Open Access Article
Open Access ArticleControllable properties of NiO nanostructures fabricated by plasma assisted-chemical vapor deposition†
Davide
Barreca
 a,
Enrico
Scattolin
a,
Enrico
Scattolin
 b,
Chiara
Maccato
b,
Chiara
Maccato
 *ab,
Alberto
Gasparotto
*ab,
Alberto
Gasparotto
 ab,
Lorenzo
Signorin
b,
Naida
El Habra
ab,
Lorenzo
Signorin
b,
Naida
El Habra
 ae,
Andraž
Šuligoj
ae,
Andraž
Šuligoj
 cd,
Urška Lavrenčič
Štangar
cd,
Urška Lavrenčič
Štangar
 d and
Gian Andrea
Rizzi
d and
Gian Andrea
Rizzi
 ab
ab
aCNR-ICMATE and INSTM, Department of Chemical Sciences, Padova University Via Marzolo 1, 35131 Padova, Italy
bDepartment of Chemical Sciences, Padova University and INSTM, 35131 Padova, Italy. E-mail: chiara.maccato@unipd.it
cNational Institute of Chemistry, Ljubljana SI-1000, Slovenia
dFaculty of Chemistry and Chemical Technology, University of Ljubljana, Ljubljana SI-1000, Slovenia
eCNR-ICMATE, 35127 Padova, Italy
First published on 17th January 2025
Abstract
An original plasma assisted vapor phase route is proposed for the low-temperature fabrication of supported NiO nanostructures on conductive glasses. The sole deposition time variation enables to tailor material properties, modulating, in turn, the system wettability and functional performances in the photodegradation of recalcitrant pollutants.
Thin films and nanomaterials of nickel(II) oxide (NiO), a p-type pluripotent semiconductor featuring a good chemical stability, stand as appealing candidates for a plethora of technological applications, encompassing solar cells,1–3 electrochromic devices,4–7 gas sensors,8–10 and heterogeneous (photo)catalysts for various processes, including hydrogen production, oxygen evolution in water splitting, and degradation of aqueous organic pollutants.11–16 In addition, NiO systems are attractive as hydrophobic coatings for protection against corrosion, offering also self-cleaning, anti-fouling, and frost-prevention properties.17,18
The aforementioned applications have significantly stimulated research activities aimed at the preparation of NiO films/nanosystems by different techniques, including sputtering,2,7,9 evaporation,10 electrodeposition,16,17 hydrothermal routes,11 sol–gel,1,3 spray pyrolysis,6,8 atomic layer deposition (ALD)5,14 and chemical vapor deposition (CVD),4,12,15,19 a potentially scalable route featuring manifold concurrent benefits. In this regard, plasma-assisted processes are appealing preparation strategies for multi-functional nanoarchitectures, owing to the unique features and activation mechanisms of non-equilibrium plasmas that enable deposition at low temperatures even on thermally labile supports.20 Nevertheless, to the best of our knowledge, only two reports on the plasma assisted-CVD of NiO films are available in the literature so far.13,21
Recently, we have proposed variously substituted diketonate–diamine Ni(II) complexes as valuable precursors for the CVD of NiO films.19,22 Herein, we report for the first time on the plasma assisted-chemical vapor deposition (PA-CVD) of Ni(II) oxide nanosystems from one of these precursors (see the ESI†). The target systems were grown at a temperature of 100 °C, much lower than those adopted in previous plasma-assisted vapor phase syntheses,13,14,21 on fluorine-doped tin oxide (FTO), focusing on the influence exerted by the deposition time (10, 30, 60, 90 min) on material chemico-physical characteristics. The latter were investigated by means of forefront complementary techniques, including ultraviolet photoelectron spectroscopy (UPS) and reflection electron energy loss spectroscopy (REELS), used for the first time on similar systems in conjunction with X-ray photoelectron spectroscopy (XPS). In fact, REELS can probe the presence of hydrogen,23 which, though being elusive, may directly affect the system behavior. In addition, functional properties were investigated with particular regard to the system wettability, by measurements of water contact angle (WCA) values.18,24 Attention was also dedicated for the first time, as a proof-of-concept, to assessing material activity in the photodegradation of aqueous diclofenac {DCF; 2-[2-(2,6-dichloroanilino)phenyl]acetic acid},22 a nonsteroidal anti-inflammatory drug, as a representative persistent pharmaceutical pollutant.25
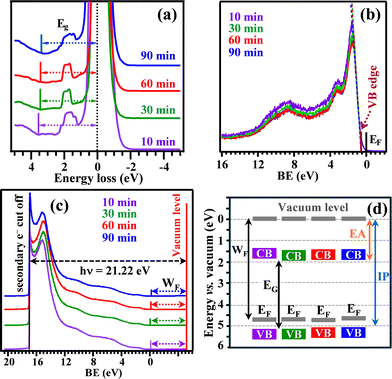
Fig. 1a (see also Fig. S1, ESI†) reports X-ray diffraction (XRD) patterns for NiO samples grown for different durations. Besides FTO reflections, all diffractograms clearly showed three signals ascribed to (111), (200) and (220) crystallographic planes of cubic NiO.19,26 The analysis of relative peak intensities indicated that crystallites were preferentially oriented along [111] and [220] directions (see also Fig. S2, ESI†). Additional efforts were dedicated to the analysis of material composition by means of XPS (see also Fig. S3–S5 and Table S1, ESI†). Ni2p peaks position and shape (Fig. 1b), featuring a peculiar multiplet structure with satellite signals at energy ≈6 eV higher than the main spin–orbit components, were in line with previous studies on Ni(II) oxide.11,19 The presence of NiO was also supported by the O1s peak, exhibiting a component at ≈529.9 eV [(I) in Fig. 1c], attributable to NiO lattice oxygen,12,27 whereas band (II) at 531.5 eV (Table S2, ESI†) was mainly due to the concurrence of –OH groups and atmospheric oxygen adsorbed onto O defect sites.19,28 The presence of tin from the FTO substrate (Fig. S6, ESI†), with a progressively lower content upon increasing the deposition time, was also observed. As demonstrated by morphological analyses (see Fig. 3 and below), this trend can be ascribed to the progressive deposit thickness increase for longer PA-CVD durations, gradually hindering the underlying SnO2 detection. Additional information on the electronic properties of the target systems was obtained by REELS and valence band spectra. For all samples, the recorded REELS spectra (Fig. 2a) featured an intense peak at zero energy loss due to elastically scattered electrons, and a signal below 2 eV due to scattering collisions with hydrogen atoms,23 whose content can be envisaged to be qualitatively higher at lower deposition times. An increase in the PA-CVD process duration resulted in a modest decrease of the measured energy gap from 3.6 to 3.3 eV (Table S3, ESI†), the latter value being in line with that reported for bulk NiO.19 The separation of the valence band edge from the Fermi level energy (see Fig. 2b and Table S3, ESI;† values are in good agreement with the p-type character of NiO19) underwent a slight decrease upon going from the 10 min sample to the 90 min one. Finally, the analysis of UPS valence band spectra (Fig. 2c), in conjunction with the other data, enabled to obtain the parameters marked in Fig. 2d and reported in Table S3, ESI.† The work function values were all very close to 4.7 eV, in accordance with the literature.29,30 A detailed inspection of the obtained data revealed only minor differences in the electronic properties of the target systems, suggesting thus that their functional properties were mainly affected by the actual material morphology (see below).
Field emission-scanning electron microscopy (FE-SEM) images of the target NiO specimens are reported in Fig. 3a–d. A careful micrograph inspection showed that a process duration of 10 min (Fig. 3a) resulted in a conformal coverage of the FTO substrate by the NiO deposit. An increase of the PA-CVD process duration, thanks to the synergy between material deposition and modification induced by the used plasmas,20 resulted in the progressive development of a nanocolumnar, open area morphology, a feature particularly evident for the longest deposition time (Fig. 3d). These results suggested the occurrence of a hybrid 2D–3D growth mode, likely favored by the inherently rough surface of the used FTO supports.31,32 The average deposit thickness linearly increased with the corresponding deposition time (Fig. 3e), indicating the possibility of exerting a fine control of this parameter and of the related properties by simple modulations of the process duration. The surface topography of the samples, investigated by atomic force microscopy (AFM) (see Fig. 3f and Fig. S7, ESI†), did not show a significant variation in the root-mean-square (RMS) roughness as a function of deposition time. In fact, the system morphology prevents from a successful probing of the materials in their real depth-profile, returning an almost constant RMS value of ≈16 nm.
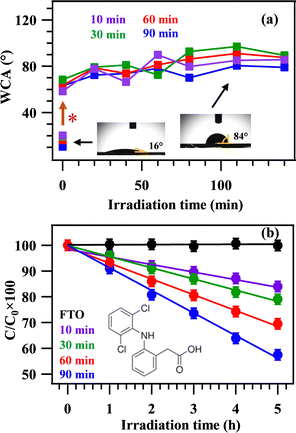
The attention was subsequently dedicated to investigating the system wetting properties as a function of PA-CVD growth duration by measuring WCA values (see Fig. 4a and Fig. S8, ESI†). Specimens deposited for 10, 30, 60, and 90 min showed pristine WCA values of 20.0, 19.5, 16.2, and 10.6°, respectively. These values showcased a higher hydrophilic character for thicker NiO systems, offering a larger surface area to the water droplet wetting the surface. A significant WCA increase up to ≈60–70° was observed after 24 days of exposure to laboratory air (Fig. 4a). This result, in line with those obtained for nickel micro-nano cones array,33 may be related to the adsorption of hydrocarbons on the target material surface.34 Concurrent issues accounting for this behavior can be associated to: (i) the existence of oxygen vacancies (as evidenced by XPS analyses, see above) promoting a progressive O2 adsorption, which, in turn, can contribute to the observed hydrophobicity; (ii) the presence of surface CFx groups (Fig. S5, ESI†), resulting in the maintenance of the hydrophobic behavior upon irradiation.35,36 In fact, during the subsequent UVA light illumination, only a slight WCA variation occurred during the first 20 min, and the measured WCAs remained in the ≈80–90° range for the whole experiment duration (140 min; Fig. 4a). These results evidenced that the obtained materials maintained their hydrophobic characteristics and did not promote photodegradation processes of surface solid contaminants, as confirmed by tests carried out using methyl stearate (see the ESI† and Fig. S9). However, as the NiO deposit thickening contributed to a better wettability and to a band gap decrease to 3.3 eV (deposition time of 90 min, Table S3, ESI†), additional efforts were dedicated to assessing the system photocatalytic activity in the degradation of DCF, whose initial adsorption might be possibly favoured by the hydrophobic surface nature.37 The obtained results (Fig. 4b) showed a noticeable activity, which underwent a progressive increase with the deposit thickness (see Fig. 3e). Interestingly, the trend in DCF degradation followed the trend of pristine hydrophilicity, highlighting that the most hydrophilic sample (the one with a PA-CVD duration of 90 min) produced the fastest DCF degradation (see also Table S4, ESI†). Although NiO as such suffers from a relatively fast recombination of photogenerated charge carriers,38 the presence of the FTO substrate (n-type) below NiO (p-type) enables electron injection into FTO, whereas holes accumulate on NiO,39 accounting thus for DCF degradation. It is worthwhile noticing that thicker deposits feature the occurrence of higher columns (Fig. 3), as well as of larger NiO grains atop the columns themselves (see Fig. S10, ESI†). These issues foster an increased UV light absorption (see Fig. S11, ESI†), leading to a more efficient charge carrier generation and, ultimately, to a faster DCF degradation in the case of the 90 min specimen. An additional contributing effect can be related to the higher active area of materials obtained for longer PA-CVD process durations.
In summary, phase-pure NiO supported nanostructures were successfully fabricated via plasma assisted-CVD on glassy substrates at temperatures of 100 °C, the lowest ever reported so far in similar processes. Modulation of the sole process duration enabled to tailor material morphology, with particular regard to grain dimensions and deposit thickness. These features directly affected material properties in terms of wettability and photodegradation efficiency of aqueous diclofenac, a persistent pharmaceutical product. This work demonstrates an amenable and straightforward strategy for the preparation of NiO nanostructures, that can be conveniently extended even to thermally labile substrates. In perspective, the system surface hydrophobicity can be exploited in the design of catalysts with improved activity and selectivity. Furthermore, the flexible control over thickness and morphology of NiO systems renders them interesting candidates for additional photoactivated end-uses, including water splitting to green hydrogen. Efforts in this direction are already in progress.
Davide Barreca: conceptualization, investigation, methodology, writing – original draft. Enrico Scattolin: data curation, software, formal analysis. Chiara Maccato: vision, formal analysis, project administration, writing – review & editing, funding acquisition. Alberto Gasparotto: supervision, methodology, project administration, writing – review & editing. Lorenzo Signorin: methodology, validation. Naida El Habra: vision, data curation. Andraž Šuligoj: investigation, methodology, writing – original draft. Urška Lavrenčič Štangar: supervision, project administration. Gian Andrea Rizzi: conceptualization, software, supervision, writing – review & editing.
Padova University (P-DiSC#02BIRD2023-UNIPD RIGENERA), CNR (Progetti di Ricerca @CNR-avviso 2020-ASSIST), PRIN 2022474YE8 (SCI-TROPHY project), and ARIS (P1-0134 core funding) assisted financially the work.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Rajesh, K. Vengatesan, M. H. Aly, R. Sitharthan, S. S. Dhanabalan and M. Karthikeyan, Opt. Quantum Electron., 2023, 55, 1167 CrossRef CAS.
- C. Aivalioti, E. G. Manidakis, N. T. Pelekanos, M. Androulidaki, K. Tsagaraki, Z. Viskadourakis, E. Spanakis and E. Aperathitis, Thin Solid Films, 2023, 778, 139910 CrossRef CAS.
- Q. S. Jiang, Y. Wu, Z. Xie, M. Wei, Y. Zhao, X. Yang, W. Xun, S. Cao and C. Wang, Mater. Today Commun., 2023, 35, 106401 CrossRef CAS.
- M. Z. Sialvi, R. J. Mortimer, G. D. Wilcox, A. M. Teridi, T. S. Varley, K. G. U. Wijayantha and C. A. Kirk, ACS Appl. Mater. Interfaces, 2013, 5, 5675–5682 CrossRef CAS PubMed.
- X. Su, Z. Tu, L. Ji, H. Wu, H. Xu and C. Liu, J. Vac. Sci. Technol., A, 2023, 41, 062407 CrossRef CAS.
- E. A. Khera, H. Ullah, F. Hussain, S. Abubakar, A. Majeed, I. Tabssum, Z. Batool, A. Nazir and G. Gilanie, ChemistrySelect, 2023, 8, e20230232 CrossRef.
- Y. Tang, H. Shen, T. Wang, S. Peng, K. Jin, Q. Qian, G. Li and Z. Gan, Thin Solid Films, 2023, 769, 139754 CrossRef CAS.
- K. Rajesh, N. Pothukanuri and M. V. R. Reddy, Chem. Phys. Impact, 2024, 8, 100397 CrossRef.
- S. Srivastava, C. Dwivedi, A. Yadav, A. Kumar, G. Gupta and P. Singh, Mater. Lett., 2023, 351, 135040 CrossRef CAS.
- D. Dastan, K. Shan, A. Jafari, T. Marszalek, M. K. A. Mohammed, L. Tao, Z. Shi, Y. Chen, X.-T. Yin, N. D. Alharbi, F. Gity, S. Asgary, M. Hatamvand and L. Ansari, Mater. Sci. Semicond. Process., 2023, 154, 107232 CrossRef CAS.
- N. Kitchamsetti, M. S. Ramteke, S. R. Rondiya, S. R. Mulani, M. S. Patil, R. W. Cross, N. Y. Dzade and R. S. Devan, J. Alloys Compd., 2021, 855, 157337 CrossRef CAS.
- D. Zywitzki, D. H. Taffa, L. Lamkowski, M. Winter, D. Rogalla, M. Wark and A. Devi, Inorg. Chem., 2020, 59, 10059–10070 CrossRef CAS PubMed.
- N. Weidler, J. Schuch, F. Knaus, P. Stenner, S. Hoch, A. Maljusch, R. Schäfer, B. Kaiser and W. Jaegermann, J. Phys. Chem. C, 2017, 121, 6455–6463 CrossRef CAS.
- S. Haghverdi Khamene, C. van Helvoirt, M. N. Tsampas and M. Creatore, J. Phys. Chem. C, 2023, 127, 22570–22582 CrossRef CAS PubMed.
- K. Munawar, M. A. Mansoor, R. Naeem, M. Rizwan, M. S. Ahmad, T. Zaharinie, M. N. M. Zubir and Z. Aspanut, Thin Solid Films, 2023, 782, 140031 CrossRef CAS.
- H. Chen, D. Ge, J. Chen, R. Li, X. Zhang, T. Yu, Y. Wang and S. Song, Chem. Commun., 2020, 56, 10529–10532 RSC.
- A. Bahramian, M. Eyraud, F. Vacandio, V. Hornebecq, T. Djenizian and P. Knauth, J. Appl. Electrochem., 2019, 49, 621–629 CrossRef CAS.
- K. Liu, M. Vuckovac, M. Latikka, T. Huhtamäki and R. H. A. Ras, Science, 2019, 363, 1147–1148 CrossRef CAS PubMed.
- M. Benedet, C. Maccato, G. Pagot, C. Invernizzi, C. Sada, V. Di Noto, G. A. Rizzi, E. Fois, G. Tabacchi and D. Barreca, J. Phys. Chem. C, 2023, 127, 22304–22314 CrossRef CAS.
- A. Gasparotto, D. Barreca, D. Bekermann, A. Devi, R. A. Fischer, C. Maccato and E. Tondello, J. Nanosci. Nanotechnol., 2011, 11, 8206–8213 CrossRef CAS PubMed.
- M. Chandrakala, S. Raj Bharath, T. Maiyalagan and S. Arockiasamy, Mater. Chem. Phys., 2017, 201, 344–353 CrossRef CAS.
- M. Benedet, D. Barreca, E. Fois, R. Seraglia, G. Tabacchi, M. Roverso, G. Pagot, C. Invernizzi, A. Gasparotto, A. A. Heidecker, A. Pöthig, E. Callone, S. Dirè, S. Bogialli, V. Di Noto and C. Maccato, Dalton Trans., 2023, 52, 10677–10688 RSC.
- S. D. Nehate, A. K. Saikumar and K. B. Sundaram, Coatings, 2021, 11, 196 CrossRef CAS.
- D. Barreca and C. Maccato, CrystEngComm, 2023, 25, 3968–3987 RSC.
- N. Aghababaei, M. Abdouss, H. Hosseini-Monfared and F. Ghanbari, J. Environ. Chem. Eng., 2023, 11, 110477 CrossRef CAS.
- Pattern no 00-0047-1049, JCPDS, 2000.
- G. Pagot, M. Benedet, C. Maccato, D. Barreca and V. Di Noto, Surf. Sci. Spectra, 2023, 30, 024028 CrossRef CAS.
- D. Barreca, F. Gri, A. Gasparotto, G. Carraro, L. Bigiani, T. Altantzis, B. Žener, U. Lavrenčič Štangar, B. Alessi, D. B. Padmanaban, D. Mariotti and C. Maccato, Nanoscale, 2019, 11, 98–108 RSC.
- Y. Zhang, J. Zuo, P. Li, Y. Gao, W. He and Z. Zheng, Physica E, 2019, 111, 75–78 CrossRef CAS.
- Y. Cheng, S. Lu, W. Xu, R. Boukherroub, S. Szunerits and W. Liang, J. Alloys Compd., 2017, 723, 225–236 CrossRef CAS.
- J. E. Prieto and I. Markov, Surf. Sci., 2017, 664, 172–184 CrossRef CAS.
- A. Baskaran and P. Smereka, J. Appl. Phys., 2012, 111, 044321 CrossRef.
- W. Geng, A. Hu and M. Li, Appl. Surf. Sci., 2012, 263, 821–824 CrossRef CAS.
- J. Bae, I. A. Samek, P. C. Stair and R. Q. Snurr, Langmuir, 2019, 35, 5762–5769 CrossRef CAS PubMed.
- G. Qi, X. Liu, C. Li, C. Wang and Z. Yuan, Angew. Chem., Int. Ed., 2019, 58, 17406–17411 CrossRef CAS PubMed.
- P. Dimitrakellis and E. Gogolides, Adv. Colloid Interface Sci., 2018, 254, 1–21 CrossRef CAS PubMed.
- N. Suriyanon, P. Punyapalakul and C. Ngamcharussrivichai, Chem. Eng. J., 2013, 214, 208–218 CrossRef CAS.
- T. Munawar, S. Fatima, M. S. Nadeem, F. Mukhtar, U. A. Akbar, A. S. Hakeem and F. Iqbal, J. Mater. Sci.: Mater. Electron., 2023, 34, 687 CrossRef CAS.
- B. Saha, K. Sarkar, A. Bera, K. Deb and R. Thapa, Appl. Surf. Sci., 2017, 418, 328–334 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc06548d |
| This journal is © The Royal Society of Chemistry 2025 |