the presence of the two dynamic equilibria in the solution: (1) MesCAT-H2O dissociation and (2) facile para-H2 activation by MesCAT. MesCAT is the key intermediate in this process, while the ability of MesCAT-H2O to dissociate is a critical feature that enables the formation of this intermediate. Considering this process from the general perspective, one must have reversible hydration (upper equilibrium for MesCAT in Fig. 1A) of AAB to observe hyperpolarization effects, a feature that is absent in the case of PhCAT-H2O (Fig. 1B).
In this context, it is essential to know the kinetic parameters of the equilibria and details of the hyperpolarization mechanism. The reaction rate measurements performed using the 1D EXSY NMR technique provided data about the exchange process between bound water in MesCAT-H2O and free water in the solution (Table 1; see ESI† for experimental details). The exchange rates are seen to increase with increasing the temperature, as expected. Generally, we can conclude also that the lifetimes of the MesCAT-H2O species in the presence of the equilibrium shown in Fig. 1A are on the order of tens to hundreds of milliseconds. Such rather short lifetimes make the exchange between MesCAT and MesCAT-H2O reversible enough to observe polarization effects for MesCAT-H2, which is formed in another reversible process of para-H2 activation, Fig. 1B. A detailed discussion of para-H2 activation for MesCAT and other AABs in acetonitrile was reported recently in ref. 15 and will not be considered here any further. However, the comparison of the dissociation rate constants for MesCAT-H2O and MesCAT-H2 (kdis) in Table 1 reveals that the lifetimes, defined as the inverse of the rate parameters, of the former species are at least an order of magnitude shorter as compared to those of the latter species. In other words, one can conclude that MesCAT-H2O is less stable than MesCAT-H2. Apparently, this feature of MesCAT facilitates the observation of nuclear spin hyperpolarization effects with para-H2 for MesCAT-H2.
and MesCAT-H2 (kdis) in Table 1 reveals that the lifetimes, defined as the inverse of the rate parameters, of the former species are at least an order of magnitude shorter as compared to those of the latter species. In other words, one can conclude that MesCAT-H2O is less stable than MesCAT-H2. Apparently, this feature of MesCAT facilitates the observation of nuclear spin hyperpolarization effects with para-H2 for MesCAT-H2.
In addition to acetonitrile-d3, MesCAT-H2O and PhCAT-H2O were examined in toluene-d8 in terms of PHIP effects. In this case, the excess of water was significantly lower (AAB-H2O÷H2O ratio was ca. 1÷7) due to the limited solubility of H2O in toluene. Similarly to acetonitrile experiments, strong hyperpolarization of the generated MesCAT-H2 species was observed in the case of MesCAT-H2O in toluene with signal enhancements exceeding two orders of magnitude at 9.4 T (Table 1; see Fig. S5 and details in ESI†). The kinetic measurements revealed even more facile reversibility of water addition in toluene than in acetonitrile since the corresponding water dissociation constant,  , was 6–8 times lower at the same temperatures in the former case. It is clear that the reversibility of water addition is an intrinsic feature of MesCAT. In contrast, the experiment with PhCAT-H2O in toluene did not result in any hyperpolarization, and there was no sign of reversibility of water addition to PhCAT for the temperatures up to 328 K examined in this study.
, was 6–8 times lower at the same temperatures in the former case. It is clear that the reversibility of water addition is an intrinsic feature of MesCAT. In contrast, the experiment with PhCAT-H2O in toluene did not result in any hyperpolarization, and there was no sign of reversibility of water addition to PhCAT for the temperatures up to 328 K examined in this study.
To gain additional insights into the observed differences of the studied AABs, we performed a density functional theory (DFT) modelling of water addition to MesCAT and PhCAT in acetonitrile and toluene. The geometry optimizations and estimations of solvation effects using the SMD model were performed at the ωB97X-D/6-311G** level of theory similarly as it was done in our previous publications (see ESI† for more details).11,15 For comparison, Fig. 3 illustrates the most stable structures of AAB-H2O and AAB-H2 adducts according to the computations. In the figure, relative standard Gibbs free energies for AAB-H2O + H2 (Fig. 3A and C) and AAB-H2 + H2O (Fig. 3B and D) are given in parentheses for acetonitrile (first number) and toluene (second number), describing the preference of hydration and dihydrogen addition, respectively. Note that the energies were calculated with respect to the corresponding AAB-H2O + H2 level.
 | ||
| Fig. 3 Comparison of thermodynamic stabilities of MesCAT (A) and (B) and PhCAT (C) and (D) adducts with water (A) and (C) and dihydrogen (B) and (D) according to the DFT calculations. Structures of the most stable water and dihydrogen adducts are shown in the figure. Relative standard Gibbs free energies, ΔrG°, in kcal mol−1, are given in parentheses with respect to AAB-H2O + H2 for acetonitrile (first number) and toluene (second number) as solvents. To preserve the total number of atoms unchanged, the energies of the AAB-H2O + H2 and AAB-H2O + H2O states are compared. In the calculation, however, all molecules were treated as separate species (see ESI† for details). | ||
Interestingly, the comparison between MesCAT-H2O + H2 (Fig. 3A) and MesCAT-H2 + H2O (Fig. 3B) reveals quite neutral thermodynamic stability of water and dihydrogen adducts since the ΔrG° between the hydrated and the hydrogenated forms of MesCAT does not exceed ±2 kcal mol−1, and both forms can be present in sensible quantities in acetonitrile and toluene. This result supports the experimentally observed thermal and hyperpolarized MesCAT-H2 in the water-containing solvents. Specifically, DFT computations imply that under standard conditions in acetonitrile (i.e., 298 K and 1 M concentrations), MesCAT-H2 is more favorable than MesCAT-H2O (ΔrG° = −1.5 kcal mol−1). In contrast, in toluene, MesCAT-H2O is more favorable (ΔrG° = 2 kcal mol−1). However, the free energies do not differ significantly, and experimentally, in both cases we have observed hyperpolarization effects. In the case of PhCAT, the situation is significantly different, since PhCAT-H2O species are at least 6 kcal mol−1 more favorable than PhCAT-H2 (ΔrG° is 6.2 kcal mol−1 in acetonitrile and 9.8 kcal mol−1 in toluene). Therefore, in both solvents, the inactive hydrated form of PhCAT must be thermodynamically stabilized. This conclusion is clearly confirmed in our experiments, highlighting the relevance of the theoretical approach utilized. We note, however, that DFT predictions have intrinsic precision, which, from our experience with reactivity modeling of AABs, amounts to ca. 1 kcal mol−1. Generally, the unique reactivity of MesCAT can be attributed to a well-balanced combination of electronic effects – facilitating the sufficient Lewis acidity of the boryl site to activate both H2 and H2O – and the steric bulkiness of mesityl substituents. In the case of H2O, the latter likely induces back strain at the boron site upon hydration, preventing full boron pyramidalization and the formation of a strong B–OH bond, making hydration reversible. The importance of back strain in water tolerance was previously demonstrated by Soós et al. for tri(aryl)boranes.24
In conclusion, we have demonstrated that nuclear spin hyperpolarization effects with amine-borane frustrated Lewis pairs can be observed in the presence of a dramatic excess of water in solution. To the best of our knowledge, this is also the first communication reporting pronounced water tolerance with this class of metal-free dihydrogen activators. Namely, orthophenylene AAB species with mesityls at the boryl site, MesCAT, showed more than three orders of magnitude of 1H NMR signal enhancement in a 9.4 T magnetic field for the MesCAT-H2 dihydrogen adduct produced in para-H2 activation in the presence of a 100-fold excess of water in acetonitrile. Alike results were obtained in the experiments with MesCAT in toluene. The performed kinetics measurement provided lifetimes of MesCAT-H2 that are significantly longer than those for MesCAT-H2O, confirming the conclusions stemming from the PHIP experiments about the comparative stability of both species. Generally, we can conclude that MesCAT can be exposed to air during sample preparation for PHIP experiments or for potential catalytic applications since its water adduct can dissociate in a facile manner, releasing the active free MesCAT species. In contrast, PhCAT AAB with less bulky phenyl substituents at the boryl site forms very stable PhCAT-H2O adducts due to the irreversible hydration, which disables any PHIP effects with PhCAT in the presence of moisture. The performed DFT modelling supported the essential role of steric effects on the way to the optimization of AAB active centers for water-tolerant metal-free dihydrogen activators. Finally, we believe that further rational design of AABs can result in significantly more pronounced shifting of underlying equilibria towards the desired dihydrogen activation and suppressing the formation of the inactive hydration products.
This work was done through the following contributions: K. K.: investigation, validation, writing – review and editing; T. R.: conceptualization, funding acquisition, resources, supervision, validation, writing – review and editing; V. V. Z.: conceptualization, funding acquisition, resources, supervision, investigation, validation, visualization, writing – original draft.
K. K. and V. V. Z. are grateful for the financial support from the Research Council of Finland (grant number 362959) and Kvantum Institute (University of Oulu). V. V. Z. wishes to acknowledge CSC – IT Center for Science, Finland, for computational resources (CSC project 2004016).
Data availability
The data supporting this article have been included as part of the ESI.† In addition, data for this article, including raw NMR data folders and output files for the DFT computations, are available at fairdata.fi at https://doi.org/10.23729/be2203c4-430c-45f6-aa43-7fb1292dc855.Conflicts of interest
There are no conflicts to declare.Notes and references
- D. W. Stephan, Science, 2016, 354, aaf7229 CrossRef PubMed.
- A. R. Jupp and D. W. Stephan, Trends Chem., 2019, 1, 35–48 CrossRef CAS.
- J. Lam, K. M. Szkop, E. Mosaferi and D. W. Stephan, Chem. Soc. Rev., 2019, 48, 3592–3612 RSC.
- D. W. Stephan, Acc. Chem. Res., 2015, 48, 306–316 CrossRef CAS PubMed.
- D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2015, 54, 6400–6441 CrossRef CAS PubMed.
- V. Sumerin, F. Schulz, M. Atsumi, C. Wang, M. Nieger, M. Leskelä, T. Repo, P. Pyykko and B. Rieger, J. Am. Chem. Soc., 2008, 130, 14117–14119 CrossRef CAS PubMed.
- F. Schulz, V. Sumerin, S. Heikkinen, B. Pedersen, C. Wang, M. Atsumi, M. Leskela, T. Repo, P. Pyykko, W. Petry and B. Rieger, J. Am. Chem. Soc., 2011, 133, 20245–20257 CrossRef CAS PubMed.
- K. Chernichenko, Á. Madarász, I. Pápai, M. Nieger, M. Leskelä and T. Repo, Nat. Chem., 2013, 5, 718–723 CrossRef CAS PubMed.
- V. Sumerin, K. Chernichenko, M. Nieger, M. Leskela, B. Rieger and T. Repo, Adv. Synth. Catal., 2011, 353, 2093–2110 CrossRef CAS.
- K. Chernichenko, M. Lindqvist, B. Kótai, M. Nieger, K. Sorochkina, I. Pápai and T. Repo, J. Am. Chem. Soc., 2016, 138, 4860–4868 CrossRef CAS PubMed.
- K. Chernichenko, B. Kótai, I. Pápai, V. Zhivonitko, M. Nieger, M. Leskelä and T. Repo, Angew. Chem., Int. Ed., 2015, 54, 1749–1753 CrossRef CAS PubMed.
- V. V. Zhivonitko, K. Sorochkina, K. Chernichenko, B. Kotai, T. Földes, I. Pápai, V.-V. Telkki, T. Repo and I. Koptyug, Phys. Chem. Chem. Phys., 2016, 18, 27784–27795 RSC.
- K. Sorochkina, V. V. Zhivonitko, K. Chernichenko, V. V. Telkki, T. Repo and I. V. Koptyug, J. Phys. Chem. Lett., 2018, 9, 903–907 CrossRef CAS PubMed.
- D. O. Zakharov, K. Chernichenko, K. Sorochkina, S. Yang, V. V. Telkki, T. Repo and V. V. Zhivonitko, Chem. – Eur. J., 2022, 28, e202103501 CrossRef CAS PubMed.
- K. Konsewicz, G. Laczkó, I. Pápai and V. V. Zhivonitko, Phys. Chem. Chem. Phys., 2024, 26, 3197–3207 RSC.
- C. R. Bowers and D. P. Weitekamp, Phys. Rev. Lett., 1986, 57, 2645–2648 CrossRef CAS PubMed.
- C. R. Bowers and D. P. Weitekamp, J. Am. Chem. Soc., 1987, 109, 5541–5542 CrossRef CAS.
- C. R. Bowers, in Encyclopedia of Nuclear Magnetic Resonance, ed. D. M. Grant and R. K. Harris, Wiley, Chichester, 2002, vol. 9, pp. 750–769 Search PubMed.
- B. J. Tickner and V. V. Zhivonitko, Chem. Sci., 2022, 13, 4670–4696 RSC.
- S. B. Duckett, in Encyclopedia of Spectroscopy and Spectrometry, ed. J. C. Lindon, G. E. Tranter and D. W. Koppenaal, Elsevier, 2016, pp. 527–534 Search PubMed.
- K. Sorochkina, K. Chernichenko, V. V. Zhivonitko, M. Nieger and T. Repo, Chem. – Eur. J., 2022, 28, e202201927 CrossRef CAS PubMed.
- K. Sorochkina, K. Chernichenko, M. Nieger, M. Leskelä and T. Repo, Z. Naturforsch., B, 2017, 72, 903–908 CrossRef CAS.
- V. Fasano and M. J. Ingleson, Synthesis, 2018, 1783–1795 CAS.
- É. Dorkó, M. Szabó, B. Kótai, I. Pápai, A. Domján and T. Soós, Angew. Chem., Int. Ed., 2017, 56, 9512–9516 CrossRef PubMed.
- Á. Gyömöre, M. Bakos, T. Földes, I. Pápai, A. Domján and T. Soós, ACS Catal., 2015, 5, 5366–5372 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental materials and methods; para-H2 experiments and 1D EXSY NMR data; supporting 1H and 11B NMR spectra; theory and DFT results; xyz structures. See DOI: https://doi.org/10.1039/d5cc00528k |
| This journal is © The Royal Society of Chemistry 2025 |