Simultaneous NIR photoluminescence and mechanoluminescence from Cr3+ activated MgGa2O4 phosphors with multifunctions for optical sensing†
Yaowu
Wang
a,
Guocheng
Pan
a,
Jianfeng
Wang
*b,
Yinyan
Li
 a,
Zhenping
Wu
a,
Zhenping
Wu
 c,
Shiqing
Xu
a and
Gongxun
Bai
c,
Shiqing
Xu
a and
Gongxun
Bai
 *a
*a
aCollege of Optical and Electronic Technology, China Jiliang University, Hangzhou 310018, China. E-mail: baigx@cjlu.edu.cn; jifewang@gmail.com
bCollege of Sciences, China Jiliang University, Hangzhou 310018, China
cLaboratory of Optoelectronics Materials and Devices, School of Science, Beijing University of Posts and Telecommunications, Beijing 100876, China
First published on 10th February 2024
Abstract
Light-emitting diodes (LEDs) combined with near-infrared (NIR) phosphors are rapidly becoming important light sources for food safety, anti-counterfeiting and biological imaging due to non-destructive and non-toxic advantages. Single-phase materials with multi-mode luminescence under different stimuli are crucial for the development and applications of smart NIR phosphors. Here, we have developed multifunctional Cr3+ activated MgGa2O4 (MGO:Cr3+) phosphors with simultaneous photoluminescence and mechanoluminescence for NIR sensing. Under 470 nm excitation, the phosphors can produce emission bands from 650 to 850 nm. Meanwhile, the flexible devices based on phosphors can exhibit strong emissions in response to mechanical deformation, such as shock and friction. In addition, optical manometers based on luminescence from phosphors can achieve pressure sensing from 7 to 64 KPa. More intriguingly, we combined the MGO:Cr3+ phosphor with a blue LED chip to fabricate an NIR phosphor-converted LED for anti-counterfeiting and biological tissue penetration. Our results suggest that the multifunctional NIR phosphors with simultaneous photoluminescence and mechanoluminescence have potential applications in various sensing devices.
1. Introduction
Near-infrared (NIR) light occupies the electromagnetic spectrum region between visible and mid-infrared wavelengths.1 Due to its notable penetration, imperceptibility, and non-destructive properties, NIR light is utilized in diverse fields such as food quality assessment, biological tissue monitoring, component analysis, and AI-based imaging. Based on photon energy, the International Commission on Illumination categorizes electromagnetic waves of 700 to 1400 nm wavelengths as near-infrared light.2–4 Conventionally, this spectral region is divided into NIR I (700–950 nm) and NIR II (1000–1400 nm). Infrared products, with their extensive utility in both industrial processes and daily life, are anticipated to see consistent growth in the future market demand.5 For instance, in food biological analysis, near-infrared spectroscopy aids in the analysis and detection of key components. This capability stems from the unique ability of NIR light to interact with C–H, O–H, and N–H chemical groups, fundamental components of water, proteins, fat, and carbohydrates.6 Near-infrared luminescent materials can be excited by a variety of excitation processes, such as photoluminescence (PL), mechanical luminescence (ML), electroluminescence (EL), and thermoluminescence (TL).7–9 Additionally, there are also some novel stimulation sources, such as modifying and improving some advanced magnetite nanomaterials to respond to external magnetic fields and produce bright near-infrared emissions.10,11 Furthermore, sonoluminescence, a fascinating phenomenon, involves tiny bubbles in liquid emitting light when exposed to strong sound waves. This phenomenon has sparked significant interest among scientists due to its intriguing physical properties and potential applications across various fields. Consequently, the development of near-infrared luminescent materials adaptable to diverse stimuli is crucial for advancing luminescent material technology.12Recent reports indicate that the active ions contributing to near-infrared (NIR) emission are mainly transition metal ions (Mn2+, Cr3+, and Ni2+),13 Ln3+ doped ions (Eu3+, Tb3+, Er3+, and Tm3+),14 and main group metal ions (Bi3+ and Bi2+).15–17 Near-infrared phosphors can be categorized into three groups based on their activating ions: those activated by rare earth ions, main group ions, and transition metal ions.18 Cr3+ is extensively investigated as an activator of near-infrared luminescence, serving as a representative transition metal ion with partially filled d orbitals. The intensity of its emission spectrum relies on the crystal field environment of the host lattice.19 Additionally, Cr3+ ions possess notable light penetration properties. They can capture light signals passing through biological tissues, indicating potential for developing narrowband ML materials for the NIR I region.20 Also, Cr3+ is efficiently excitable by blue light. Using blue LED chips with Cr3+ doped near-infrared phosphors can notably boost near-infrared emission efficiency.21–23
In this study, we synthesized the MGO:Cr3+ phosphor emitting near-infrared light through a high-temperature solid-phase reaction, capitalizing on the luminescence characteristics of Cr3+ ions. Our investigation comprehensively analyzed the material's crystal structure, microstructure, and durability. Importantly, we investigated the phosphor's dual-mode response (PL and ML), specifically its pressure-sensing capabilities in ML mode when combined with polydimethylsiloxane (PDMS). Additionally, we combined MGO:Cr3+ with a commercial LED chip, forming a new pc-LED capable of penetrating biological tissues and expanding its potential applications, particularly in security.
2. Experimental section
Materials and synthesis: MgGa2O4:x%Cr3+ (0.5 ≤ x ≤ 2.0) powder was synthesized via the traditional high-temperature solid-state reaction method, where x represents the atomic percentage. MgO (99.99% purity, Aladdin), Ga2O3 (99.99% purity, Aladdin), and Cr2O3 (99.99% purity, Aladdin) were chosen as the initial raw materials to guarantee sample quality. Subsequently, the samples were poured into an agate mortar, fully and completely ground for 30 minutes, and finally transferred to a corundum crucible and placed in a tubular sintering furnace for 8 h. After the sample was naturally cooled to room temperature, it was removed from the furnace and poured into the mortar to continue to fully grind, and finally the required sample MgGa2O4:Cr3+ was obtained.Preparation of MGO:x%Cr3+/PDMS composite elastomers: Elastic ML films were produced by blending the prepared phosphor and PDMS (Dow Corning) in a weight ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Initially, 8 g of the PDMS substrate, 0.8 g of a curing agent, and 12 g of MGO:2%Cr3+ were accurately weighed into specialized Petri dishes with a 55 mm diameter, thoroughly mixed, and continuously stirred for 15 minutes. Subsequently, the solution was dried and cured in an oven at 70 °C for 1 hour to yield an elastomer exhibiting ML properties.
2. Initially, 8 g of the PDMS substrate, 0.8 g of a curing agent, and 12 g of MGO:2%Cr3+ were accurately weighed into specialized Petri dishes with a 55 mm diameter, thoroughly mixed, and continuously stirred for 15 minutes. Subsequently, the solution was dried and cured in an oven at 70 °C for 1 hour to yield an elastomer exhibiting ML properties.
3. Results and discussion
3.1 Crystal structure and microstructure
Both crystal structure and microstructure are essential for material characterization. The X-ray diffraction (XRD) patterns of prepared MGO:x%Cr3+ (x = 0, 0.5, 1, 1.5, 2) are shown in Fig. 1a. The results shown align well with the MGO's standard card (PDF#10-0113). At the same time, no secondary phase and out of phase responses of the material were observed, indicating that Cr3+ doping on this surface did not affect the composition of the main phase of the material, and its purity and crystallinity were also high. A clear angular shift is observed when compared with the standard spectrum, indicating the successful doping of Cr3+ ions into MGO. MGO is a typical spinel structure, and its crystal structure is shown in Fig. 1b. Mg2+, Ga3+ and O2− ions are represented by blue, yellow and red spheres respectively. The Ga3+ ion has 6 coordination and the Mg2+ ion has 4 coordination. The [GaO6] octahedra are interconnected by shared edges, in addition to which they are connected to the [MgO4] tetrahedra by sharing vertexes to form a rigid lattice structure.24 Considering that the doping process follows the principle of similar ionic radii, Cr3+ (r = 0.615 Å) is more inclined to occupy the Ga3+ (r = 0.662 Å) site.25–29 We conjecture the presence of [CrO6] octahedra within the MGO:Cr3+ phosphor. The crystal structure diagram illustrates the sequential coordination of Ga3+ ions with Ga–O, Ga–Ga, and Ga–Mg shells.Examination of the SEM images of the MGO:2%Cr3+ sample (Fig. S1, ESI†) reveals that the phosphor we synthesized possesses a regular cylindrical shape with a central hollow core. Overall, MGO exhibits a combination of agglomeration and dispersion. Fig. 1c presents the energy dispersion spectrum of MGO, clearly indicating the presence of Cr element in the prepared phosphor. Analysis of the elemental diagram in Fig. 1d reveals that Cr3+ ions are present in small quantities in MGO, with Mg, Ga, Cr, and O elements uniformly dispersed in a columnar form. X-ray photoelectron spectroscopy (XPS) analysis reveals the site occupancy and localized structure of Cr3+. The XPS spectra of Cr3+ in MGO reveal a peak at 578.6 eV (Fig. S2, ESI†), aligning closely with the binding energy characteristics of trivalent chromium ions within the [CrO6] octahedra.30,31 We also analyzed and measured the XPS patterns of other elements, which enhances our understanding of the energy levels of other elements.
3.2 Photoluminescence properties
We employed the conventional solid-phase sintering method to prepare the MGO:Cr3+ phosphor. For a more in-depth exploration of its optical properties, Fig. 2a displays the excitation and emission spectra of the MGO:Cr3+ phosphor. When pumped with a 460 nm laser, it is found that the PL spectra of MGO:Cr3+ are composed of wide-band near-infrared light in the range of 600 to 900 nm, with a peak emission peak centered at 710 nm, which is in line with the 2E → 4A2 transition of Cr3+. At the same time, the PLE spectrum of MGO:Cr3+ detected at 710 nm consists of two strong absorption bands centered at 440 (4A2 → 4T1) and 590 nm (4A2 → 4T2), which matches the commercial blue LED chip and can be better used as a device in the later stage.32–34Fig. 2b shows the PL spectrum of MGO:x%Cr3+ (x = 0–2). It can be seen from the figure that the emission intensity increases when the concentration of Cr3+ increases. At the same time, in order to better know the concentration of Cr3+ ions with the best emission intensity, we increase x from 2 to 4. The emission intensity of the phosphor gradually increases, and then, due to the concentration quenching effect, reaches a maximum value at x = 2 and begins to gradually decrease (Fig. S3, ESI†). Thus, MGO:2%Cr3+ achieves the strongest overall emission intensity. In addition, we also observed that when we excited at 460 nm, the full-width at half-maximum (FWHM) also changed with the increase of Cr3+ concentration. As shown in Fig. 2c, when x = 0.2, FWHM reaches a maximum value of about 158 nm, which is the result of the combined contribution of 2E → 4A2 and 4T2 → 4A2 in Cr3+. Fig. 2d shows the diffuse reflection spectra of MGO:Cr3+ samples. It is evident from Fig. 2d that the peaks of MGO:Cr3+ at 440 and 590 nm correspond to two different absorptions, belonging to the 4A2 → 4T1 and 4A2 → 4T2 transitions of Cr3+, which are highly consistent with the emission peaks in the PLE spectrum. At the same time, with the increase of Cr3+ doping concentration in the sample, the reflection intensity of MGO:Cr3+ also gradually increased. Based on the diffuse reflection spectral data shown in Fig. S4 (ESI†), we calculated the band gap of the MGO:2%Cr3+ sample to be 2.43 eV.In order to better understand the luminescence mechanism of MGO:Cr3+, we explained it theoretically by using the Tanabe–Sugano theory and using Dq/B to appraise the crystal field intensity. The crystal field parameters are represented as Dq, and the Racah parameters are represented as B.
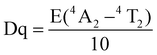 | (1) |
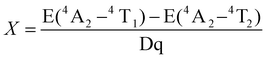 | (2) |
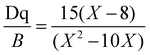 | (3) |
According to the calculation, we finally determine that Dq/B = 3.2, as shown in Fig. 2e. According to the data, when Dq/B > 2.3, the main body is a strong crystal field and 2E → 4A2 of Cr3+ ions dominates.35–37 At this point, the NIR light exhibits narrowband emission. When Dq/B < 2.3, indicating a weak crystal field, a 4T2 → 4A2 transition occurs, leading to broadband emission. Simultaneously, the emission spectra of Cr3+ doped NIR phosphors predominantly exhibit narrow bands (2E → 4A2 transition) in strong crystal fields and wide bands (4T2 → 4A2 transition) in weak crystal fields.38 In medium-intensity crystal fields, the coexistence of these two bands can be observed in the spectrograph, as demonstrated by our preparation of MGO:Cr3+. In addition, we monitored the correlation between the Cr3+ content and the photoluminescence lifetime of the prepared MGO:x%Cr3+ samples (Fig. 2f). We observed that when x = 2, the fluorescence lifetime of MGO reached its maximum value. Subsequently, with the continuous increase in concentration, the lifetime gradually decreased due to the concentration quenching effect.39–41 The specific reason is that the non-radiative transition between Cr3+ ions increases with the continuous increase of Cr3+ concentration, leading to the shortening of its lifetime. In summary, MGO:Cr3+ has the best performance when the concentration of Cr3+ is 2%.
3.3 Thermal stability
Thermal stability is one of the important parameters to characterize phosphors. In this work, in order to evaluate the thermal stability, we studied the temperature-dependent PL spectrum of MGO:Cr3+ (Fig. 3a). As temperature rises, the luminous intensity gradually decreases, but the emission wavelength of the MGO:Cr3+ NIR phosphor, prepared by us, remains relatively constant. This suggests that the crystal field intensity of Cr3+ in the phosphor does not significantly change with temperature. At the same time, we studied the spectral intensity of MGO:Cr3+ at 710 nm under 460 nm excitation, and cumulatively drew the data into a histogram (Fig. 3b). With the gradual increase of temperature, the spectral emission intensity of MGO:Cr3+ gradually decreases. The emission intensity at 303 K is 87.42% of the initial level, at 473 K is 52.31% of the initial level, and at 523 K is 38.34% of the initial level. This indicates that the prepared MGO:Cr3+ phosphor also has a certain stability at ultra-high temperatures. In addition to stability, reversibility and repeatability checks are also extremely important for phosphor testing. We repeated 8 cycles of heating and cooling of MGO:2%Cr3+ at 303, 473 and 523 K, and it can be seen from Fig. 3c that after several cycles of experiments, the spectral intensity is basically at the same level, and the samples prepared on this surface are also reproducible.To better understand the factors affecting the thermal stability of phosphors, we extensively reviewed the data. Our investigation shows that lower concentrations of active ions tend to increase structural rigidity and lead to wider main band gaps, which helps develop phosphors with stronger thermal stability.42–44 In addition to this, another key factor affecting thermal stability is the thermal quenching performance of the material. The thermal quenching performance of MGO:Cr3+ can be illustrated by the configuration diagram. As can be seen from Fig. 3d, at high temperatures, the electrons of the 4T2 state will reach the intersection of the 4T2 excited state and the 4A2 ground state (process IV, activation energy is provided in the form of ΔE2) and then return to the ground state by non-radiative transition (process V), which is the thermal quenching process of the 4T2 state.45–47 The heat quenching process of the 2E state is completely different. After excitation, it is easier to lift the electrons of the 2E state to the intersection of the 2E and 4T2 excited states (process III, where the activation energy is provided in the form ΔE1), after which the electrons will return to the ground state via radiative transitions (process II) and non-radiative transitions (processes IV and V). For MGO:Cr3+, due to large Dq/B (Fig. 2e), we can clearly observe the narrowband emission of 2E → 4A2. As temperature increases, the 2E state will not have enough electrons to compensate for the electrons lost through the non-radiative transition, resulting in a decrease in thermal stability. However, we found that when 4T2 → 4A2 is dominant, thermal stability is higher than when 2E → 4A2 is dominant. Therefore, we can allow the coexistence of sharp 2E → 4A2 and wideband 4T2 → 4A2 to significantly improve the thermal stability of Cr3+ activated NIR light emission, which also provides a new idea for our subsequent work.
3.4 ML properties
In order to explore whether MGO:Cr3+ has multi-mode response, we further examined its ML characteristics. Using the special stress sensing characteristics of the PDMS film, we mixed PDMS and MGO:Cr3+ to produce a new near-infrared fluorescent film. We applied different forces to the film and measured its spectrum through a fiber optic spectrometer. Fig. 4a shows that as the force we applied increased, the ML strength of MGO:Cr3+ also increased. Meanwhile, we measured its FWHM and observed that FWHM increased with the increasing force (Fig. 4b). In addition to the applied stress, the concentration of doping ions is also a key factor that can affect the strength of ML. Fig. 4c shows that with increasing Cr3+ ion concentration, the ML strength of MGO:x%Cr3+ (0 ≤ x ≤ 2.0) first increases and then decreases, reaching a maximum at x = 2 (at which point the force fixed to the material is 10 N).Based on the above results, we can conclude the potential ML mechanism of MGO:Cr3+ (Fig. 4d). Upon external mechanical stimulation of MGO:Cr3+, it produces an internal piezoelectric field, capturing charges through the piezoelectric effect. The piezoelectric field-induced band bending enables the release of electrons from the trap to the conduction band. Additionally, they can directly achieve the dopant level of Cr3+ by leveraging the tunneling effect. Subsequently, non-radiative energy is released through electron–hole recombination, exciting Cr3+ ions and facilitating electron relaxation to achieve ML. In summary, the ML phenomenon in MGO:Cr3+ is contingent on the induction of electrons into the trap state, disrupting the electron distribution.48–52 This process leads to the creation of a localized piezoelectric field within the inter-electronic energy level, facilitating electron transfer and energy conversion.
3.5 Stress sensing
Additionally, it should be noted that certain fluorescent powders exhibit sensitivity to pressure, such as Sr8Si4O12Cl8:Eu2+, which emits yellow light above 7 GPa and undergoes a significant red shift with increasing pressure.53 Utilizing upconversion, sub-micron-sized YVO4:Yb3+ Er3+ demonstrates high sensitivity and accuracy in ultra-low-pressure ranges.54 A single lanthanide-doped upconversion material, YPO4:Yb3+ Er3+, can also be employed for optical sensing of low and high-pressure values.55 At the same time, the MGO:Cr3+ fluorescent powder we prepared is also sensitive to dynamic loading. By leveraging this characteristic, we can transform this fluorescent powder into a pressure sensor. As shown in Fig. 5a, the MGO:Cr3+ phosphor and PDMS solvent are fully mixed in a mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and then cut into a normal rectangle (50 × 18 × 2 mm) after the process of drying and cooling. After fixing one end, the other part was connected to a tensiometer, and different degrees of tensile force were applied to it. At the same time, we irradiated pre-made PDMS + MGO:Cr3+ films with a 460 nm laser and measured their spectral data by placing a fiber optic spectrometer in the vertical direction. To counteract the 460 nm laser, we placed a 550 nm filter in front of the fiber probe to eliminate the inherent effects of the 460 nm laser. With a fixed 460 nm laser power, the tension force applied to the film was systematically varied, and the resulting data were collected using a fiber spectrometer. The obtained results are presented in Fig. 5b and c. From the above figure, it can be seen that the spectral intensity of the film gradually increases as the pressure applied to the side of the film increases. The emission bands at 710 and 770 nm showed a linear increment trend. The spectral intensity data of 710 and 770 nm were taken out separately for linear fitting. It can be seen from Fig. 5d that the spectral intensity and force basically have a linear relationship, and the final fitting value is R2 = 0.998. The better fitting results can prove that our prepared phosphors have good application prospects in stress sensing in the future. By harnessing the sensitivity of MGO:Cr3+ thin film to dynamic loading and the imperceptibility of near-infrared light, we captured the real-time single-point dynamic pressure trajectory of personal handwriting on the thin film, as depicted in Fig. S5 (ESI†). The pressure visualization of handwritten “CJLU” is indistinguishable to the naked eye but becomes discernible under near-infrared cameras.
2 and then cut into a normal rectangle (50 × 18 × 2 mm) after the process of drying and cooling. After fixing one end, the other part was connected to a tensiometer, and different degrees of tensile force were applied to it. At the same time, we irradiated pre-made PDMS + MGO:Cr3+ films with a 460 nm laser and measured their spectral data by placing a fiber optic spectrometer in the vertical direction. To counteract the 460 nm laser, we placed a 550 nm filter in front of the fiber probe to eliminate the inherent effects of the 460 nm laser. With a fixed 460 nm laser power, the tension force applied to the film was systematically varied, and the resulting data were collected using a fiber spectrometer. The obtained results are presented in Fig. 5b and c. From the above figure, it can be seen that the spectral intensity of the film gradually increases as the pressure applied to the side of the film increases. The emission bands at 710 and 770 nm showed a linear increment trend. The spectral intensity data of 710 and 770 nm were taken out separately for linear fitting. It can be seen from Fig. 5d that the spectral intensity and force basically have a linear relationship, and the final fitting value is R2 = 0.998. The better fitting results can prove that our prepared phosphors have good application prospects in stress sensing in the future. By harnessing the sensitivity of MGO:Cr3+ thin film to dynamic loading and the imperceptibility of near-infrared light, we captured the real-time single-point dynamic pressure trajectory of personal handwriting on the thin film, as depicted in Fig. S5 (ESI†). The pressure visualization of handwritten “CJLU” is indistinguishable to the naked eye but becomes discernible under near-infrared cameras.
At the same time, we studied the sensitivity of the phosphor to static load. We placed 20 g weights on the MGO:Cr3+/PDMS film to provide pressure. After the 460 nm laser excitation, the data were measured with the fiber spectrometer, as shown in Fig. 5e. It can be seen that the obtained spectra were basically similar to those in Fig. 5b. Repetitive experiments were conducted by varying the applied weight (20–150 g), and the results are presented in Fig. 5f. As can be seen from the figure, as the weight of the weight applied to the film increases, the light intensity presented by MGO:Cr3+ also increases, which is highly similar to the above experimental conclusions obtained by us and better proves that the MGO:Cr3+ phosphor has the function of stress sensing. This provides new ideas for our follow-up work.
3.6 Near infrared applications
In view of the excellent NIR luminescence performance of the MGO:Cr3+ phosphor we prepared, we combined it with a blue LED chip (460 nm) to fabricate an NIR LED device. The preparation process is as follows: (1) MGO:Cr3+ and PDMS were merged evenly according to a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of mass, and bubbles were removed at rest. (2) Then, they were smeared on the LED chip. The blue LED chip was energized to facilitate subsequent experiments. Fig. 6a shows the emission spectra of the fabricated NIR pc-LED chip, whose spectral intensity is gradually increased by changing the current applied to the chip. The bright deep red emission light can be observed by using a near-infrared camera. In order to explore the application of this device in anti-counterfeiting, we designed an experiment as shown in Fig. 6b. Firstly, we used a black ball-point pen to cover our designed pattern completely, and then irradiated it with a near-infrared pc-LED chip afterward, and finally with the help of a near-infrared camera, we observed the original pattern clearly. Secondly, we surrounded the reagent bottle with black gauze and again irradiated it with the NIR pc-LED chip, so that the reagent capacity in the original reagent bottle could be observed under the NIR camera. Finally, we irradiated eggs with NIR light from the NIR pc-LED. The separation of egg yolk and egg white could not be observed with the naked eye, but could be clearly observed using the NIR camera, which was consistent with our expected idea.
2 ratio of mass, and bubbles were removed at rest. (2) Then, they were smeared on the LED chip. The blue LED chip was energized to facilitate subsequent experiments. Fig. 6a shows the emission spectra of the fabricated NIR pc-LED chip, whose spectral intensity is gradually increased by changing the current applied to the chip. The bright deep red emission light can be observed by using a near-infrared camera. In order to explore the application of this device in anti-counterfeiting, we designed an experiment as shown in Fig. 6b. Firstly, we used a black ball-point pen to cover our designed pattern completely, and then irradiated it with a near-infrared pc-LED chip afterward, and finally with the help of a near-infrared camera, we observed the original pattern clearly. Secondly, we surrounded the reagent bottle with black gauze and again irradiated it with the NIR pc-LED chip, so that the reagent capacity in the original reagent bottle could be observed under the NIR camera. Finally, we irradiated eggs with NIR light from the NIR pc-LED. The separation of egg yolk and egg white could not be observed with the naked eye, but could be clearly observed using the NIR camera, which was consistent with our expected idea.
Fig. 6c provides the output power and photoelectric conversion efficiency of our prepared blue LED chip in the driving current range of 20–310 mA. As the driving current increased, the output power of the NIR pc-LED also rose gradually from 92.45 to 952.3 mW. However, the photoelectric conversion efficiency of the NIR pc-LED decreased proportionally. This is presumed to be primarily due to the reduction in the luminous efficiency of the blue LED chip caused by the surge in current. As shown in Fig. 6d and e, the NIR light emitted by our prepared MGO:Cr3+ was also used to illuminate palms, fruits, and flowers. When observed through the NIR camera, the blood vessels in palms are clearly visible. The experimental results indicate that the MGO:Cr3+ near-infrared pc-LED prepared by us has significant potential applications in penetrating animal and plant tissues for area coverage and anti-counterfeiting purposes.
4. Conclusions
In summary, we have successfully synthesized MGO:Cr3+ NIR phosphors using the high temperature solid phase method. The optimal doping concentration of Cr3+ was experimentally determined to be 2%. The phosphors can be efficiently excited by a 460 nm laser to produce an emission band centered at 710 nm. In addition to traditional photoluminescence, MGO:Cr3+ exhibits ML properties. Varying the applied force yields different spectral patterns, enabling a dual-mode response. It also has good temperature stability and repeatability. In addition, the device fabricated by mixing the phosphor and PDMS film shows excellent stress sensing performance. Most importantly, we encapsulated MGO:Cr3+/PDMS on a blue LED chip. Under the illumination of the NIR pc-LED chip and by using NIR camera, different hidden objects are clearly observable, demonstrating its penetration capability. This study enhances the potential applications of NIR phosphors and introduces novel theoretical concepts for designing multi-mode responsive NIR luminescent materials.Author contributions
Yaowu Wang: conceptualization, methodology, investigation, data curation, writing original draft; Gongxun Bai: resources, writing – reviewing and editing, supervision, project administration, funding acquisition, formal analysis; Guocheng Pan: methodology, investigation; Yinyan Li: resources, data curation; Jianfeng Wang: funding acquisition, investigation; Zhenping Wu: project administration, formal analysis; and Shiqing Xu: resources, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LR24F050002), the Provincial Professional Degree Graduate Student Research Program (012013) and Tunable Intelligent Luminescent Material Design and its Optical Detection Application (230034).References
- J. Qiao, G. Zhou, Y. Zhou, Q. Zhang and Z. Xia, Nat. Commun., 2019, 10, 5267 CrossRef PubMed.
- B. Su, M. Li, E. Song and Z. Xia, Adv. Funct. Mater., 2021, 31, 2105316 CrossRef CAS.
- S. Yu, S. Fang, L. Zhao, Y. Bai, R. Wang and Z. Wang, Chem. Eng. J., 2023, 474, 145542 CrossRef CAS.
- S. Liu, Y. Zheng, D. Peng, J. Zhao, Z. Song and Q. Liu, Adv. Funct. Mater., 2023, 33, 2209275 CrossRef CAS.
- X. Zou, H. Zhang, W. Li, M. Zheng, M. S. Molokeev, Z. Xia, Y. Zheng, Q. Li, Y. Liu, X. Zhang and B. Lei, Adv. Opt. Mater., 2022, 10, 2200882 CrossRef CAS.
- B. Kumar Rajwar, J. Manam and S. Kumar Sharma, Spectrochim. Acta, Part A, 2023, 293, 122512 CrossRef CAS PubMed.
- Y. Zhang, S. Miao, Y. Liang, C. Liang, D. Chen, X. Shan, K. Sun and X. J. Wang, Light: Sci. Appl., 2022, 11, 136 CrossRef CAS PubMed.
- R. S. Liu, Chem. Mater., 2023, 35, 6179–6183 CrossRef CAS.
- R. Shi, S. Miao, Y. Zhang, X. Lv, D. Chen and Y. Liang, J. Mater. Chem. C, 2023, 11, 2748–2755 RSC.
- M. Runowski and S. Lis, J. Lumin., 2016, 170, 484–490 CrossRef CAS.
- M. Skwierczyńska, P. Woźny, M. Runowski, M. Perzanowski, P. Kulpiński and S. Lis, J. Alloys Compd., 2020, 829, 154456 CrossRef.
- J. Xiang, X. Zhou, X. Zhao, Z. Wu, C. Chen, X. Zhou and C. Guo, Laser Photonics Rev., 2023, 17, 2200965 CrossRef CAS.
- S. M. Jeong, S. Song, K. I. Joo, J. Kim, S. H. Hwang, J. Jeong and H. Kim, Energy Environ. Sci., 2014, 7, 3338–3346 RSC.
- M. Runowski, A. Shyichuk, A. Tymiński, T. Grzyb, V. Lavín and S. Lis, ACS Appl. Mater. Interfaces, 2018, 10, 17269–17279 CrossRef CAS PubMed.
- G. H. Li, J. B. Huang, Q. H. Yang, J. Wang, G. M. Cai and X. J. Wang, J. Mater. Chem. C, 2023, 11, 16578–16586 RSC.
- X. Zhang, X. Hou, J. Gao, Z. Wang, X. Zhao, C. Xu and D. Gao, J. Mater. Chem. C, 2023, 11, 16631–16637 RSC.
- E. Song, H. Ming, Y. Zhou, F. He, J. Wu, Z. Xia and Q. Zhang, Laser Photonics Rev., 2021, 15, 2000410 CrossRef CAS.
- J. Xue, Z. Yu, H. M. Noh, B. R. Lee, B. C. Choi, S. H. Park, J. H. Jeong, P. Du and M. Song, Chem. Eng. J., 2021, 415, 128977 CrossRef CAS.
- C. Jin, R. Li, Y. Liu, L. Zhang, J. Zhang, P. Sun, Z. Luo and J. Jiang, Adv. Opt. Mater., 2023, 11, 2300772 CrossRef CAS.
- S. Liu, R. Yang, H. Cai, Y. Zhuang, Z. Song, L. Ning and Q. Liu, Laser Photonics Rev., 2023, 17, 2200999 CrossRef CAS.
- K. Ye, Z. Yan, X. Yang and S. Xiao, Opt. Mater., 2021, 121, 111480 CrossRef CAS.
- J. Deng, Z. Guan, D. Fu, Y. Zheng, Z. Chen, H. Li and X. Liu, Adv. Opt. Mater., 2023, 11, 2300207 CrossRef CAS.
- J. H. Han, Y. Yoon, Y. M. Park, H. J. Kim, N. S. M. Viswanath, H. B. Cho, S. H. Jang, Y. R. Kim, J. M. Seo, J. Y. Moon, K. Kim and W. B. Im, ECS J. Solid State Sci. Technol., 2023, 12, 076006 CrossRef.
- S. Hajra, S. Panda, S. Song, B. K. Panigrahi, P. Pakawanit, S. M. Jeong and H. J. Kim, Nano Energy, 2023, 114, 108668 CrossRef CAS.
- G. Tiwari, N. Brahme, R. Sharma, D. P. Bisen, S. K. Sao and A. Khare, J. Lumin., 2017, 183, 89–96 CrossRef CAS.
- A. Abdukayum, J. T. Chen, Q. Zhao and X. P. Yan, J. Am. Chem. Soc., 2013, 135, 14125–14133 CrossRef CAS PubMed.
- E. Song, X. Jiang, Y. Zhou, Z. Lin, S. Ye, Z. Xia and Q. Zhang, Adv. Opt. Mater., 2019, 7, 1901105 CrossRef CAS.
- J. Zuo and X. Lin, Laser Photonics Rev., 2022, 16, 2270025 CrossRef.
- G. Wei, P. Li, R. Li, Y. Wang, S. He, J. Li, Y. Shi, H. Suo, Y. Yang and Z. Wang, Adv. Opt. Mater., 2023, 11, 2301794 CrossRef CAS.
- J. Ning, Y. Zheng, Y. Ren, L. Li, X. Shi, D. Peng and Y. Yang, Sci. Bull., 2022, 67, 707–715 CrossRef CAS PubMed.
- T. Zheng, M. Runowski, I. R. Martín, K. Soler-Carracedo, L. Peng, M. Skwierczyńska, M. Sójka, J. Barzowska, S. Mahlik, H. Hemmerich, F. Rivera-López, P. Kulpiński, V. Lavín, D. Alonso and D. Peng, Adv. Mater., 2023, 35, 2304140 CrossRef CAS PubMed.
- T. Y. Hwang, Y. Choi, Y. Song, N. S. A. Eom, S. Kim, H. B. Cho, N. V. Myung and Y. H. Choa, J. Mater. Chem. C, 2018, 6, 972–979 RSC.
- T. Gao, Y. Liu, R. Liu and W. Zhuang, Materials, 2023, 16, 3145 CrossRef CAS PubMed.
- C. Chen, Z. Lin, H. Huang, X. Pan, T. Zhou, H. Luo, L. Jin, D. Peng, J. Xu, Y. Zhuang and R. Xie, Adv. Funct. Mater., 2023, 33, 2304917 CrossRef CAS.
- L. Li, C. Cai, X. Lv, X. Shi, D. Peng, J. Qiu and Y. Yang, Adv. Funct. Mater., 2023, 33, 2301372 CrossRef CAS.
- B. Malysa, A. Meijerink and T. Jüstel, J. Lumin., 2018, 202, 523–531 CrossRef CAS.
- B. Chen, C. Li, D. Deng, F. Ruan, M. Wu, L. Wang, Y. Zhu and S. Xu, J. Alloys Compd., 2019, 792, 702–712 CrossRef CAS.
- M. Szymczak, M. Runowski, V. Lavín and L. Marciniak, Laser Photonics Rev., 2023, 17, 2200801 CrossRef CAS.
- Y. Zhuang, D. Chen, W. Chen, W. Zhang, X. Su, R. Deng, Z. An, H. Chenand and R. J. Xie, Light: Sci. Appl., 2021, 10, 132 CrossRef CAS PubMed.
- L. Guo, P. Xia, T. Wang, A. N. Yakovlev, T. Hu, F. Zhao, Q. Wang and X. Yu, Adv. Funct. Mater., 2023, 33, 2306875 CrossRef CAS.
- Z. Liu, X. Yu, Q. Peng, X. Zhu, J. Xiao, J. Xu, S. Jiang, J. Qiu and X. Xu, Adv. Funct. Mater., 2023, 33, 2214497 CrossRef CAS.
- Q. Zhang, D. Liu, P. Dang, H. Lian, G. Li and J. Lin, Laser Photonics Rev., 2022, 16, 2100459 CrossRef CAS.
- Y. Yan, S. Fang, Y. Li, Y. Xu, Y. Song, Z. Ma, Y. Shi, L. Zhao and Z. Wang, J. Mater. Chem. C, 2023, 11, 11509–11517 RSC.
- Q. Du, J. Ueda and S. Tanabe, J. Mater. Chem. C, 2023, 11, 16225–16233 RSC.
- Z. Zhu, Z. Luo, Y. Xie, Y. Sun, L. Xu and Q. Wu, Adv. Funct. Mater., 2023, 24, 2313701 CrossRef.
- D. Huang, H. Zhu, Z. Deng, H. Yang, J. Hu, S. Liang, D. Chen, E. Ma and W. Guo, J. Mater. Chem. C, 2021, 9, 164–172 RSC.
- E. Song, J. Wang, S. Ye, X. Yang, M. Peng, Q. Zhang and L. Wondraczek, Adv. Opt. Mater., 2017, 5, 1700070 CrossRef.
- J. Lai, W. Shen, J. Qiu, D. Zhou, Z. Long, Y. Yang, K. Zhang, I. Khan and Q. Wang, J. Am. Ceram. Soc., 2020, 103, 5067–5075 CrossRef CAS.
- M. Jiao, N. Guo, W. Lü, Y. Jia, W. Lv, Q. Zhao, B. Shao and H. You, Inorg. Chem., 2013, 52, 10340–10346 CrossRef CAS PubMed.
- D. Liu, G. Li, P. Dang, Q. Zhang, Y. Wei, L. Qiu, M. S. Molokeev, H. Lian, M. Shang and J. Lin, Light: Sci. Appl., 2022, 11, 112 CrossRef CAS PubMed.
- A. Lay, C. Siefe, S. Fischer, R. D. Mehlenbacher, F. Ke, W. L. Mao, A. P. Alivisatos, M. B. Goodman and J. A. Dionne, Nano Lett., 2018, 18, 4454–4459 CrossRef CAS PubMed.
- Q. Zhang, X. Wei, J. Zhou, B. Milićević, L. Lin, J. Huo, J. Li, H. Ni and Z. Xia, Adv. Opt. Mater., 2023, 11, 2300310 CrossRef CAS.
- T. Zheng, M. Runowski, J. Xue, L. Luo, U. R. Rodríguez-Mendoza, V. Lavín, I. R. Martín, P. Rodríguez-Hernández, A. Muñoz and P. Du, Adv. Funct. Mater., 2023, 33, 2214663 CrossRef CAS.
- M. Runowski, P. Woźny, S. Lis, V. Lavín and I. R. Martín, Adv. Mater. Technol., 2020, 5, 1901091 CrossRef CAS.
- M. Runowski, P. Woźny and I. R. Martín, J. Mater. Chem. C, 2021, 9, 4643–4651 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc00059e |
| This journal is © The Royal Society of Chemistry 2024 |






