Prudently designed Se@fMWCNT as a peroxidase mimicking nanozyme for distinctive electrochemical detection of H2O2 and glutathione†
Vadakke Purakkal
Sruthi
 and
Sellappan
Senthilkumar
and
Sellappan
Senthilkumar
 *
*
Department of Chemistry, School of Advanced Sciences, Vellore Institute of Technology (VIT), Vellore-632014, India. E-mail: senthilkumar.s@vit.ac.in; senthilanalytical@gmail.com
First published on 22nd May 2024
Abstract
Nanozymes are nanomaterials with enzymatic characteristics that are used to overcome the challenges associated with naturally occurring enzymes. A peroxidase mimicking nanozyme has been developed in this work, which has ability in the reduction of hydrogen peroxide (H2O2) to water, by oxidising the substrates in the presence of H2O2. We have designed and developed a neoteric Se@fMWCNT nanocomposite with peroxidase mimicking activity for electrochemical detection of H2O2 and glutathione (GSH). The choice of selenium (Se) was inspired by the natural enzymatic antioxidant glutathione peroxidase, which has Se as its active centre. Before preparation of the nanocomposite, MWCNT was functionalized with acid to obtain functionalized MWCNT, in order to improve biocompatibility and to increase conductivity by providing abundant active sites for the successful incorporation of Se. The as-synthesized nanocomposite was immobilized on a glassy carbon electrode, which could then be used for amperometric detection of the analytes H2O2 and GSH at neutral pH. The fabricated sensor exhibits a linear detection range of 50 nM–1.4 μM and a limit of detection (LOD) of 18.23 nM for H2O2, and a range of 50–450 μM and 500 μM–1.5 mM for GSH determination, with an LOD of 19.2 μM.
1. Introduction
Scrimin and Pasquato's team first coined the term ‘nanozyme’ in order to illustrate the ribonuclease-like activity of their gold nanoparticles functionalized with triazacyclononan.1 Noticeably, following the discovery of intrinsic peroxidase mimetic characteristics of Fe3O4 by Yan and co-workers in 2007,2 these nanozymes have been in the limelight in wide variety of areas of research, such as biomedicine, environmental monitoring and food safety.3 They have been exploited to replace the frailties of naturally occurring enzymes and exisiting artificially obtained/synthesized enzymes. Of several existing nanozymes, the four most explored nanozymes are for mimicking oxidase, peroxidase, catalase and superoxide dismutase (SOD), because of their vital role in biomedical science in protecting cells by altering the levels of reactive oxygen species (ROS) in the cells. Briefly, oxidases catalyse the oxidation of substrates by making use of oxygen (O2) as the electron acceptor, eventually reducing O2 to water (H2O) or hydrogen peroxide (H2O2). Similarly, SODs catalyse the conversion of superoxide into H2O2, which is further oxidised into H2O and O2. The decomposition of H2O2 into H2O and O2 is further aided by the enzyme catalase. Meanwhile, peroxidases oxidise substrates mostly in the presence of H2O2, where the substrates act as electron donors.4–6 Due to the outstanding catalytic properties of peroxidase enzymes, they are widely applied in diverse domains, including sensing, catalysis, bioimaging, and wound healing.3,7Peroxidase enzymes have acquired marked significance in bioscience due to their superior catalytic activity. They can oxidise a wide range of chromogenic substrates, such as o-phenylenediamine (OPD), 3,3′,5,5′-tetramethylbenzidine (TMB), and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), in the presence of H2O2.2,8 They play a vital role in biological processes by efficiently detoxifying ROS and protecting cells from attack by pathogens. However, natural enzymes are prone to being denatured under harsh conditions, such as high temperature and variable pH. These limitations have given rise to peroxidase mimicking enzymes, or peroxidase nanozymes. Thus, natural peroxidases can be prudently substituted with peroxidase nanozymes for sustained bioanalytical applications in electrochemical, fluorescence and colorimetric sensing.9–11 Being a potent member of the ROS family, detection of H2O2 is crucial in order to monitor cell health. The optimum level of intracellular H2O2 should be less than 10 nM. The presence of excessive H2O2 in cells is known to cause damage to the central nervous system, resulting in detrimental diseases, including cancer.12 Hence, selective and sensitive detection of H2O2 is necessary to elevate quality of life.
Of late, several nanomaterials, especially carbon materials13–15 (graphene oxide (GO), multi-walled carbon nanotube (MWCNT), single-walled carbon nanotube (SWCNT), metal organic frameworks (MOFs),16–18 and nanomaterials,19 were identified for their ability to act as enzyme mimics. However, these materials have not yet been explored for electrochemical sensing in this context. Although there are numerous reports of peroxidase mimics for colorimetric sensing, only a handful of sensors have been reported as enzyme mimics in electrochemical sensing. However, these reported sensors have drawbacks, such as high sensing potential and failure to detect at neutral pH. Therefore, it is important to design and develop an enzyme mimicking material with superior analytical performance, in order to realize electrochemical detection in real-time, complex environments.
Glutathione (GSH) is a thiol-containing bioactive tripeptide, which consists of three amino acids, namely cysteine, glycine and glutamic acid.20 GSH acts as an antioxidant that aids the enzyme glutathione peroxidase (GSHPx) in maintaining the intracellular and extracellular concentrations of H2O2.21,22 GSHPx protects the cells from oxidative damage by catalysing the reduction of H2O2. Selenium (Se) is the core moiety in GSHPx, which makes use of GSH as a cofactor in order to alter the oxidative damage caused by reactive oxygen species.23–26 The optimum level of GSH in blood should lie in the range 1–10 × 10−3 mol L−1 for a healthy individual.27 Abnormal concentrations of GSH are known to cause several diseases, including cancers, diabetes, Parkinson's disease and Alzheimer's disease. Therefore, the detection of GSH is pivotal in order to ameliorate living standards.28 Se present in GSHPx plays a pivotal role in its catalytic cycle and thus several Se-based nanozymes that act as GSHPx mimics have been reported, for diverse applications.29 For example, Li and co-workers reported a selenium-doped g-C3N4 nanocomposite with peroxidase mimicking activity and used it for colorimetric detection of H2O2 and xanthine.30 Later, in 2017, Huang et al. reported a GO-Se nanocomposite that acts as a GSHPx mimetic and used it for cytoprotection applications.31 Recently, Tian and co-workers synthesized a binuclear Fe-containing Se MOF nanozyme with tetraenzyme characteristics and successfully applied it as an antioxidant agent for the treatment of ischemic stroke.32 Although Se nanozymes and their efficacy have been investigated in detail, no attempts have been made, to the best of our knowledge, towards the electrochemical sensing of GSH, and this prompted us to develop a Se nanocomposite that could serve as a peroxidase mimic for the electrochemical determination of H2O2 and GSH subsequently.
MWCNTs are popular for their exceptional chemical stability, electronic conductivity, mechanical properties, moderate anti-bacterial nature and large surface to volume ratio, which have secured them a distinctive spot in sensing applications.33–39 Yet, they have downsides, such as poor solubilities and difficulties with regard to uniform dispersion, which could be overcome by functionalization of the MWCNTs. Introduction of functional groups containing oxygen (–OH, –COOH and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) by acid functionalization can lead to better dispersion and surface activation, thereby increasing the interfacial interactions between MWCNTs and other nanoparticles for the formation of nanocomposites.40–42
O) by acid functionalization can lead to better dispersion and surface activation, thereby increasing the interfacial interactions between MWCNTs and other nanoparticles for the formation of nanocomposites.40–42
Owing to the robust nature and ease of synthesis of nanozymes, they can act as substitutes for natural enzymes in disparate sensing applications. While natural enzymes are highly selective in nature, their activity depends on several parameters that include pH and temperature, making their application in sensors a tedious process. Taking into account the disadvantages of natural enzymes and significance of Se nanozymes, herein, we have fabricated a novel Se@fMWCNT peroxidase mimetic by a simple hydrothermal method. The functionalized MWCNT (fMWCNT) can serve as a host for Se as well as enhance the conducting properties of the nanocomposite. The synthesized material was employed for electrochemical sensing of H2O2 and GSH. Impressively, the developed sensor displayed wide linear ranges and low detection limits with high values of sensitivity towards both analytes.
2. Experimental section
2.1 Materials and methods
Selenium powder with approx. 100 mesh size and dopamine hydrochloride (DA) were purchased from Sigma Aldrich, India. Multi-walled carbon nanotubes, sodium phosphate monobasic anhydrous, sodium phosphate dibasic anhydrous, glutathione (GSH), uric acid (UA), tryptophan (L-Tryp), cholesterol (Chol), cystine (Cys), glucose (Glu) and potassium chloride (KCl) were acquired from SRL Chemicals, India. Leucine (Leu) was acquired from Sd Fine Chemicals, India. Hydrogen peroxide (H2O2) and ascorbic acid (AA) were purchased from Merck, India. Potassium perchlorate (KClO3) was obtained from Alfa Aesar, India, and potassium nitrite (KNO3) was bought from Avra Chemicals, India. Valine (Val) was purchased from Spectrochem, India. Glutone-Hydra tablets containing L-GSH were obtained from CRIUS life sciences and milk samples were obtained from a local vendor. All the chemicals obtained were of analytical grade and used without further purification. Milli-Q water was used to prepare all the sample solutions.2.2 Instrumentation
Powder X-ray diffraction (PXRD) spectra were obtained using a Bruker D8 advance instrument with iron-filtered Cu Kα radiation (λ = 1.5406 Å). Field emission scanning electron microscopy (FESEM) analysis was carried out on a Thermo-Fisher FEI QUANTA 250 FEG under high vacuum conditions, at an operating voltage range of 5–30 kV. High-resolution transmission electron microscopy (HR-TEM) studies were performed on a JEOL JEM 2100 with LaB6 as the electron source. X-ray photoelectron spectroscopic (XPS) studies were carried out using a PHI5000 Version Probe III. All electrochemical studies were performed using a CHI-760E electrochemical workstation. A conventional three-electrode system, with glassy carbon electrode (GCE) as the working electrode, Ag/AgCl as the reference electrode and platinum coil as the counter electrode, was used to perform the electrochemical studies.2.3 Functionalization of MWCNT
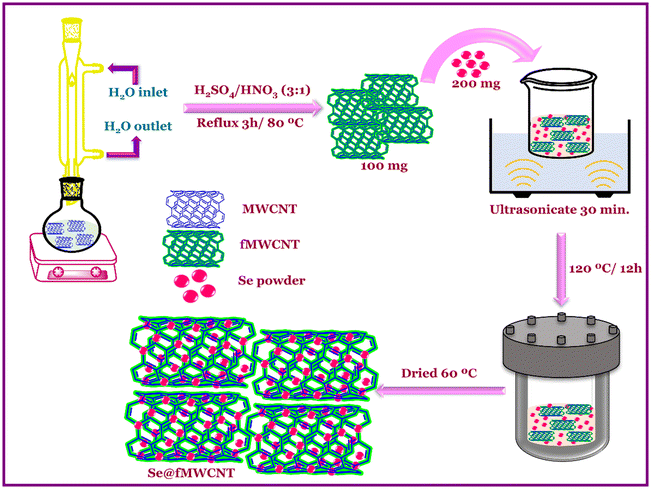
MWCNT was acid-functionalized using a previously reported literature method with minor modifications.43 Typically, 1 g of MWCNT was added into a round-bottomed flask consisting of a mixture of 20 mL HNO3 and 60 mL H2SO4, and the mixture was refluxed for 6 hours at 80 °C (Scheme 1). Later, the resultant mixture was washed continuously with distilled water until the filtrate attained a neutral pH value. Thereafter, the obtained sample was centrifuged and later dried overnight at 60 °C to obtain fMWCNT.2.4 Synthesis of Se@fMWCNT nanocomposite
After a few attempts, the procedure for the synthesis of Se@fMWCNT nanocomposite as a peroxidase mimic was optimized (Scheme 1) and is as follows. Initially, 200 mg of Se powder and 100 mg of fMWCNT were sonicated separately in 35 mL of H2O for 30 minutes, and then, the solutions were mixed and sonicated for 30 minutes. The concomitant mixture was taken in a 100 mL autoclave and heated for 12 hours at 120 °C. Then, the obtained precipitate was centrifuged multiple times with water and ethanol and was allowed to dry in a hot air oven at 60 °C overnight to attain Se@fMWCNT.2.5 Modification of working electrode
To begin with the GCE was polished with slurries of alumina of size 1 μm, 0.3 μm and 0.05 μm. Then, 10 μL of Se@fMWCNT (1 mg in 50 μL EtOH, 49 μL H2O and 1 μL Nafion) was drop-coated onto the pre-cleaned GCE and allowed to dry at room temperature to obtain Se@fMWCNT/GCE. Prior to performing the electrochemical studies, Se@fMWCNT/GCE was stabilized by conducting cyclic voltammetry (CV) in the potential range +0.2 to −0.8 V (vs. Ag/AgCl electrode).3. Results and discussion
3.1 Characterization of Se@fMWCNT
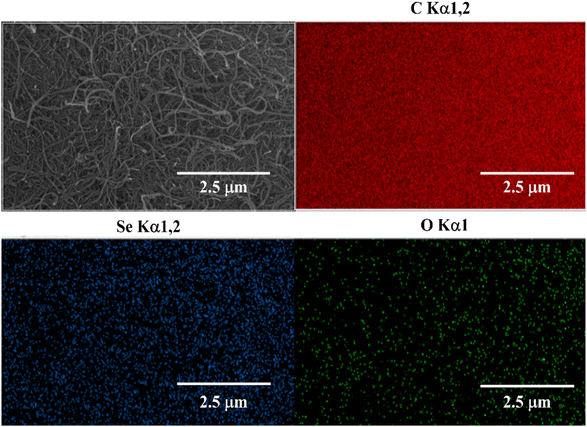
The formation of Se@fMWCNT nanocomposite has been investigated by recording the PXRD patterns of Se powder, fMWCNT and Se@fMWCNT, and the patterns are shown in Fig. 1a. The peaks at 23.4°, 29.6°, 41.1°, 43.6°, 45.2°, 51.4° and 55.8° are attributed to the (100), (101), (110), (102), (100), (201) and (112) planes of Se (blue curve) (JCPDS 06-0362). The XRD pattern of fMWCNT (green curve) shows two peaks at 25.8° and 42.6°, which can be correlated to the (002) and (100) planes, respectively, corresponding to graphitic carbon. The formation of Se@fMWCNT nanocomposite (orange curve) was confirmed with the appearance of an additional peak at 26.2° representing the (002) plane of fMWCNT along with the characteristic peaks of Se. Due to the higher intensity of the (102) plane of Se, the peak at 42.6° corresponding to the (100) plane of fMWCNT has diminished in the nanocomposite. Additionally, the HR-TEM images (Fig. 1b and c) and selected area electron diffraction (SAED) pattern (Fig. 1d) support that Se has been successfully anchored onto the fMWCNT. Fig. 1c shows the HR-TEM image of Se@fMWCNT and exhibits d-spacings of 0.26 nm and 0.33 nm, which can be attributed to the (102) and (002) planes. In addition, Fig. 1d discloses the SAED pattern, consisting of rings that can be assigned to the amorphous nature of fMWCNT (002) and crystalline nature of Se atoms, which can be indexed to the (101) and (201) planes. Moreover, the size of the Se nanoparticles on fMWCNT was obtained from the HR-TEM images, with the average size of Se found to be 7.9 nm. Furthermore, the morphology of the prepared Se@fMWCNT was probed using FESEM and elemental mapping, and the results are displayed in Fig. 2. The FESEM images show the uniform size of the Se nanoparticles, and the elemental mapping proved that the elements are uniformly distributed.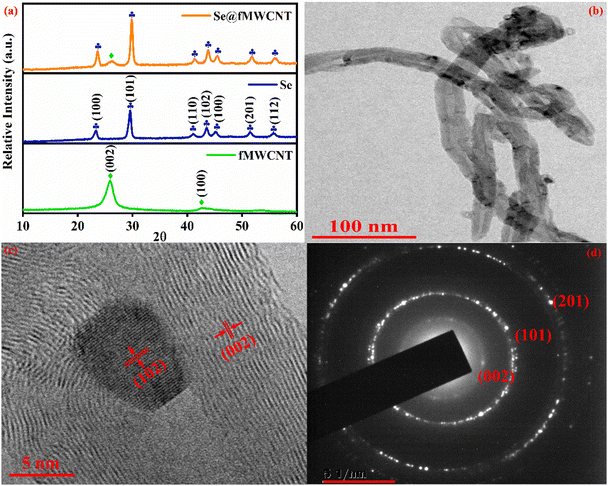 | ||
| Fig. 1 (a) XRD patterns of fMWCNT (green), Se (blue) and Se@fMWCNT (orange). (b) and (c) HR-TEM images and (d) SAED pattern of Se@fMWCNT. | ||
The surface composition of Se@fMWCNT (Fig. 3a) was identified using XPS, which reveals the presence of Se, carbon (C) and oxygen (O). In the Se 3d spectrum (Fig. 3b), the two major peaks at binding energy (B.E.) values of 55.7 eV and 54.8 eV represent the 3d3/2 and 3d5/2 states of Se, affirming the −2 oxidation state.44–46 The small peaks at 56.5 eV and 58.4 eV are due to the formation of C–Se bonds.45,47 The O 1s spectrum (Fig. 3c) shows the presence of C–OH at a B.E. value of 533.48 eV, HO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at a B.E. value of 532.58 eV and C
O at a B.E. value of 532.58 eV and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at a B.E. value of 531.38 eV.48,49 The deconvoluted spectrum of C 1s (Fig. 3d) unravels the presence of functional groups, such as C–O, C
O at a B.E. value of 531.38 eV.48,49 The deconvoluted spectrum of C 1s (Fig. 3d) unravels the presence of functional groups, such as C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COOH. The peaks at B.E. values of 284.48 eV and 284.98 eV could be assigned to C
O and COOH. The peaks at B.E. values of 284.48 eV and 284.98 eV could be assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (sp2) and C–C/C–Se (sp3), respectively.50,51 The peaks correlating to C–O species are found to occur between 286 and 289 eV, and the satellite peak around 291 eV could be assigned to the π–π* transition.50 Thus, the XPS results clearly infer the formation of Se@fMWCNT, which could subsequently be used for electrochemical detection.
C (sp2) and C–C/C–Se (sp3), respectively.50,51 The peaks correlating to C–O species are found to occur between 286 and 289 eV, and the satellite peak around 291 eV could be assigned to the π–π* transition.50 Thus, the XPS results clearly infer the formation of Se@fMWCNT, which could subsequently be used for electrochemical detection.
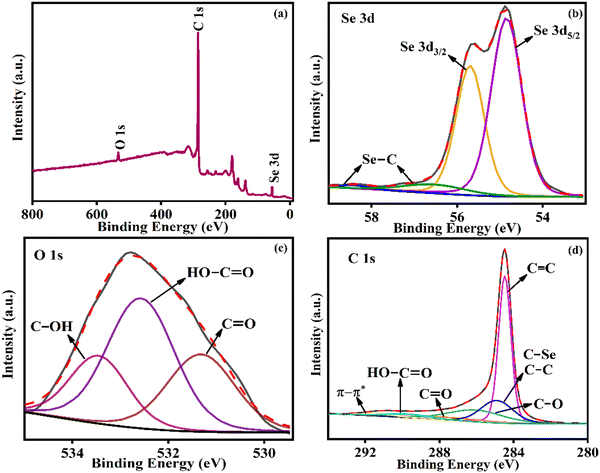 | ||
| Fig. 3 (a) XPS survey spectrum of Se@fMWCNT and core-level spectra of (b) Se 3d, (c) O 1s and (d) C 1s. | ||
3.2 Electrochemical and electrocatalytic behaviour of Se@fMWCNT/GCE
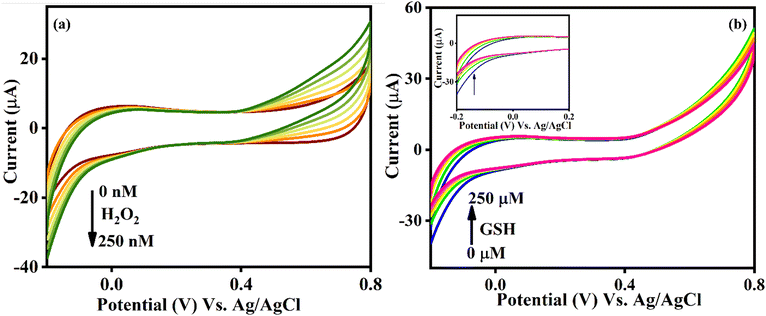
The electrochemical behaviour of the newly synthesized nanocomposite has been probed using CV. As anticipated, the Se@fMWCNT/GCE did not exhibit any redox activity in 0.1 M PBS (pH = 7; N2 saturated) in the potential window from −0.2 V to +0.8 V. Since redox behaviour was not observed for Se@fMWCNT/GCE, the scan rate effect was studied in the presence of a 2.5 mM ferricyanide/ferrocyanide redox couple. Both oxidation and reduction peak currents showed a gradual increment with increase in the scan rate. As shown in Fig. S1 (ESI†), the square root of scan rate vs. peak current had a linear relationship, indicating that the redox process occurring at the modified electrode surface is diffusion controlled. The electrochemical active surface area (ECSA) of the Se@fMWCNT/GCE and fMWCNT/GCE were also calculated using the Randles–Sevcik equation,52–54 as displayed below:| ip = 2.69 × 105 × A × C × n3/2 × D3/2 × v3/2, |
3.3 Amperometric detection of H2O2 and GSH
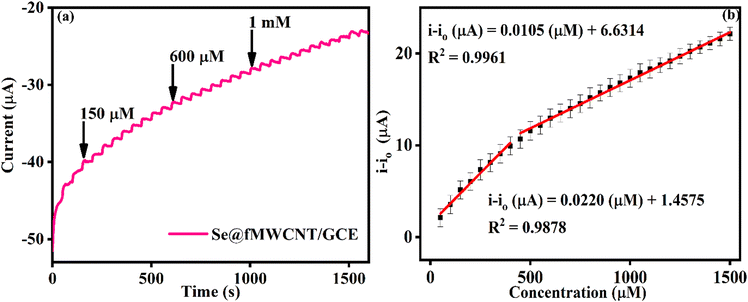
The impressive electrocatalytic activity of Se@fMWCNT/GCE towards H2O2 and GSH prompted us to investigate its performance under dynamic conditions using the amperometric technique. Hence, amperometric measurements were carried out in N2-saturated 0.1 M PBS (pH = 7) with an operating potential of −0.2 V and solution rotation speed of 350 rpm. Fig. 5a shows the amperometric (i–t) curve of Se@fMWCNT/GCE for different concentrations of H2O2 spiked at an addition time interval of 50 s. The Se@fMWCNT/GCE shows a uniform catalytic current response for successive addition of 50 nM H2O2, which clearly depicts the tremendous peroxidase enzyme mimicking property of the fabricated sensor obtained, resulting from the selection of Se metal and fMWCNT. The corresponding calibration plot (Fig. 5b) displays exceptional linearity in the concentration range from 50 nM to 1.4 μM with a regression coefficient (R2) value of 0.9992. The detection limit and sensitivity of Se@fMWCNT/GCE towards H2O2 sensing were found to be impressively, as 18.23 nM and 0.1585 μA μM cm−2, respectively. The linear range, detection limit and sensitivity thus obtained using Se@fMWCNT/GCE for the detection of H2O2 are comparable or better than for other recently reported nanozyme-based H2O2 sensors.55–58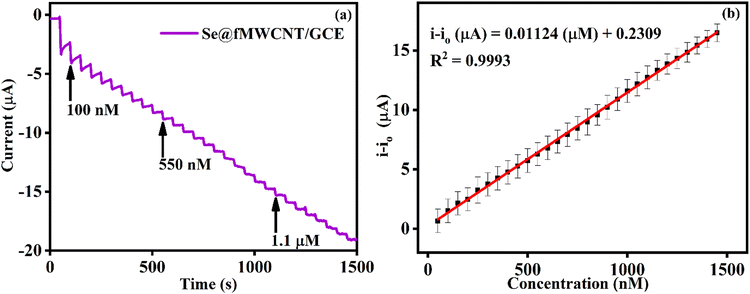 | ||
| Fig. 5 (a) Amperometric response of Se@fMWCNT/GCE for sequential addition of H2O2 in 0.1 M PBS (pH = 7; N2 saturated) at −0.2 V. (b) Calibration plot obtained from amperometric response. | ||
Similarly, GSH was detected amperometrically in 0.1 M PBS (pH = 7; N2 saturated) consisting of 1 mM H2O2. The operating potential was fixed at −0.2 V, and the solution was stirred at 350 rpm, and, as expected, a linear decrease in current was observed for every addition of GSH. This decrease in catalytic current is due to the oxidation of GSH to GSSH, and the sudden increase in current response is because of the availability of large numbers of active sites at Se@fMWCNT/GCE. The experiment was also carried out in the absence and presence of varying concentrations of H2O2 (0.1 mM, 1 mM and 10 mM), and the results are presented in Fig. S3 (ESI†). As anticipated, no reasonable difference in current was seen in the absence of H2O2 (Fig. S3a, ESI†) and a significant decrease in current response was noted in the presence of H2O2 (Fig. S3(b–d), ESI†). These results clearly infer that the Se@fMWCNT sensor detected GSH only when H2O2 is present, revealing that the sensor demonstrates GSHPx-like characteristics. Although obvious decrease in the current response was noticed at different concentrations of H2O2, a distinct and linear response was obtained only in the presence of 1 mM H2O2 (Fig. S3c, ESI†) and hence this concentration was chosen as the optimum concentration for amperometric determination of GSH (Fig. 6a). The mechanism of sensing of GSH is similar to that observed in the enzyme GSHPx and can be expressed as follows:
| H2O2 + R − Se− → R − SeOH + OH− | (i) |
| R − SeOH + GSH → R − Se − SG + H2O | (ii) |
| R − Se − SG + GSH → GSSG + H+ + R − Se− | (iii) |
The first step involves the formation of a selenic acid derivative (R−SeOH) by oxidation of R−Se− and generation of OH− anion by the reduction of H2O2. In the second step, R−SeOH reacts with the added analyte GSH and forms the selenyl-sulfide intermediate (R−Se−SG) and H2O. Then, in the third step, the R−Se–SG intermediate immediately reacts with another molecule of GSH to form GSSH, during which R−Se− is regenerated. These reactions occur in a cycle as long as the analyte GSH is available at the electrode surface, which results in a linear current response. The calibration plot corresponding to the amperometric response obtained is shown in Fig. 6b. The developed sensor had two linear ranges, from 50 to 450 μM and from 500 μM to 1.5 mM, and the LOD was calculated to be 19.2 μM. The sensitivities of the Se@fMWCNT/GCE sensor towards GSH detection were calculated to be 0.1465 and 0.2873 μA μM cm−2. The obtained analytical parameters are comparable or better than for the recently reported sensors for GSH detection.27,28,57,59–61 Furthermore, fMWCNT/GCE was employed for its ability to detect H2O2 and GSH as shown in Fig. S4(a and b) (ESI†). In detail, 50 nM of H2O2 was added to a solution of N2-saturated 0.1 M PBS (pH = 7) at a time interval of 50 s. Similarly, 50 μM of GSH was added to a solution of N2-saturated 0.1 M PBS (pH = 7) containing 1 mM H2O2 at a time interval of 50 s. From the figures it can be seen that fMWCNT/GCE (without Se) does not show any linear or reproducible response, and hence it cannot be employed for detection of H2O2 and GSH. This investigation also evidences that the enzyme mimicking activity could not be observed in the absence of Se and is attained only with Se@fMWCNT/GCE.
3.4 Peroxidase mimetic activity of Se@fMWCNT
The Michaelis–Menten constants (Km and Imax) are important in the case of biological enzymatic systems, as they provide vital information about the magnitude of the affinity between the enzyme and substrate. Lower values of Km indicate effective binding of the enzyme to the substrate and a higher value of Imax implies the better catalytic activity of the enzyme. Hence, the enzyme mimicking efficacy of the newly designed nanozymes could be understood by comparing these parameters with those of natural enzymes. The peroxidase-like activity of nanozymes can be evaluated by their ability to oxidise substrates such as TMB, ABTS, and OPD, in the presence of H2O2.62–64 Herein, the peroxidase mimicking characteristics of Se@fMWCNT were assessed primarily using UV-vis spectroscopy.63 Precisely, to a solution containing 1 mM H2O2 in 0.1 M PBS (pH = 7), 1 mg TMB and 1 mg mL−1 of the synthesized Se@fMWCNT were added (Fig. S5a inset, ESI†). As expected, the colour of the solution changed to blue, indicating the peroxidase mimicking activity of the synthesized Se@fMWCNT nanocomposite. Furthermore, on addition of 1 mM GSH, the solution turned colourless, restricting the further oxidation of TMB by Se@fMWCNT. The UV-vis spectrum (Fig. S5a, ESI†) of TMB in 1 mM H2O2 showed a weak absorbance, which was enhanced upon addition of 1 mg of Se@fMWCNT, proving the ability of Se@fMWCNT to oxidise TMB. Moreover, on introduction of 1 mM GSH to the aforementioned solution, the absorbance diminished, since GSH acts as a reductant, prohibiting further oxidation of TMB by the Se@fMWCNT nanocomposite.65 Furthermore, the peroxidase mimicking efficacy of the Se@fMWCNT nanozyme has been examined using amperometric measurements, and the Michaelis–Menten parameters were quantified using the Lineweaver–Burk equation, as follows:66where I represents anodic or cathodic current, C represents the concentration of analyte and Imax is the maximum electrocatalytic current. Km and Imax were calculated from the 1/I versus 1/C plots of H2O2 (Fig. S5b, ESI†) and were found to be 4.42 μM and 60.98 μA, respectively. The Km value of Se@fMWCNT nanocomposite with H2O2 as substrate was found to be remarkably less than that of the natural horseradish peroxidase (HRP) enzyme, which is indicative of a larger affinity of the synthesized nanocomposite towards H2O2.2
3.5 Interference and stability studies
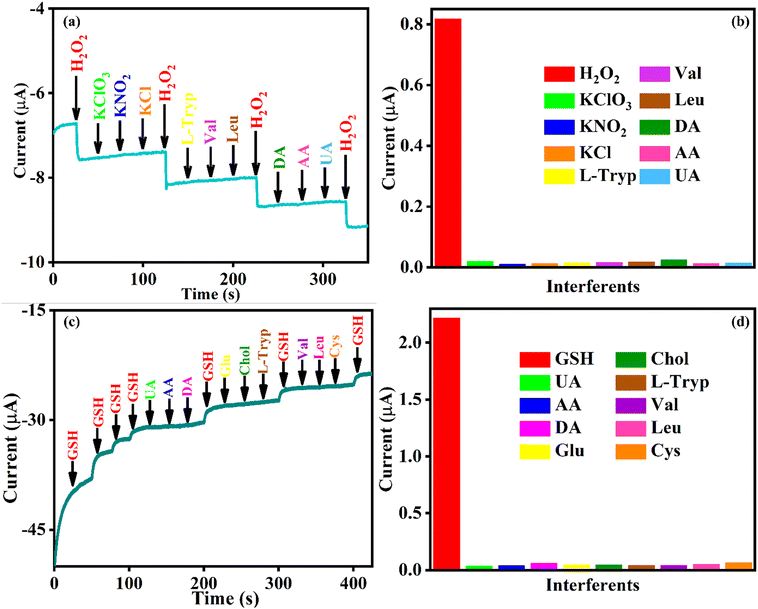
With the aim of investigating the selectivity for the real-time applicability of the constructed sensor towards electrochemical determination of H2O2 and GSH, the effect of interferents was also studied by performing amperometry in 0.1 M PBS (pH = 7), at an applied potential of −0.2 V. The interfering species, namely, KClO3, KNO2, L-Tryp, Val, Leu and Cys, were chosen since they have high tendency to interfere with H2O2 and GSH at an applied potential of −0.2 V. Further, DA, UA, AA, Chol and Glu are some well-known interfering species in biological systems, and hence were used as interferents in order to study the selectivity of the sensor. Fig. 7a shows the amperometric current response of Se@fMWCNT/GCE towards H2O2 along with a tenfold excess of other, concomitant interfering species. The corresponding columnar diagram of the current response (Fig. 7b) depicts that the developed sensor is highly selective towards H2O2. Similarly, the selectivity of Se@fMWCNT/GCE towards GSH was determined by carrying out amperometry at −0.2 V in 0.1 M PBS comprising of 1 mM H2O2. Fig. 7c shows the amperometric current response of Se@fMWCNT/GCE towards GSH along with a tenfold excess of other interferents. The respective columnar diagram of the current response (Fig. 7d) illustrates the high selectivity of Se@fMWCNT/GCE towards GSH, since no, or negligible, signal was obtained for any other interferents. The results clearly validate that the Se@fMWCNT/GCE sensor has exceptional selectivity towards biomimetic electrochemical detection of H2O2 and GSH.The stability of this newly proposed nanozyme-based sensor was also probed by recording cyclic voltammetry at a scan rate of 50 mV s−1 for 100 continuous cycles in 0.1 M PBS (pH = 7; N2 saturated), and the results are shown in Fig. S6 (ESI†). Fig. S6 (ESI†) clearly demonstrates that the constructed sensor was stable up to 100 cycles without any decline in current response, both in the absence and presence of 1 mM H2O2. The stability studies confirm the robust, lasting nature of the sensing probe Se@fMWCNT, which is an essential quality for real-time applications.
3.6 Real-time analysis
The sensor was tested for its ability to detect H2O2 and GSH in real-time samples. For this purpose, the fabricated sensor was employed towards sensing of H2O2 and GSH in milk samples and glutathione tablets, respectively, and this is shown in Fig. S7(a and b) (ESI†). Briefly, milk samples were diluted using 0.1 M PBS (pH = 7) and a known concentration of H2O2 was spiked by a standard addition method. Similarly, GSH tablets were diluted to 10 mM using 0.1 M PBS (pH = 7) and a known concentration was spiked to a solution of 0.1 M PBS (pH = 7), consisting of 1 mM H2O2. All the measurements were carried out in triplicate, and the fabricated sensor showed a good recovery percentage and low RSD values, as presented in Table 1.4. Conclusion
In summary, a simple viable Se@fMWCNT nanocomposite with intrinsic peroxidase mimicking activity has been synthesized via a hydrothermal method. The as-prepared nanocomposite showed excellent nanozyme activity and was utilized to construct an electrochemical biosensor for the determination of H2O2 and GSH. The system shows extraordinary Michaelis–Menten values, which reveals the unprecedented peroxidase-like activity. The Se@fMWCNT/GCE sensor functions by electrochemical reduction of H2O2 and subsequently facilitates the electrochemical oxidation of GSH. Selectivity of the sensor towards H2O2 and GSH was achieved by appropriate selection of Se and the presence of fMWCNT. In addition, fMWCNT served as a good host for Se and enhanced the conductivity, thereby contributing significantly towards the peroxidase activity of the sensor. Furthermore, the Se@fMWCNT/GCE sensor is expected to show a sensing mechanism with close resemblance to the naturally occurring GSHPx enzyme. This work exemplifies a new, simple and judiciously designed nanocomposite as an enzyme mimic, with outstanding peroxidase mimicking activity for the selective detection of H2O2 and GSH. The present investigation also opens up an avenue towards the design and engineering of novel nanomaterials with biomimetic activity for application in bioelectrocatalysis, clinical diagnosis and environmental screening.Author contributions
Vadakke Purakkal Sruthi: conceptualization, methodology, investigation, validation, writing – original draft. Sellappan Senthilkumar: conceptualization, resources, supervision, project administration, writing – review & editing.Conflicts of interest
There are no conflicts to declare.References
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Chem. Soc. Rev., 2019, 48, 3683–3704 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- B. Das, J. Lou Franco, N. Logan, P. Balasubramanian, M. Il Kim and C. Cao, Nanozymes in Point-of-Care Diagnosis: An Emerging Futuristic Approach for Biosensing, Nano-Micro Lett., 2021, 13, 193 CrossRef CAS.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- N. Waris, A. Hasnat, S. Hasan, S. Bano, S. Sultana, A. O. Ibhadon and M. Z. Khan, J. Mater. Chem. B, 2023, 11, 6762–6781 RSC.
- L. X. Yn, B. B. Wang, X. Zhao, L. J. Chen and X. P. Yan, ACS Appl. Mater. Interfaces, 2021, 13, 60955–60965 CrossRef PubMed.
- M. Bilal, N. Khaliq, M. Ashraf, N. Hussain, Z. Baqar, J. Zdarta, T. Jesionowski and H. M. N. Iqbal, Colloids Surf., B, 2023, 221, 112950 CrossRef CAS PubMed.
- Q. Wang, H. Wei, Z. Zhang, E. Wang and S. Dong, TrAC, Trends Anal. Chem., 2018, 105, 218–224 CrossRef CAS.
- Z. Chen, Y. Yu, Y. Gao and Z. Zhu, ACS Nano, 2023, 17, 13062–13080 CrossRef CAS PubMed.
- D. Mohanapriya, J. Satija, S. Senthilkumar, V. Kumar Ponnusamy and K. Thenmozhi, Coord. Chem. Rev., 2024, 507, 215746 CrossRef CAS.
- Y. Song, X. Wang, C. Zhao, K. Qu, J. Ren and X. Qu, Chem. – Eur. J., 2010, 16, 3617–3621 CrossRef CAS PubMed.
- H. Sun, Y. Zhou, J. Ren and X. Qu, Angew. Chem., 2018, 130, 9366–9379 CrossRef.
- X. Wang, H. Wang and S. Zhou, J. Phys. Chem. Lett., 2021, 12, 11751–11760 CrossRef CAS PubMed.
- D. Wang, D. Jana and Y. Zhao, Acc. Chem. Res., 2020, 53, 1389–1400 CrossRef CAS PubMed.
- X. Niu, X. Li, Z. Lyu, J. Pan, S. Ding, X. Ruan, W. Zhu, D. Du and Y. Lin, Chem. Commun., 2020, 56, 11338–11353 RSC.
- X. Huang, S. Zhang, Y. Tang, X. Zhang, Y. Bai and H. Pang, Coord. Chem. Rev., 2021, 449, 214216 CrossRef CAS.
- Y. Lin, J. Ren and X. Qu, Acc. Chem. Res., 2014, 47, 1097–1105 CrossRef CAS PubMed.
- J. Nemhauser and J. Chory, Arab. B., 2002, 2011, 1–32 Search PubMed.
- H. Ungati, V. Govindaraj, M. Narayanan and G. Mugesh, Angew. Chem., Int. Ed., 2019, 58, 8156–8160 CrossRef CAS PubMed.
- A. Iqubal, A. Khan, A. laeeq, K. Malhotra, M. A. Ansari and S. E. Haque, Curr. Pharm. Des., 2021, 27, 3881–3900 CrossRef CAS.
- J. Chaudiere, O. Courtin and J. Leclaire, Arch. Biochem. Biophys., 1992, 296, 328–336 CrossRef CAS PubMed.
- G. S. Heverly-Coulson and R. J. Boyd, J. Phys. Chem. A, 2010, 114, 1996–2000 CrossRef CAS PubMed.
- R. Brigelius-Flohé and M. Maiorino, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830, 3289–3303 CrossRef.
- S. Ghosh, P. Roy, N. Karmodak, E. D. Jemmis and G. Mugesh, Angew. Chem., Int. Ed., 2018, 57, 4510–4515 CrossRef CAS PubMed.
- G. Liu, T. Xia, J. Wei, S. Hou and S. Hou, ChemElectroChem, 2022, 9, 1–8 Search PubMed.
- T. Zhang, N. Lu, C. Wang, H. Jiang, M. Zhang, R. Zhang, Y. Zhong and D. Xing, ACS Appl. Mater. Interfaces, 2023, 40, 46738–46746 CrossRef PubMed.
- Y. Lai, J. Wang, N. Yue, Q. Zhang, J. Wu, W. Qi and R. Su, Biomater. Sci., 2023, 11, 2292–2316 RSC.
- F. Qiao, J. Wang, S. Ai and L. Li, Sens. Actuators, B, 2015, 216, 418–427 CrossRef CAS.
- Y. Huang, C. Liu, F. Pu, Z. Liu, J. Ren and X. Qu, Chem. Commun., 2017, 53, 3082–3085 RSC.
- S. L. Ruizhen Tian, H. Ma, W. Ye, Y. Li, S. Wang, Z. Zhang, F. B. Mingsong Zang, J. Hou, J. Xu, Q. Luo, H. Sun, J. Liu and Y. Yang, Adv. Funct. Mater., 2022, 32, 2204025 CrossRef.
- T. Thomas, R. J. Mascarenhas, P. Martis, Z. Mekhalif and B. E. K. Swamy, Mater. Sci. Eng., C, 2013, 33, 3294–3302 CrossRef CAS PubMed.
- W. Yuan, G. Jiang, J. Che, X. Qi, R. Xu, M. W. Chang, Y. Chen, S. Y. Lim, J. Dai and M. B. Chan-Park, J. Phys. Chem. C, 2008, 112, 18754–18759 CrossRef CAS.
- H. Z. Zardini, A. Amiri, M. Shanbedi, M. Maghrebi and M. Baniadam, Colloids Surf., B, 2012, 92, 196–202 CrossRef CAS PubMed.
- M. Kumar, B. E. Kumara Swamy, S. Reddy, W. Zhao, S. Chetana and V. Gowrav Kumar, J. Electroanal. Chem., 2019, 835, 96–105 CrossRef CAS.
- S. A. Kumar, S. F. Wang, Y. T. Chang, H. C. Lu and C. T. Yeh, Colloids Surf., B, 2011, 82, 526–531 CrossRef CAS PubMed.
- N. Murugan, T. H. V. Kumar, N. R. Devi and A. K. Sundramoorthy, New J. Chem., 2019, 43, 15105–15114 RSC.
- Y. Wang, W. Li, H. Li, M. Ye, X. Zhang, C. Gong, H. Zhang, G. Wang, Y. Zhang and C. Yu, Chem. Eng. J., 2021, 414, 128925 CrossRef CAS.
- S. Mallakpour, A. Abdolmaleki and S. Borandeh, Prog. Org. Coat., 2014, 77, 1966–1971 CrossRef CAS.
- F. Avilés, J. V. Cauich-Rodríguez, L. Moo-Tah, A. May-Pat and R. Vargas-Coronado, Carbon, 2009, 47, 2970–2975 CrossRef.
- L. Y. Jun, N. M. Mubarak, L. S. Yon, C. H. Bing, M. Khalid and E. C. Abdullah, J. Environ. Chem. Eng., 2018, 6, 5889–5896 CrossRef CAS.
- Z. Zhao, Z. Yang, Y. Hu, J. Li and X. Fan, Appl. Surf. Sci., 2013, 276, 476–481 CrossRef CAS.
- D. Cheng, L. Yang, R. Hu, J. Liu, R. Che, J. Cui, Y. Wu, W. Chen, J. Huang, M. Zhu and Y. J. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 36685–36696 CrossRef CAS PubMed.
- S. Xiao, Z. Li, J. Liu, Y. Song, T. Li, Y. Xiang, J. S. Chen and Q. Yan, Small, 2020, 16, 1–9 Search PubMed.
- A. S. Kshirsagar, C. Hiragond, A. Dey, P. V. More and P. K. Khanna, ACS Appl. Energy Mater., 2019, 2, 2680–2691 CrossRef CAS.
- Z. Li, L. Yuan, Z. Yi, Y. Liu and Y. Huang, Nano Energy, 2014, 9, 229–236 CrossRef CAS.
- J. Yuan, J. X. Mi, R. Yin, T. Yan, H. Liu, X. Chen, J. Liu, W. Si, Y. Peng, J. Chen and J. Li, ACS Catal., 2022, 12, 1024–1030 CrossRef CAS.
- J. Li, P. Yu, J. Xie, J. Liu, Z. Wang, C. Wu, J. Rong, H. Liu and D. Su, ACS Catal., 2017, 7, 7305–7311 CrossRef CAS.
- L. Ma, H. L. Zhuang, S. Wei, K. E. Hendrickson, M. S. Kim, G. Cohn, R. G. Hennig and L. A. Archer, ACS Nano, 2016, 10, 1050–1059 CrossRef CAS PubMed.
- S. Pal, S. Bawari, T. Veettil Vineesh, N. Shyaga and T. N. Narayanan, ACS Appl. Energy Mater., 2019, 2, 3624–3632 CrossRef CAS.
- L. Qian, A. R. Thiruppathi, J. Van Der Zalm and A. Chen, ACS Appl. Nano Mater., 2021, 4, 3696–3706 CrossRef CAS.
- M. Zahirul Kabir, C. Erkmen, S. Kurbanoglu, G. Aydogdu Tig and B. Uslu, J. Electroanal. Chem., 2023, 944, 117651 CrossRef CAS.
- N. Mavis Xhakaza, R. Chokkareddy and G. G. Redhi, J. Mol. Liq., 2022, 368, 120444 CrossRef CAS.
- S. N. Bhaduri, D. Ghosh, S. Chatterjee, R. Biswas, A. Bhaumik and P. Biswas, J. Mater. Chem. B, 2023, 11, 8956–8965 RSC.
- M. Xu, W. Wang, H. Han, Y. Wei, J. Sha and G. Liu, Chem. Eng. J., 2023, 475, 146324 CrossRef CAS.
- C. Liu, Y. Cai, J. Wang, X. Liu, H. Ren, L. Yan, Y. Zhang, S. Yang, J. Guo and A. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 42521–42530 CrossRef CAS PubMed.
- M. Sherazee, S. R. Ahmed, P. Das, S. Srinivasan and A. R. Rajabzadeh, Colloids Surf., A, 2023, 679, 132576 CrossRef CAS.
- Z. Xu, P. Li, X. Liu, X. Zhu, M. Liu, Y. Zhang and S. Yao, Electrochim. Acta, 2022, 434, 141273 CrossRef CAS.
- A. M. Mahmoud, M. H. Mahnashi and M. M. El-Wekil, Anal. Chim. Acta, 2023, 1272, 341498 CrossRef CAS.
- A. M. Mahmoud, B. A. Alyami, M. H. Mahnashi, F. M. Alshareef, Y. S. AlQahtani and M. M. El-Wekil, Microchem. J., 2023, 187, 108419 CrossRef CAS.
- W. Song, B. Zhao, C. Wang, Y. Ozaki and X. Lu, J. Mater. Chem. B, 2019, 7, 850–875 RSC.
- X. Zhou, L. Kong, J. Hao, J. Feng, S. Sun, C. Zhou, Y. Liu, Z. Yan, X. Zhu and L. Hu, J. Mater. Chem. C, 2023, 11, 13047–13055 RSC.
- M. Sun, K. Pu, X. Hao, T. Liu, Z. Lu, G. Su, C. Wu, Y. Wang, S. Cai, X. Zhao and H. Rao, J. Mater. Chem. C, 2023, 12, 221–231 RSC.
- A. R. Hormozi Jangi, M. R. Hormozi Jangi and S. R. Hormozi Jangi, Chin. J. Chem. Eng., 2020, 28, 1492–1503 CrossRef.
- N. Lu, Y. Liu, X. Yan, Z. Xu, Y. Xing, Y. Song, P. Zhao, M. Liu, Y. Gu, Z. Zhang and S. Zhai, ACS Appl. Nano Mater., 2022, 8, 11361–11370 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Effect of scan rate, electrocatalytic activity, amperometric curve (i–t) in different concentrations of H2O2, Lineweaver–Burk plot, stability studies. See DOI: https://doi.org/10.1039/d4tc01231c |
| This journal is © The Royal Society of Chemistry 2024 |