 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Slow magnetic relaxation in a {CoIICoIII2} complex containing a high magnetic anisotropy trigonal bipyramidal CoII centre†
Alexandra
Collet
a,
Gavin A.
Craig‡
 a,
María José
Heras Ojea§
a,
María José
Heras Ojea§
 a,
Lakshmi
Bhaskaran
b,
Claire
Wilson
a,
Lakshmi
Bhaskaran
b,
Claire
Wilson
 a,
Stephen
Hill
a,
Stephen
Hill
 b and
Mark
Murrie
b and
Mark
Murrie
 *a
*a
aWestCHEM, School of Chemistry, University of Glasgow, University Avenue, Glasgow, G12 8QQ, UK. E-mail: mark.murrie@glasgow.ac.uk
bDepartment of Physics and NHMFL, Florida State University, Tallahassee, FL32310, USA
First published on 18th June 2018
Abstract
We report a trinuclear mixed-valence {CoIICoIII2} complex, where the CoII centre adopts a trigonal bipyramidal geometry, leading to a large, easy-plane magnetic anisotropy and field-induced slow magnetic relaxation with a Raman-like relaxation process.
Single-Molecule Magnets (SMMs) show promise in a number of technological applications such as molecular spintronics,1 high-density information storage,2 and qubits for quantum computation.3 When the magnetic properties arise from a single paramagnetic ion in an appropriate ligand field, then these molecules are often referred to as Single-Ion Magnets (SIMs). The origin of SMM and SIM behaviour is the presence of an energy barrier (ΔE/kB) that prevents the reorientation of magnetisation between the MS = ±S components of the ground state S. Magnetic anisotropy, which depends on the spin–orbit contribution,4 is difficult to control in polymetallic systems, and hence recent research has been focused on monometallic systems. Monometallic CoII complexes have been found to exhibit both large negative (easy-axis) and large positive (easy-plane) magnetic anisotropy when found in tetracoordinate, pentacoordinate and hexacoordinate geometries.5 More specifically, CoII ions with an ideal trigonal bipyramidal geometry are expected to exhibit a high easy-plane anisotropy.6
We are working towards the synthesis of coordination complexes containing at least one high magnetic anisotropy centre, such as trigonal bipyramidal (TBP) CoII, in order to boost the barrier ΔE/kB. Herein, we report the complex [CoIICoIII2(μ3-OH)(μ-pz)4(DBM)3]·2MeCN (1·2MeCN) (Fig. 1), which is a new solvate of a previously reported {Co3} complex, obtained by a different synthetic procedure.7
Complex 1 is a mixed-valence isosceles triangle of CoII/CoIII ions synthesised from the reaction of CoCl2·6H2O with Hpz (pyrazole) and HDBM (dibenzoylmethane) in MeOH/MeCN in the presence of NEt3 (see the ESI†). The crystallographic data can be found in Table S1 (see the ESI†). The CoII centre (Co1) is five-coordinate adopting a slightly distorted TBP geometry, while the two diamagnetic CoIII ions (Co2 and Co3) adopt an octahedral geometry. The oxidation states of CoII and CoIII were confirmed using Bond Valence Sum (BVS) analysis.8 Continuous shape measures (CShMs),9 which provide an estimate of the distortion from the ideal TBP geometry for Co1, give a value of 0.33 (where 0 corresponds to the ideal polyhedron), confirming a small distortion (Table S2 and Fig. S3 ESI†). The crystal packing is shown in Fig. S4.† Intermolecular interactions are present through hydrogen–π and π–π interactions between the phenyl and pyrazolate rings of neighbouring molecules, while the shortest intermolecular Co1⋯Co1′ distance is ∼9.3 Å.
Direct current (dc) magnetic susceptibility measurements were performed on a polycrystalline sample of 1 restrained in eicosane in the 290–2 K temperature range in an applied magnetic field of 1000 Oe (Fig. 2). The χMT value at room temperature (2.45 cm3 mol−1 K) corresponds to a high-spin CoII ion and indicates a spin–orbit coupling contribution (the CoIII ions are diamagnetic so for g = 2, S = 3/2, χMT = 1.88 cm3 mol−1 K). Upon cooling, χMT decreases slowly until ∼50 K to reach 2.16 cm3 mol−1 K and then decreases rapidly below ∼50 K to reach 1.40 cm3 mol−1 K at 2 K, indicating zero-field splitting of the ground state. Additionally, magnetisation versus field plots at 2, 4 and 6 K did not saturate at the highest available field of 5 T, a further indication of the presence of magnetic anisotropy (Fig. 2 inset).
Microanalysis and powder X-ray diffraction carried out on ground and non-ground samples (Fig. S1 and S2†) show that the lattice solvent is easily lost. Such desolvation could cause changes in the crystal packing, resulting in small changes of the local cobalt environment and hence small changes to the CoIIg values and zero-field splitting (ZFS) parameters. High frequency EPR studies on 1 (Fig. S5†) are in agreement and suggest the presence of two discrete species within the microcrystalline powder sample in an ∼50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio, having distinct ZFS parameters. Analysis of the EPR data gives the parameters for the two species as: gx = gy = 2.18, gz = 2.07 with an E/D ratio ∼0.13 and gx = gy = 2.23, gz = 2.08 with an E/D ratio ∼0.17 (see the ESI†). Using the average of these two sets, gx = gy = 2.205, gz = 2.075, the dc magnetic susceptibility data and the magnetisation curves of 1 were fitted simultaneously using the program PHI10 (Fig. 2), as described by the following effective Hamiltonian equation (1):
50 ratio, having distinct ZFS parameters. Analysis of the EPR data gives the parameters for the two species as: gx = gy = 2.18, gz = 2.07 with an E/D ratio ∼0.13 and gx = gy = 2.23, gz = 2.08 with an E/D ratio ∼0.17 (see the ESI†). Using the average of these two sets, gx = gy = 2.205, gz = 2.075, the dc magnetic susceptibility data and the magnetisation curves of 1 were fitted simultaneously using the program PHI10 (Fig. 2), as described by the following effective Hamiltonian equation (1):
 | (1) |
The first and second terms represent the axial and rhombic ZFS terms, parameterised by D and E, respectively, Ŝ is the spin operator with components Ŝi (i = x, y, z), and the final term denotes the Zeeman interaction with the local magnetic field,  , parameterised through the Landé
, parameterised through the Landé 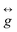 tensor. Fixing the values of gx = gy = 2.205 and gz = 2.075, and χTIP = 0.0009 cm3 mol−1, where χTIP stands for the contribution of temperature-independent paramagnetism arising from two CoIII and one TBP CoII,11 we were able to extract the ZFS parameters D = +23.85 (±0.17) cm−1 and E = +4.04 (±0.09) cm−1. The E/D ratio extracted from the fitting of the magnetic data is ∼0.17, consistent with the EPR studies. The magnitude of D is also consistent with previously reported CoII centres in TBP geometry with easy-plane anisotropy.12,13
tensor. Fixing the values of gx = gy = 2.205 and gz = 2.075, and χTIP = 0.0009 cm3 mol−1, where χTIP stands for the contribution of temperature-independent paramagnetism arising from two CoIII and one TBP CoII,11 we were able to extract the ZFS parameters D = +23.85 (±0.17) cm−1 and E = +4.04 (±0.09) cm−1. The E/D ratio extracted from the fitting of the magnetic data is ∼0.17, consistent with the EPR studies. The magnitude of D is also consistent with previously reported CoII centres in TBP geometry with easy-plane anisotropy.12,13
The relatively high value of the transverse anisotropy indicates significant mixing of the MS = ±3/2 and ±1/2 levels, and can be attributed to the small deviation from the ideal TBP geometry and/or the different nature of the ligands in the equatorial positions.13 Only a few examples of CoII in TBP geometry with easy-plane anisotropy have been found to show slow relaxation of the magnetisation.13 Therefore, we performed alternating current (ac) magnetic susceptibility measurements. In zero applied dc field, 1 does not display any out-of-phase ac signals, due to efficient zero-field quantum tunnelling. However, by using an applied dc field to suppress tunnelling, compound 1 does display slow magnetic relaxation at low temperature. Variable dc fields (500–5000 Oe) were applied to 1 at 2 K in order to obtain the optimum dc field at which the characteristic relaxation time of the magnetisation (τ) possesses the largest value (Fig. S7, ESI†). The characteristic relaxation times for each field were calculated using CC-FIT,14 and the τmax value was obtained at 1000 Oe. The frequency dependence of the in-phase and out-of-phase magnetic susceptibility was measured under the optimum dc field for the range of temperatures 1.8–8 K (Fig. 3).
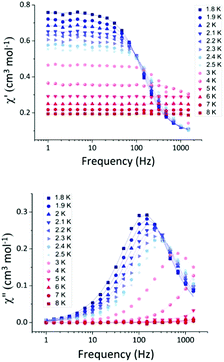 | ||
| Fig. 3 Frequency dependent in-phase (top) and out-of-phase (bottom) susceptibility signals for complex 1 in a 1000 Oe dc applied magnetic field, in the temperature range 1.8–8 K. The solid lines correspond to the fit (CC-FIT).14 | ||
The fitting of the Cole–Cole plot (out-of-phase versus in-phase signals) for 1 was performed using CC-FIT14 (Fig. 4), resulting in small values of the Cole–Cole parameter α (0.08–0.02) indicative of a relatively narrow distribution of relaxation times. The τ values were used to construct an Arrhenius plot for the temperatures 1.8–5 K, from which the relaxation parameters of ΔE/kB (energy barrier) and τ0 (pre-exponential factor) at higher temperatures were extracted for 1 (Fig. S8, ESI†). Fitting within the linear region (Orbach relaxation mechanism), the values ΔE/kB = 23.18 (±2.2) K and τ0 = 1.14 × 10−7 s were extracted. However, the value of ΔE/kB is smaller than the calculated energy difference between the ground and first excited state of ∼50 cm−1 , a clear indication that other relaxation processes need to be considered. Using eqn (2), we attempted to fit the τ−1versus T data but we were not able to extract reasonable values. The terms are the direct, tunnelling, Raman and Orbach contributions, in that order.15
, a clear indication that other relaxation processes need to be considered. Using eqn (2), we attempted to fit the τ−1versus T data but we were not able to extract reasonable values. The terms are the direct, tunnelling, Raman and Orbach contributions, in that order.15
 | (2) |
| τ−1 = B + CTn | (3) |
 | ||
| Fig. 4 The Cole–Cole plot of the ac magnetic susceptibility of 1 at 1000 Oe (top). The solid lines correspond to the fit (CC-FIT). The plot of τ−1versus T for 1 in the temperature range 1.8–5 K (bottom). The red solid line corresponds to the fit using eqn (3) (see the text for details). | ||
In order to avoid over-parameterisation we attempted to fit the τ versus field (H) plot using only the terms for direct and tunnelling processes (which are dependent on the field) to extract the parameters A, B1, and B2. However, all efforts were unsuccessful, an indication that there is a more complicated dependence of τ with the field. Therefore, using only the tunnelling (expressed as the parameter B) and Raman contributions (see eqn (3)) we were able to fit the τ−1versus T plot (Fig. 4) affording the values B = 926 s−1, C = 2.3 K−n s−1 and n = 6.6. The exponent factor n in the Raman process should be equal to 9 for Kramers' ions, or 5 in the presence of low-lying states. However, lower values for n have been reported in cases where acoustic and optical phonons are involved.15,16
In conclusion, [CoIICoIII2(μ3-OH)(μ-pz)4(DBM)3] is the only reported example of a mixed-valence CoII/CoIII polynuclear complex containing a single trigonal bipyramidal CoII centre that gives rise to slow magnetic relaxation.17 In the case of 1, this arises from a large, easy-plane magnetic anisotropy. To obtain the zero-field splitting parameters, we used high frequency EPR measurements to extract the g factors. These were then fixed in the simultaneous fitting of the dc magnetic susceptibility and magnetisation data to give the parameters D = +23.85 (±0.17) cm−1 and E = +4.04 (±0.09) cm−1. Furthermore, it has been demonstrated recently how the magnetic anisotropy of octahedral CoII is transferred to the overall magnetic anisotropy of a polymetallic {Cr7Co} system. This is of interest for quantum information processing, especially in relation to molecules with a large spin ground state that is characterised by a large, easy-plane anisotropy.18 Hence, the next step is to develop a route to incorporate high magnetic anisotropy trigonal bipyramidal CoII centres into exchange-coupled polymetallic systems that contain multiple paramagnetic centres.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
MM acknowledges the support from The UK Engineering and Physical Sciences Research Council (EP/J018147/1). A portion of this work was performed at the NHMFL, which is supported by the NSF (DMR-1157490 and DMR-1644779) and the State of Florida. SH also acknowledges the support from the NSF (DMR-1610226). The data that underpin this work are available at http://dx.doi.org/10.5525/gla.researchdata.618.Notes and references
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179 CrossRef PubMed.
- J. Bartolome, F. Luis and J. F. Fernández, in Molecular Magnets: Physics and Applications, Springer, Verlag, Berlin Heidelberg, 2014 Search PubMed.
- R. Vincent, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Nature, 2012, 488, 357 CrossRef PubMed.
- B. N. Figgis, Introduction to ligand fields, Wiley-VCH, New York, 1966 RSC; B. N. Figgis, M. Gerloch, J. Lewis, F. E. Mabbs and G. A. Webb, J. Chem. Soc. A, 1968, 2086 RSC; R. B. Bentley, M. Gerloch, J. Lewis and P. N. Quested, J. Chem. Soc. A, 1971, 3751 RSC; A. K. Gregson, R. L. Martin and S. Mitra, J. Chem. Soc., Dalton Trans., 1976, 1458 RSC.
- Y. Rechkemmer, F. D. Breitgoff, M. van der Meer, M. Atanasov, M. Hakl, M. Orlita, P. Neugebauer, F. Neese, B. Sarkar and J. van Slageren, Nat. Commun., 2016, 7, 10467 CrossRef PubMed; V. V. Novikov, A. A. Pavlov, Y. V. Nelyubina, M. Boulon, O. A. Varzatskii, Y. Z. Voloshin and R. E. P. Winpenny, J. Am. Chem. Soc., 2015, 137, 9792 CrossRef PubMed; C. Rajnák, J. Titiš, J. Moncol′, F. Renz and R. Boča, Eur. J. Inorg. Chem., 2017, 11, 1520 CrossRef; C. Rajnák, J. Titiš, O. Fuhr, M. Ruben and R. Boča, Inorg. Chem., 2014, 53, 8200 CrossRef PubMed; F. Shao, B. Cahier, E. Rivière, R. Guillot, N. Guihéry, V. E. Campbell and T. Mallah, Inorg. Chem., 2017, 56, 1104 CrossRef PubMed; F. Shao, B. Cahier, N. Guihéry, E. Rivière, R. Guillot, A. Barra, Y. Lan, W. Wernsdorfer, V. E. Campbell and T. Mallah, Chem. Commun., 2015, 51, 16475 RSC; D. Sertphon, K. S. Murray, W. Phonsri, J. Jover, E. Ruiz, S. G. Telfer, A. Alkaş, P. Harding and D. J. Harding, Dalton Trans., 2018, 47, 859 RSC.
- S. Gomez-Coca, E. Cremades, N. Aliaga-Alcalde and E. Ruiz, J. Am. Chem. Soc., 2013, 135, 7010 CrossRef PubMed.
- M. Łukasiewicz, Z. Ciunik, J. Mazurek, J. Sobczak, A. Staroń, S. Wołowiec and J. J. Ziółkowski, Eur. J. Inorg. Chem., 2001, 1575 CrossRef.
- N. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192 CrossRef.
- M. Pinsky and D. Avnir, Inorg. Chem., 1998, 37, 5575 CrossRef PubMed; S. Alvarez and M. Llunell, J. Chem. Soc., Dalton Trans., 2000, 3288 RSC.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164 CrossRef PubMed.
- R. Herchel and R. Boča, Dalton Trans., 2005, 1352 RSC; J. S. Wood, J. Chem. Soc. A, 1969, 1582 RSC.
- R. Boča, L. Dlháň, W. Linert, H. Ehrenberg, H. Fuess and W. Haase, Chem. Phys. Lett., 1999, 307, 359 CrossRef; R. Herchel and R. Boča, Dalton Trans., 2005, 1352 RSC; C. Rajnák, J. Titiš, I. Šalitroš, R. Boča, O. Fuhr and M. Ruben, Polyhedron, 2013, 65, 122 CrossRef; B. Cahier, M. Perfetti, G. Zakhia, D. Naoufal, F. ElKhatib, R. Guillot, E. Rivière, R. Sessoli, A.-L. Barra, N. Guihéry and T. Mallah, Chem. – Eur. J., 2017, 23, 3648 CrossRef PubMed; D. Schweinfurth, J. Krzystek, M. Atanasov, J. Klein, S. Hohloch, J. Telser, S. Demeshko, F. Meyer, F. Neese and B. Sarkar, Inorg. Chem., 2017, 56, 5253 CrossRef PubMed.
- T. J. Woods, M. F. Ballesteros-Rivas, S. Gómez-Coca, E. Ruiz and K. R. Dunbar, J. Am. Chem. Soc., 2016, 138, 16407 CrossRef PubMed; N. Nedelko, A. Kornowicz, I. Justyniak, P. Aleshkevych, D. Prochowicz, P. Krupiński, O. Dorosh, A. Ślawska-Waniewska and J. Lewiński, Inorg. Chem., 2014, 53, 12870 CrossRef PubMed; A. K. Mondal, T. Goswami, A. Misra and S. Konar, Inorg. Chem., 2017, 56, 6870 CrossRef PubMed; C. Rajnák, J. Titiš, I. Šalitroš, R. Boča, O. Fuhr and M. Ruben, Polyhedron, 2013, 65, 122 CrossRef.
- CC-FIT Copyright © 2014 Nicholas F. Chilton; Y.-N. Guo, G.-F. Xu, Y. Guo and J. Tang, Dalton Trans., 2011, 40, 9953 RSC.
- Y. Zhang, S. Gomez-Coca, A. J. Brown, M. R. Saber, X. Zhanga and K. R. Dunbar, Chem. Sci., 2016, 7, 6519 RSC.
- D. Shao, L. Zhang, L. Shi, Y. Zhang and X. Wang, Inorg. Chem., 2016, 55, 10859 CrossRef PubMed; L. Chen, S. Chen, Y. Sun, Y. Guo, L. Yu, X. Chen, Z. Wang, Z. W. Ouyang, Y. Songa and Z. Xued, Dalton Trans., 2015, 44, 11482 RSC; E. Colacio, J. Ruiz, E. Ruiz, E. Cremades, J. Krzystek, S. Carretta, J. Cano, T. Guidi, W. Wernsdorfer and E. K. Brechin, Angew. Chem., Int. Ed., 2013, 52, 9130 CrossRef PubMed.
- Y. Zhu, C. Cui, Y. Zhang, J. Jia, X. Guo, C. Gao, K. Qian, S. Jiang, B. Wang, Z. Wang and S. Gao, Chem. Sci., 2013, 4, 1802 RSC; V. Chandrasekhar, A. Dey, A. J. Mota and E. Colacio, Inorg. Chem., 2013, 52, 4554 CrossRef PubMed; D. Wu, X. Zhang, P. Huang, W. Huang, M. Ruan and Z. W. Ouyang, Inorg. Chem., 2013, 52, 10976 CrossRef PubMed; E. A. Buvaylo, V. N. Kokozay, O. Y. Vassilyeva, B. W. Skelton, A. Ozarowski, J. Titiš, B. Vranovičová and R. Boča, Inorg. Chem., 2017, 56, 6999 CrossRef PubMed; S. Manna, A. Bhunia, S. Mistri, J. Vallejo, E. Zangrando, H. Puschmann, J. Cano and S. C. Manna, Eur. J. Inorg. Chem., 2017, 19, 2585 CrossRef; S. Mandal, S. Mondal, C. Rajnák, J. Titiš, R. Boča and S. Mohanta, Dalton Trans., 2017, 46, 13135 RSC.
- E. Garlatti, T. Guidi, A. Chiesa, S. Ansbro, M. L. Baker, J. Ollivier, H. Mutka, G. A. Timco, I. Vitorica-Yrezabal, E. Pavarini, P. Santini, G. Amoretti, R. E. P. Winpenny and S. Carretta, Chem. Sci., 2018, 9, 3555 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Further experimental details. CCDC 1838443. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8dt01997e |
| ‡ Current address: Institute for Integrated Cell-Material Sciences (WPI-iCeMS), Kyoto University, Yoshida, Sakyo-ku, Kyoto 606-8501, Japan. |
| § Current address: School of Chemistry, The University of Manchester, Manchester M13 9PL, U.K. |
| This journal is © The Royal Society of Chemistry 2018 |


