Solution-cast self-assembled films of perchlorate-doped oligo(3-methoxythiophene) showing a gold-like luster†
Reo Tagawaa,
Hyuma Masub,
Tsutomu Itohb and
Katsuyoshi Hoshino*a
aGraduate School of Advanced Integrated Science, Chiba University, Chiba 263-8522, Japan. E-mail: k_hoshino@faculty.chiba-u.jp
bCenter for Analytical Instrumentation, Chiba University, Chiba 263-8522, Japan
First published on 23rd May 2014
Abstract
Perchlorate-doped 3-methoxythiophene oligomer was chemically synthesized, and its solutions were applied to produce films characterized by their gold-like luster which showed no degradation in the ambient atmosphere for at least one year. The oligomer is characterized by its good solvent-soluble, good film-forming, and moderate electrical conducting properties. More importantly, wide-angle and grazing incidence X-ray diffraction measurements revealed that the gold-like films have a compact periodic layered structure with a lamellar interlayer spacing of 1.10 nm and an intralayer spacing of 0.34 nm, though the oligomer involves no long alkyl chains. It was proposed that the strong gold-like luster could be related to the highly regular and compact nature of the lamellae in the oligomer films.
1. Introduction
Metal effect pigments,1 such as the flakes or lamellae of aluminum, copper, copper–zinc alloys (gold bronzes), zinc, etc., have found applications in automotive coatings, roof coatings, printing inks, cosmetics, and plastic materials for protective and decorative functions.2 The flakes of these pigments are produced by grinding metal granules in a ball mill. After filtration, the pigment flakes are supplied in the form of a powder or paste with organic compounds. Their use in our daily life has become increasingly important due to our recent interests in global environmental problems and the reinforcement of security. The application of the strong lustrous color effect of metal effect pigments in cars and buildings retards the buildup of heat by reflecting the incoming solar radiation and reduces the use of air-conditioning for cooling.3 Lustrous printings using metal effect pigments cannot easily be copied using reprographic techniques, thus making forgings much more difficult to carry out.1 Among these metal effect pigments, imitation gold pigments have been distinguished from the other pigments in the field of Kansei engineering and imaging science/technology since their origin dates back to the goldbeating technology by the ancient Egyptians who appreciated the peculiar qualities of gold in art and industry (at least 3000 B.C.).4,5 Due to the prohibitive cost of gold, gold-colored copper alloys were developed.6 The modern popular method for achieving imitation gold is the combination of aluminum pigments with yellow transparent chromatic pigments or dyes.5 Industrially, aluminum flakes are dispersed in a polymer-binder solution containing yellow colorants, and the gold coatings are obtained by applying the dispersion. However, sedimentation of the flakes in the paint and the resultant color shift and a loss of hiding power, color and lightness deviations due to the differences in the flake orientation in the coating layers, corrosive nature of the flakes, and heavy weight of the coating films are drawbacks of these industrial paints and their coatings.1,2,5 In order to overcome these drawbacks, the search for organic materials for use in the gold-like coatings have been ongoing. The crystalline powders of α,α′-bis(dithieno[3,2-b:2′,3′-d]thiophene)7 and 2,5-bis[2-(5,2′-thienyl)-furyl]-thiophene8 were found to exhibit a gold-like luster. Ogura et al. synthesized gold-colored derivatives of 1-aryl-2-(2-thienyl)-5-[5-(tricyanoethenyl)-2-thienyl]pyrroles which are soluble in CHCl3, and a gold luster was observed in the residues formed by dropping the solutions of the above pyrrole derivatives on a glass plate followed by drying.9 Depending on the substituent, the residues of the pyrrole derivatives exhibited lustrous colors of bronze, orange, and reddish purple.10–14 Additionally, replacement of the pyrrole ring by a furan ring produced a green shiny crystal. Kondo et al. synthesized 4,4′-bis{1-[2-(N,N-dimethylamino)]ethoxy}azo-benzene and found that a gold coating was obtained by applying its chloroform solution.15 However, the color of the coating turned nonlustrous yellow in ca. 24 h.In this paper, we report the synthesis of oligo(3-methoxythiophene) doped with perchlorate anion and the finding that the films prepared by applying the oligomer solutions show a gold luster. To the best of our knowledge, this is the first organic material capable of forming air-stable gold-like coatings.
2. Experimental
2.1. Chemicals and materials
3-Methoxythiophene (Wako Pure Chemical Industries, Ltd, >98%), Fe(ClO4)3·nH2O (Wako Pure Chemical Industries, Ltd, the content as an anhydride was 70.7%), and nitromethane (Tokyo Chemical Industry, >98.0%) were used as supplied. Methanol (>99.8%, reagent grate) and acetonitrile (>99.7%, spectroscopic grade) were purchased from Kanto Chemical Co., Inc. The glass plate (MATSUNAMI GLASS IND., S1225) and polyester film (PANAC, Lumirror 50-T60) were cleaned by sonication in acetone for 10 min prior to use.2.2. Sample characterization
The FT-IR transmission spectra of the samples (KBr pellets) were recorded using a Jasco FT/IR-410 spectrometer. The 1H NMR spectra were recorded in a DMSO-d6 solution by a Varian NMR system. All the chemical shifts (δ in ppm) were referenced to the solvent signal. Gel permeation chromatography (GPC) measurements were conducted using a Waters system equipped with a PDA 996 photodiode array detector (UV270 nm) and two Shodex KF-806M columns. NMP (1-methy-2-pyrrolidone) was used as the eluent containing 0.01 M lithium bromide (elution rate, 0.4 ml min−1), and polystyrene standards were used for calibration. The carbon and hydrogen contents of the product were measured by a Perkin-Elmer 2400 CHN elemental analyzer. The sulfur and chlorine contents were determined by a TOA DKK ion chromatograph ICA-2000 combined with a Yanaco New Science combustion furnace (model SQ-1) and absorption unit (model HSU-35). The powder of the product was used for the thermogravimetry and differential scanning calorimetry (TG-DSC) analyses. The product sample (ca. 6 mg) was placed in an aluminum pan and heated from 13 to 520 °C at the rate of 30 °C min−1 under a nitrogen flow of 60 ml min−1 in a Shimadzu® Thermogravimetry DTG-60H. The thermoanalytical data were analyzed using software-TA 60 WS® (Thermal Analysis) version 2.2.1 from Shimadzu®.2.3. Film characterization
UV-vis reflection spectra were obtained using a JASCO MSV-370 spectrometer in which the incident and reflection angles were at 23° from the vertical position. Observation of films was made using an optical microscope (VHX-2000, KEYENCE). The lustrous feature of the films was demonstrated by the optical micrographic images. Wide-angle X-ray diffraction measurements were performed using a diffractometer (Rigaku SmartLab) equipped with a CuKα (λ = 1.54 Å) source. Grazing-incidence XRD measurements were made at an incident angle of 0.15° in order to avoid any contribution of the glass substrate to the scattering profile. UV-vis transmission and absorption spectra measurements were carried out using a Hitachi U-3000 spectrophotometer. The electric conductivity of the films was measured by a four-point-probe method using a resistivity meter (Mitsubishi Chemical Analytech, model Loresta-GP MCP-T600 with an MCP-TP06P probe). Each electric conductivity value was averaged from five measurements made at different positions on the film surface (ESI, Fig. S1†). The thickness of the film was measured using a surface profile measuring system (Sloan Co., model Dektak 3030).3. Results and discussion
3.1. Synthesis and film formation
The oligo(3-methoxythiophene) doped with perchlorate anion (1) was prepared by reference to the method for the preparation of poly(3-methoxythiophene) doped with chloride anion,16 but with some modifications. To a 20 ml stirred acetonitrile solution of 3-methoxythiophene (0.1 M) was dropwise added a 20 ml acetonitrile solution of Fe(ClO4)3 (0.2 M) under a nitrogen atmosphere at 22 °C. The color of the solution rapidly changed from transparent colorless to dark blue. The obtained solution was then stored for 2 h, and the dark blue precipitate was isolated by suction filtration. The product was washed five times with methanol and then dried under vacuum for 90 min at 45 °C to give a powder with a partial gold luster. The yield was 0.22 g (76%).The 1H NMR spectrum of the product showed that its monomer unit was 3-methoxylthiophene-2,5-diyl (ESI, Fig. S2–S4†). Its GPC (gel permeation chromatography) analysis indicated an average molecular weight of 1.6 × 103 (14.3 monomer units) and a broad distribution extending to 104 g mol−1. The product gave a FT-IR transmission spectrum showing the characteristic signals due to the partially-doped poly(3-methoxythiophene) and perchlorate anion (ESI, Fig. S5†). The elemental analysis of the product (see ESI†), i.e., C, 38.8%; H, 2.96%; Cl, 6.96%; S, 20.9%, is consistent with the calculated formula for the product, (C5H4OS)10·3ClO4−·H2O·3O2 (C, 39.1%; H, 2.85%; Cl, 6.95%; S, 20.9%). The doping level, defined as the number of ClO4− associated with one 3-methoxythiophene unit, was calculated to be 30% as the ratio of the Cl and S compositions. All the results of the 1H NMR, FT-IR, GPC, and elemental analyses revealed that the product was the 3-methoxythiophene oligomer doped with ClO4−. The thermal gravimetry and differential scanning calorimetry traces of oligomer 1 showed a sharp exotherm at 220 °C and the sudden weight loss curve indicated its decomposition.
The oligomer 1 is easily soluble in nitromethane, acetonitrile, γ-butyrolactone, and propylene carbonate producing a blue solution, and is soluble in 2-butanone, ethanol, methanol, tetrahydrofuran, etc., but insoluble in water, hexane, chloroform, etc. The nitromethane solution of 1 (11 mg ml−1, Fig. 1d) was dropped on a glass plate and allowed to stand under ambient conditions to yield a gold-like lustrous film with a thickness of 2.8 μm (abbreviated as film A, Fig. 1a). Also, the same film was successfully prepared on a polyester sheet (Fig. 1b), favoring the applications to next-generation flexible organic electronics. For comparison, the photograph of an evaporated gold film on a glass plate (Fig. 1a, 0.1 μm thick) is also included in Fig. 1. The film prepared in the same manner using the acetonitrile solution of 1 (12 mg ml−1) exhibited a dark gold-like brown color (3.0 μm thick, film B, Fig. 1c). However, its color turned into lustrous gold simply by rubbing with a cloth (2.2 μm thick, film C, Fig. 1c). Judging from the quality of produced films, nitromethane and acetonitrile were chosen as the solvent. It was also possible to prepare gold-like lustrous films using γ-butyrolactone (b.p. = 204 °C) and propylene carbonate (b.p. = 240 °C); the concentration of the coating solutions was 10 mg ml−1. However, the films partially collapsed in shape during their drying in vacuum at 100 °C for 1 h.
3.2. Light reflection and absorption characteristics
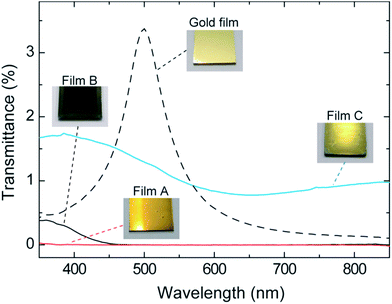
Fig. 2 shows the reflection spectra (incident light angle: 23°) of films A (red curve in part a), B (black curve in part b), and C (blue curve in part b) together with the evaporated gold film (black curve in part a). Films A and C showed a rapid increase in reflectance at 482 and 478 nm, respectively. This feature was nearly the same as that of the black curve in part a with a threshold wavelength of 486 nm,17 demonstrating that the recognized color of films A and C is gold. The reflectance above ca. 650 nm of the films (11–17% versus standard evaporated aluminum plate) is lower than that of the evaporated gold film (100–110%), exhibiting a brightness having a deepness, but much higher than that of film B (ca. 2%). Films A and C are quite stable in air and no change in their lustrous gold appearance was observed after being stored under ambient conditions for at least one year. The UV-vis transmission spectra of the gold-like films A (red) and C (blue) on the glass plate showed a very low transmittance in the visible light region (Fig. 3), in spite of their thin thicknesses (3.0 and 2.2 μm). This effect of hiding the background (hiding power) is comparable to those exhibited by the coatings of the metal effect pigments several tens of μm thick and by the evaporated gold film (0.1 μm thick, dotted curve in Fig. 3) though its spectral shape18 differs from those of films A and C.The reflection spectra of films A and C in Fig. 2 demonstrated comparatively strong reflection of red, orange, yellow, yellowish green, and green light, and weak reflection of blue and violet light. These spectral characteristics make the film look yellow. In order for the film to look like gold, however, its high light reflection is required. Therefore, we prepared the thin film of 1 (film D) and characterized its UV-vis absorption spectrum, with the purpose of getting information on the mechanistic aspect. Film D of 68 nm thick was prepared by spin-coating (1000 rpm, 10 s) a solution of 1 (10 mg ml−1) to a glass plate. Fig. 4 shows the UV-vis absorption spectrum of film D. The inset in the figure is the photograph of film D. The thin film looked deep blue when seen from a direction perpendicular to the film surface, and a slight gold-like luster was visible when the film was viewed from an oblique direction. The absorption coefficient of the maximum absorption wavelength of 632 nm, calculated from Fig. 4, ranged as high as 2.0 × 105 cm−1. This strong light absorption might be responsible for the lustrous feature of films A and C, since too intense absorption prevent light from penetrating into the film and facilitate the reflection of light. Thus, the gold-like appearance of the films A and C can be tentatively explained on the basis of the above selective reflection and strong absorption characteristics. The role of the dopant, ClO4−, is to impart a blue color to the oligomer by electrically neutralizing cationic species19 which is the chromophore involved in 1.
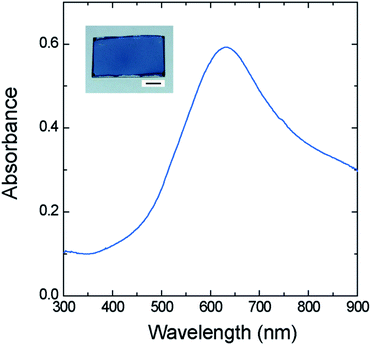 | ||
| Fig. 4 UV-vis absorption spectrum of film D on a glass plate. Film thickness: 68 nm. The inset shows the photograph of the film. Scale bar: 5 mm. | ||
According to the previous studies on the thiophene derivatives, the higher absorption coefficient value of film D is most probably relevant to the molecular packing and structural order of the films. Mondal et al.20 synthesized some copolymers containing thiophene enriched fused-aromatic thienopyridine and revealed that the copolymers with tighter inter-chain packing exhibited higher absorption coefficients. Zhokhavets et al.21 prepared poly(3-hexylthiophene)/fullerene films and found that the absorption coefficients of the films in the photon energy region 2.0–2.5 eV increased with increasing the crystallinity of the poly(3-hexylthiophene) moiety. These reports imply that the molecule of 1 in the films have an ordered structure with a tight inter-chain packing. Molecular packing in the films of 1 was thus investigated using X-ray diffraction measurements in the following section.
3.3. Film structure analysis
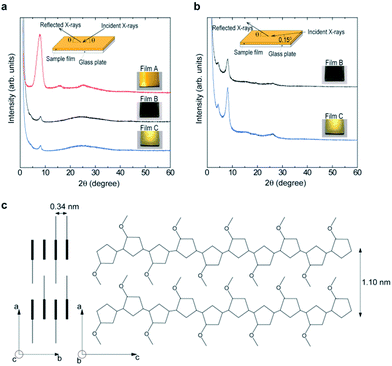
The XRD (X-ray diffraction) patterns for films A, B, and C are shown in Fig. 5a. It is generally accepted that poly(3-alkylthiophenes) have crystalline domains surrounded by amorphous ones. The crystalline domains have an orthorhombic unit cell with lattice parameters along the direction of the alkyl chain, a, the stacking direction of the molecule, b, and the direction of the backbone of the molecule, c.22–31 Based on previous reports on the structural analyses of the poly(3-alkylthiophenes),22–31 reflections at 2θ = 8.04, 16.14, and 24.82° in the red curve (film A) indicate a well-defined lamellar structure.22,23,27,30 The peaks at 2θ = 8.04 and 16.14° correspond to first-order and higher order reflections from the lamellar interlayer spacing, 1.10 nm (Fig. 5c). The 24.82° peak is a superposition of the reflections from the lamellar intralayer spacing and the glass plate substrate. In contrast to film A, the intensity of the signal at 8.04° for films B (black) and C (blue) is depressed, suggesting their lower structural order. The broad signal centered at 24.22° mainly comes from the glass substrate and may become evident as compared to the film A due to the reduced reflection from the film bulk. However, almost the same XRD pattern as that for film A were recorded when the incident angle of X-ray was fixed at 0.15° (Fig. 5b, black curve for film B and blue curve for film C). Considering that the penetration depth of the X-rays for this incident angle is estimated to be 0.26 μm,30,32 these results indicated that the oligomers are oriented in the vicinity of the film surfaces. Comparison of the signal intensity at 8.04° between the two patterns indicates the higher structural order of film C than that of film B. This result implies that the rubbing with a cloth should orient more oligomers in the film and increase the structural order. Also, considering the above experimental finding that the gold-like luster increased with an increase in the structural order, the extent to which the oligomers are aligned in the film is partly responsible for the development of the gold-like luster.Considering that the boiling point of nitromethane (b.p. = 101 °C) is higher than that of acetonitrile (b.p. = 82 °C), an evaporation rate may affect the crystallization of the films during their drying, resulting in the difference in the extent to which the molecules of 1 are aligned in the films, and in turn, the color difference of films A and B. On the other hand, the color difference of films B and C may be caused by the difference in the extent of molecular alignment before and after the rubbing of the film as described above.
In accordance with the structural order, the electric conductivity measured by a four-point-probe method was in the decreasing order of film A (1.1 × 10−2 S cm−1), film C (5.5 × 10−3 S cm−1), and film B (3.0 × 10−3 S cm−1), which can be explained by the enhanced carrier mobility associated with an improved crystallinity.33–35 The signal at 26.1° in Fig. 5b allowed us to determine the stacking distance of the thiophene rings, 0.34 nm (Fig. 5c), which is shorter than that for the poly(3-hexylthiophene) and poly(3-octylthiophene), 0.38 nm, and indicated a very compressed molecular packing and strong intermolecular interactions. When the film is viewed from the back surface of film A, a gold luster was also observed though it is slightly dull compared to that of the film surface, whereas, the back surfaces of films B and C were deep blue and showed no luster. This observation again provides evidence that the development of the gold luster is closely related to the orientation of the oligomers when considered along with the XRD measurement results. Ogura et al. insisted that the development of the gold luster for their powdered crystals is closely associated with the high coplanarity of the molecular layers and their highly compact nature developed by the intralayer interaction.9,10,14 The present oligomer-sized 3-methoxythiophene linkage structure most likely forms such a highly coplanar and compact lamellae through the π–π interactions between the thiophene rings.
Fig. 6 shows the photograph (a), reflection spectrum (b), UV-vis transmission spectrum (c), and XRD pattern (d) of film A after long-term storage for a year. The film was stored in an ambient atmosphere (ordinary pressure and temperature, no direct sunlight). Little change in the color (Fig. 6a vs. 1a), lustrous feature (Fig. 6b vs. 2a), and hiding power (Fig. 6c vs. 3) of film A with elapsed time was observed. On the other hand, in Fig. 6d, sharp signals (2θ = 15.3, 19.4, 22.7, 24.6, 26.9, 27.5, 30.2, 30.8, and 34.5°) were superimposed on the initial XRD pattern in Fig. 5a. This suggests that highly ordered structures with different alignment directions are formed. However, considering that the spectroscopic characteristics (reflection and transmission spectra) and external appearance did not change much for a long time, the amount of the newly formed ordered structures should be very small.
 | ||
| Fig. 6 Photograph (a), reflection spectrum (b), UV-vis transmission spectrum (c), and XRD pattern (d) of film A taken after a year. | ||
4. Conclusion
In summary, we have successfully obtained the first total organic paints which can be cast into air-stable films with a gold-like luster. We believe that they open up new scientific and technological fields of lustrous materials and coatings. We are now beginning to use dopants and substituents of different chemical structures and investigate their effects on the lustrous properties of the cast films, the details of which will be reported elsewhere.Acknowledgements
This work was supported by Japan Science and Technology Agency (JST) through its funding program for Adaptable and Seamless Technology Transfer Program through Target-driven R&D (no. AS251Z01269M).Notes and references
- F. J. Maile, G. Pfaff and P. Reynders, Prog. Org. Coat., 2005, 54, 150–163 CrossRef CAS PubMed.
- H. Liu, H. Ye and Y. Zhang, Colloids Surf., A, 2008, 315, 1–6 CrossRef CAS PubMed.
- G. B. Smith, A. Gentle, P. Swift, A. Earp and N. Mronga, Sol. Energy Mater. Sol. Cells, 2003, 79, 163–177 CrossRef CAS.
- T. G. H. James, Gold Bull., 1972, 5, 38–42 CrossRef.
- R. Schoppe, Aluminum Pigments for Plastics, August, 2012, pp. 1–17, http://www.silberline.com/BasicTemplate.aspx?id=1790, accessed December 2013.
- A.-Y. Zhu, J.-L. Chen, Z. Li, L.-Y. Luo, Q. Lei, L. Zhang and W. Zhang, Trans. Nonferrous Met. Soc. China, 2013, 23, 1349–1355 CrossRef CAS.
- X.-C. Li, H. Sirringhaus, F. Garnier, A. B. Holmes, S. C. Moratti, N. Feeder, W. Clegg, S. J. Teat and R. H. Friend, J. Am. Chem. Soc., 1998, 120, 2206–2207 CrossRef CAS.
- J. P. Parakka and M. P. Cava, Synth. Met., 1995, 68, 275–279 CrossRef CAS.
- K. Ogura, R. Zhao, H. Yanai, K. Maeda, R. Tozawa, S. Matsumoto and M. Akazome, Bull. Chem. Soc. Jpn., 2002, 75, 2359–2370 CrossRef CAS.
- R. Zhao, M. Akazome, S. Matsumoto and K. Ogura, Tetrahedron, 2002, 2, 10225–10231 CrossRef.
- R. Zhao, S. Matsumoto, M. Akazome and K. Ogura, Tetrahedron, 2002, 58, 10233–10241 CrossRef CAS.
- K. Ogura, R. Zhao, M. Jiang, M. Akazome, S. Matsumoto and K. Yamaguchi, Tetrahedron Lett., 2003, 44, 3595–3598 CrossRef CAS.
- K. Ogura, R. Zhao, T. Mizuoka, M. Akazome and S. Matsumoto, Org. Biomol. Chem., 2003, 1, 3845–3850 CAS.
- K. Ogura, K. Ooshima, M. Akazome and S. Matsumoto, Tetrahedron, 2006, 62, 2484–2491 CrossRef CAS PubMed.
- A. Matsumoto, M. Kawaharazuka, Y. Takahashi, N. Yoshino, T. Kawai and Y. Kondo, J. Oleo Sci., 2010, 59, 151–156 CrossRef CAS.
- Z. Han, J. Zhang, X. Yang, H. Zhu and W. Cao, Sol. Energy Mater. Sol. Cells, 2010, 94, 755–760 CrossRef CAS PubMed.
- T. Ung, L. M. Liz-Marzán and P. Mulvaney, Colloids Surf., A, 2002, 202, 119–126 CrossRef CAS.
- J. Siegel, O. Lyutakov, V. Rybka, Z. Kolská and V. Švorčík, Nanoscale Res. Lett., 2011, 6, 96–104 CrossRef PubMed.
- G. Tourillon, in Handbook of Conducting Polymers, ed. T. A. Skotheim, Marcel Dekker, New York and Basel, 1986, vol. 1, ch. 9, pp. 293–350 Search PubMed.
- R. Mondal, H. A. Becerril, E. Verploegen, D. Kim, J. E. Norton, S. Ko, N. Miyaki, S. Lee, M. F. Toney, J.-L. Brédas, M. D. McGehee and Z. Bao, J. Mater. Chem., 2010, 20, 5823–5834 RSC.
- U. Zhokhavets, T. Erb, G. Gobsch, M. Al-Ibrahim and O. Ambacher, Chem. Phys. Lett., 2006, 418, 347–350 CrossRef CAS PubMed.
- M. J. Winokur, P. Wamsley, J. Moulton, P. Smith and A. J. Heeger, Macromolecules, 1991, 24, 3812–3815 CrossRef CAS.
- T. J. Prosa, M. J. Winokur, J. Moulton, P. Smith and A. J. Heeger, Macromolecules, 1992, 25, 4364–4372 CrossRef CAS.
- R. D. McCullough, S. Tristram-Nagle, S. P. Williams, R. D. Lowe and M. Jayaraman, J. Am. Chem. Soc., 1993, 115, 4910–4911 CrossRef CAS.
- K. E. Aasmundtveit, E. J. Samuelsen, M. Guldstein, C. Steinsland, O. Flornes, C. Fagermo, T. M. Seeberg, L. A. A. Pettersson, O. Inganäs, R. Feidenhans'l and S. Ferrer, Macromolecules, 2000, 33, 3120–3127 CrossRef CAS.
- C. Visy, G. Bencsik, Z. Németh and A. Vértes, Electrochim. Acta, 2008, 53, 3942–3947 CrossRef CAS PubMed.
- J. Jose Abad, B. Pérez-García, A. Urbina, J. Colchero and E. Palacios-Lidón, Eur. Polym. J., 2008, 44, 2506–2515 CrossRef PubMed.
- Z. Yu, H. Yan, K. Lu, Y. Zhang and Z. Wei, RSC Adv., 2012, 2, 338–343 RSC.
- Y. Qu, L. Li, G. Lu, X. Zhou, Q. Su, W. Xu, S. Li, J. Jidong Zhang and X. Yang, Polym. Chem., 2012, 3, 3301–3307 RSC.
- J. Abad, N. Espinosa, P. Ferrer, R. García-Valverde, C. Miguel, J. Padilla, A. Alcolea, G. R. Castro, J. Colchero and A. Urbina, Sol. Energy Mater. Sol. Cells, 2012, 97, 109–118 CrossRef CAS PubMed.
- A. Pron, P. Gawrys, M. Zagorska, D. Djurado and R. Demadrille, Chem. Soc. Rev., 2010, 39, 2577–2632 RSC.
- R. Feidenhans'l, Surf. Sci. Rep., 1989, 10, 105–188 CrossRef.
- I. Mcculloch, M. Heeney, C. Bailey, K. Genevicius, I. Macdonald, M. Shkunov, D. Sparrowe, S. Tierney, R. Wagner, W. Zhang, M. L. Chabinyc, R. J. Kline, M. Mcgehee and M. F. Toney, Nat. Mater., 2006, 5, 328–333 CrossRef CAS PubMed.
- M. Ikawa, T. Yamada, H. Matsui, H. Minemawari, J. Tsutsumi, Y. Horii, M. Chikamatsu, R. Azumi, R. Kumai and T. Hasegawa, Nat. Commun., 2012, 3, 1176 CrossRef PubMed.
- Y. Diao, B. C.-K. Tee, G. Giri, J. Xu, D. H. Kim, H. A. Becerril, R. M. Stoltenberg, T. H. Lee, G. Xue, S. C. B. Mannsfeld and Z. Bao, Nat. Mater., 2013, 12, 665–671 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR spectra, FT-IR spectrum, elemental analyses, electric conductivity measurement. See DOI: 10.1039/c4ra03548h |
| This journal is © The Royal Society of Chemistry 2014 |