DOI:
10.1039/C4RA01845A
(Paper)
RSC Adv., 2014,
4, 24406-24411
Development of a sensitive indirect competitive enzyme-linked immunosorbent assay based on the monoclonal antibody for the detection of benzothiostrobin residue†
Received
3rd March 2014
, Accepted 15th May 2014
First published on 16th May 2014
Abstract
An indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) based on monoclonal antibodies (MAbs) for benzothiostrobin has been developed. The hapten of benzothiostrobin was synthesized and conjugated to bovine serum albumin and ovalbumin to generate an immunogen and a coating antigen. The immunogen was used to immunize BALB/c mice, resulting in anti-benzothiostrobin MAb. Under optimal conditions, the half maximal inhibition concentration (IC50) and the limit of detection (IC10) of the developed ic-ELISA were 7.55 and 0.428 μg L−1, respectively. The cross-reactivity (CR) was less than 0.05% for the tested structural analogues and regarded as negligible except for pyraclostrobin, which exhibited a CR of 0.34%. The recoveries of benzothiostrobin ranged from 80.43 to 113.83% in environmental and agricultural samples, respectively, which conformed to the requirements for residue detection. The amount of benzothiostrobin detected by ic-ELISA in the samples was significantly (R2 = 0.9894, y = 1.0867x + 0.0318) correlated with that detected by high-performance liquid chromatography (HPLC). The current study indicates that the established ic-ELISA is a potentially useful tool for detecting benzothiostrobin in environmental and agricultural samples.
1. Introduction
Strobilurin fungicide belongs to a new class of fungicides that exhibits broad-spectrum, efficient and environmentally friendly features. This new class of fungicides has attracted considerable attention from researchers. Benzothiostrobin, which is a fungicide developed by the Central China Normal University, is a novel strobilurin fungicide that exhibits highly efficient control of most fungal diseases in crops,1 and the patents of preparing and using benzothiostrobin have been applied in China, United States, Europe, and worldwide (Pub. no.: CN 102302012 B, CN 101379967 A, CN 101268780 B, US 2010/0292285 A1, 09741682.0, WO 2007/073637 A1, WO 2009/135407 A1). To the best of our knowledge, benzothiostrobin was transferred to Jiangsu Seven Continent Green Chemical Co., Ltd. in 2010. Therefore, benzothiostrobin, at least in China, has potential prospective applications. In order to keep its potential residual risk under control, the development of a rapid, sensitive, and economical method for detecting benzothiostrobin in agricultural produce and the environment as a technical support is imperative and important.
High-performance liquid chromatography (HPLC) has been used successfully for the detection of benzothiostrobin and other pesticides,2 which is characterized by low limits of detection (LOD) as well as high precision and sensitivity. However, the analytical methods are costly, time-consuming, and not suitable for the analysis of large numbers of samples.3,4 During the past decade, many enzyme-linked immunosorbent assays (ELISAs) have been published for the analysis of residues for a broad variety of pesticides in different types of food samples,5–11 and several immunochemical methodologies have been approved to analyze chemical residues in food, feed and environmental samples. In comparison, ELISAs provide a fast, sensitive, cost-effective, and selective method for the detection of pesticide residues. Several immunoassays for strobilurin fungicides have been reported.12–18 However, studies on antibodies and the development of immunoassays for benzothiostrobin residue have not been published.
In this paper, an indirect competitive ELISA (ic-ELISA) method was developed based on monoclonal antibodies for the detection of benzothiostrobin. Due to this method's high sensitivity and accuracy, ic-ELISA provides an alternate method for the detection of benzothiostrobin in environmental and agricultural samples.
2. Materials and methods
2.1. Materials and instruments
Benzothiostrobin (99.02%) was obtained from the Central China Normal University (Wuhan, China). The pesticide standards used for cross-reactivity studies were purchased from Dr Ehrenstorfer GmbH (Germany). Bovine serum albumin (BSA), ovalbumin (OVA), complete and incomplete Freund's adjuvants, N-hydroxysuccinimide (NHS), N,N′-dicyclohexylcarbodiimide (DCC), o-phenylenediamine (OPD), and polyoxyethylene sorbitan monolaurate (Tween-20) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG was purchased from DingGuo ChangSheng Biotechnology Co. (Beijing, China). All the other reagents were of analytical grade. The BALB/c mice were purchased from the Center of Comparative Medicine of Yangzhou University (Yangzhou, China). All the animal studies were performed in accordance with institutional guidelines.
Mass spectral (MS) data were obtained with an HPLC-QTOF instrument (Bruker, Germany). Nuclear magnetic resonance (NMR) spectra were recorded on a DRX500 spectrometer (Bruker, Germany). Ultraviolet-visible (UV-Vis) spectra were obtained on a UV-2550 spectrophotometer (Shimadzu, Japan). The 96-well transparent microplates were purchased from Nunc (Roskilde, Denmark). The absorbances were read with an I-mark microtiter plate reader (Bio-RAD, USA) at 490 nm, and the ELISA plates were washed with a Wellwash Plus (Thermo, USA). Benzothiostrobin was detected using Agilent 1200 HPLC (Agilent, USA). The antibody was freeze-dried using a vacuum freeze-drying machine (Thermo Savant, USA). The protein A column was purchased from the Pall Corporation (Pall, USA).
2.2. Buffers and solutions
Phosphate-buffered saline (PBS, 0.01 mol L−1, pH 7.4), carbonate-buffered saline (CBS, 0.05 mol L−1, pH 9.6), and phosphate-buffered saline containing 0.05% Tween-20 (PBST) were used. The tetramethylbenzidine (TMB) solution contained 0.4 mmol L−1 TMB and 3 mmol L−1 H2O2 in a citrate buffer (pH 5.0).
2.3. Hapten synthesis
The synthesis route of the hapten is shown in Fig. 1. Here, 8.02 g of benzothiostrobin (0.02 mol) was added to 10 mL of 10 mol L−1 LiOH (0.1 mol), and the mixture was stirred at room temperature for 4 h. Then, 30 mL of water was added to the mixture followed by extraction with ethyl acetate. The aqueous phase was adjusted to pH 3.0 with 10% HCl and extracted with ethyl acetate, and the organic phase was dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain a yellow oil, which was purified on a silica gel column eluted using 150 mL of methanol–dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) and 200 mL of methanol–dichloromethane (1
50 v/v) and 200 mL of methanol–dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v).19 The second fraction was collected and concentrated to obtain hapten, which was characterized by ESI-MS and NMR: ESI-MS, m/z: 346 [M + H+] and 368 [M + Na+]; 1H NMR (400 MHz, DMSO) δ: 3.79 (s, 2H, CH2COO), 3.86 (s, 3H, OCH3), 4.67 (s, 2H, SCH2), 7.01 (dd, 1H, ArH), 7.32–7.22 (m, 3H, ArH), 7.47 (d, 1H, ArH), 7.52 (m, 1H, ArH), 7.88 (m, 1H, ArH), 12.52 (s, 1H, COOH).
30 v/v).19 The second fraction was collected and concentrated to obtain hapten, which was characterized by ESI-MS and NMR: ESI-MS, m/z: 346 [M + H+] and 368 [M + Na+]; 1H NMR (400 MHz, DMSO) δ: 3.79 (s, 2H, CH2COO), 3.86 (s, 3H, OCH3), 4.67 (s, 2H, SCH2), 7.01 (dd, 1H, ArH), 7.32–7.22 (m, 3H, ArH), 7.47 (d, 1H, ArH), 7.52 (m, 1H, ArH), 7.88 (m, 1H, ArH), 12.52 (s, 1H, COOH).
 |
| | Fig. 1 Synthetic route of the benzothiostrobin hapten. | |
2.4. Preparation of hapten–protein conjugates
The hapten was conjugated with BSA via the carbodiimide method to produce an immunogen and conjugated with OVA via the mixed anhydride method to produce a coating antigen.20 The conjugates were dialyzed in PBS over 72 h at 4 °C and stored at −20 °C. The formations of conjugates were confirmed by UV-Vis spectroscopy, and the hapten densities (the number of hapten molecules per molecule of protein) of the conjugates were estimated according to the following formula:
| Ca/Cb = (εCa × KBb − εCb × KBa)/(εCb × KAa − εCa × KAb) |
where A, B, and C represent the hapten, protein and conjugation, respectively; x represents a and b; εAa, εBa and εCa are their ultraviolet absorbances at the characteristic absorption wavelength of hapten and εAb, εBb and εCb are their ultraviolet absorbances at the characteristic absorption wavelength of the protein; ρ is the concentration; and M is the molar mass.21,22
2.5. Immunization and monoclonal antibody preparation
Six-week-old female BALB/c mice were immunized with immunogen via an intraperitoneal injection according to the method described by Kishiro et al.23 The dosage of the first immunogen for each mouse was 100 μg (7.93 mg mL−1) dissolved in physiological saline and emulsified with an equal volume of Freund's complete adjuvant. After 3 weeks, four subsequent injections were given at 2-week intervals using immunogen emulsified with incomplete Freund's adjuvant. The antisera were obtained from the tail vein of the mouse one week after the immunization, and the sera were tested by ic-ELISA to determine their ability to conjugate with benzothiostrobin. The mouse that exhibited the strongest response was used for the peritoneal cavity injections of 200 μg of immunogen in PBS 1 week after the last immunization. Three days after the booster injection, cell fusion was performed according to Nowinski et al.24 Mouse spleen lymphocytes were fused with SP2/0 myeloma cells in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The fused cells were cultured in a hypoxanthine–aminopterin–thymidine (HAT) medium at 37 °C in an atmosphere of 5% CO2. Half of the media in the wells were replaced by fresh HAT media every third day. Fourteen days after cell fusion, the HAT media were changed to HT media. Culture supernatants were screened for their ability to recognize benzothiostrobin, and hybridoma cells in the ELISA positive wells were cloned using the limiting dilution method. Stable antibody-producing clones were expanded. Ascites obtained from BALB/c mice were purified using a protein A column and stored at −20 °C after freeze-drying. The subtyping of MAb was identified by “mouse monoclonal antibody isotyping reagents” (Sigma).
1 ratio. The fused cells were cultured in a hypoxanthine–aminopterin–thymidine (HAT) medium at 37 °C in an atmosphere of 5% CO2. Half of the media in the wells were replaced by fresh HAT media every third day. Fourteen days after cell fusion, the HAT media were changed to HT media. Culture supernatants were screened for their ability to recognize benzothiostrobin, and hybridoma cells in the ELISA positive wells were cloned using the limiting dilution method. Stable antibody-producing clones were expanded. Ascites obtained from BALB/c mice were purified using a protein A column and stored at −20 °C after freeze-drying. The subtyping of MAb was identified by “mouse monoclonal antibody isotyping reagents” (Sigma).
2.6. Immunoassay procedure
ELISA was performed on 96-well polystyrene microplates. The coating antigen (benzothiostrobin–OVA) was diluted with CBS, pipetted into the wells (100 μL per well), and incubated overnight at 4 °C. The plate was washed 5 times with PBST and blocked by adding 1% OVA/PBS (200 μL per well), followed by incubation for 50 min at 37 °C. After 5 repeated washings with PBST, either the sample or the standard in the PBS containing methanol (50 μL per well) was added, followed by the addition of the diluted antibody (50 μL per well, in PBS) and incubated for 1 h at 37 °C. After an additional wash, the diluted goat anti-mouse IgG-HRP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in PBS, 100 μL per well) was dispensed into each well and incubated for 1 h at 37 °C. Then, the plates were washed again, and the substrate solution was added (100 μL per well). The reaction was terminated with sulfuric acid (2 mol L−1, 50 μL per well) after 15 min of incubation. The absorbance was measured at 490 nm.
000 in PBS, 100 μL per well) was dispensed into each well and incubated for 1 h at 37 °C. Then, the plates were washed again, and the substrate solution was added (100 μL per well). The reaction was terminated with sulfuric acid (2 mol L−1, 50 μL per well) after 15 min of incubation. The absorbance was measured at 490 nm.
2.7. Assay optimization
The optimal concentrations of coating antigen and antibody were confirmed using the two-dimensional checkerboard method.25 The experimental parameters including the organic solvent, ionic strength, and buffer pH value were sequentially studied to improve the sensitivity of the immunoassay. Competitive curves were run in PBS solutions containing different concentrations of methanol (from 5 to 30%, v/v) and Na+ (0.1, 0.2, 0.3, 0.4 and 0.5 mol L−1) as well as with various pH values (5.5, 6.5, 7.5 and 8.5), and the evaluations of the parameters were based on the Amax/IC50, IC50 and the squared coefficients of correlation (R2) of the linear equation.26
A standard curve for benzothiostrobin was obtained under the optimum conditions by plotting the percent binding (% B/B0) as a function of the concentration of benzothiostrobin (C). The % B/B0 was calculated using the following formula:
| % B/B0 = [(Ax − Amin)/(Amax − Amin)] × 100 |
where
Ax is the absorbance of the sample,
Amax is the absorbance in the absence of the analyte, and
Amin is the absorbance of the background.
2.8. Cross-reactions
The cross-reactivity (CR) of ic-ELISA was studied by detecting the analogues of benzothiostrobin.27 The CR values were calculated with the following formula:
| CR% = (IC50 of benzothiostrobin/IC50 of analogue) × 100 |
2.9. Analysis of spiked samples
To evaluate the accuracy and precision of the immunoassays, the recoveries of the spiked samples were studied by ELISA. Ten grams of the blank samples (including river water, soil, rice, pear, or tomato sample) were spiked with benzothiostrobin at 0.01, 0.05, or 0.5 mg kg−1 and stored overnight. The river water samples were mixed with the same volume of PBS containing 10% methanol and analyzed by ELISA. Other samples were extracted twice by sonication in 20 mL of methanol for 10 min and centrifuged for 10 min at 4000 rpm.28 After dilution at appropriate multiple steps, the solutions were analyzed via the immunoassays. The spiked recoveries were used to confirm the accuracy of ic-ELISA.
The matrix interferences of the samples were analyzed by testing 2 to 20-fold dilutions with PBS containing methanol.29 The matrix interference was determined by comparing the benzothiostrobin standard curve prepared in matrix-free PBS with calibration curves prepared with a series of diluted matrix extracts.
2.10. Practical application of the immunoassays
Soil and pear (1, 3, 7, 14 days after sprayed) were collected from farms where benzothiostrobin had been used in Nanjing, China. The concentrations of benzothiostrobin in the samples were simultaneously analyzed by ELISA and HPLC to evaluate the correlation between the two methods. For ELISA, the extraction and analysis of the samples were performed as described above. For HPLC, the samples were extracted twice by sonicating in 20 mL of acetonitrile for 10 min and centrifuging for 10 min at 4000 rpm. The supernatant was filtered through anhydrous sodium sulfate and concentrated. Then, the concentrates were diluted with 2 mL of acetonitrile and detected by HPLC with SB-C18 column (250 mm × 4.6 mm i.d.) at 230 nm, and acetonitrile–0.05% trifluoroacetic acid (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v) was used as the mobile phase at a flow rate of 0.8 mL min−1.2
35, v/v) was used as the mobile phase at a flow rate of 0.8 mL min−1.2
3. Results and discussion
3.1. Identification of artificial antigens and coupling ratio
Under alkaline conditions, the ethylene bond of the reactant dissociated. With the identification by ESI-MS and NMR, the structure of the obtained hapten was similar to benzothiostrobin, and the hapten contained a carboxyl group that could be conjugated to a carrier protein. In addition, the next study confirmed that the obtained hapten could be used to prepare specific anti-benzothiostrobin antibodies. The UV-Vis spectra indicated qualitative differences between the hapten, carrier protein and conjugates, especially at 280 nm, and confirmed that the carrier protein and hapten had been successfully coupled. The molar ratios (hapten/protein) were 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 8
1 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the immunogen and coating antigen, respectively.
1 for the immunogen and coating antigen, respectively.
3.2. Subtyping of MAb
Two cell lines that can stably produce MAbs against benzothiostrobin were obtained and named 4E8 and 2B4. By determining the immunoglobulin subclass, the results indicated that 4E8 and 2B4 were of the lgG2a and lgM subclasses with a kappa light chain, respectively. In this study, 4E8 exhibited higher sensitivity than 2B4 and was used in further studies.
3.3. Optimization of immunoassays
The optimal concentrations of the coating antigen and antibody were 500 and 35 μg L−1, respectively. For solvent optimization, methanol was used to improve the solubility of the analyte and evaluate its effect on the ELISA method of benzothiostrobin. With the methanol concentrations being 5%, the ic-ELISA method showed the highest Amax/IC50 and lowest IC50. The ionic strength and pH of the buffer also affected the sensitivity of ic-ELISA; when the PBS buffer contained 0.3 mol L−1 Na+ at pH 6.5, the assay exhibited the lowest IC50 and highest sensitivity. Therefore, the optimum parameters included 5% methanol and 0.3 mol L−1 Na+ at pH 6.5, resulting in ELISA with the lowest IC50 value and maximum Amax/IC50.
3.4. Sensitivities
Under the optimum conditions, the calibration curve was obtained using the relationship between the percent binding (% B/B0) and the concentration of benzothiostrobin shown in Fig. 2. The assay indicated that IC50 was 7.55 μg L−1 and LOD (IC10) was 0.428 μg L−1. The linear range (IC10–IC90) was 0.428–54 μg L−1. In a published article, the linear range was 0.1–10 mg L−1 for benzothiostrobin by HPLC analysis.2 Comparing the two methods, ELISA was more sensitive than HPLC.
 |
| | Fig. 2 Standard curve for benzothiostrobin by ELISA. | |
3.5. Specificity
Table 1 shows the CRs result for the analogues of benzothiostrobin. The highest cross-reactivity was observed for pyraclostrobin (0.34%). The antibody exhibited negligible cross-reactivity (CR% < 0.05%) for the other analogues. Therefore, the assays exhibited strong specificity for benzothiostrobin.
Table 1 Cross-reactivity of a set of analogs related to benzothiostrobin by ELISA
| Compound |
Structure |
IC50 (mg L−1) |
CR (%) |
| Benzothiostrobin |
 |
0.00755 |
100 |
| Pyraclostrobin |
 |
2.25 |
0.34 |
| Azoxystrobin |
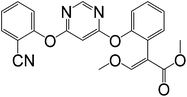 |
>100 |
<0.05 |
| Kresoxim-methyl |
 |
>100 |
<0.05 |
| Picoxystrobin |
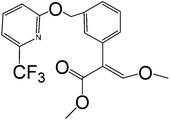 |
>100 |
<0.05 |
| Chloropiperidine ester |
 |
>100 |
<0.05 |
| Fluoxastrobin |
 |
>100 |
<0.05 |
| Fenaminstrobin |
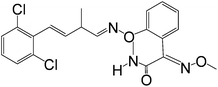 |
>100 |
<0.05 |
3.6. Matrix effects
The results of the matrix effects study indicated that different sample matrices had different effects on the sensitivity of the immunoassays. The matrices of the rice and tomato samples had a substantial impact, and their effects were reduced to acceptable levels by a 10-fold dilution. The matrices of the pear and soil samples exhibited a small impact. For a 5-fold dilution of the pear and soil samples, the interference had no effect on the sensitivity of the immunoassays. Because too much dilution would eventually reduce the limit of quantification of the samples, a 5-fold dilution (soil and pear) and 10-fold dilution (tomato and rice) were selected for subsequent immunoassays.
3.7. Analysis of spiked samples
As shown in Table 2, the immunoassay method had acceptable recoveries from 83.4 to 115.6%, and the relative standard deviations (RSDs) ranged from 1.8 to 6.8%. The test results were confirmed by HPLC, and good correlations between the HPLC results and the immunoassay results were obtained. These results indicated that the precision of ic-ELISA met the requirement of detecting residual benzothiostrobin in the samples.
Table 2 Recovery of benzothiostrobin in spiked samples (n = 3)
| Sample |
Spiked concentration (mg L−1 or mg kg−1) |
Average recovery ± SD (%) |
RSD% |
| River water |
0.01 |
85.3 ± 2.2 |
2.6 |
| 0.05 |
93.5 ± 1.7 |
1.8 |
| 0.5 |
100.2 ± 3.8 |
3.8 |
| Soil |
0.01 |
89.7 ± 1.2 |
1.3 |
| 0.05 |
89.4 ± 3.6 |
4.0 |
| 0.5 |
95.5 ± 3.2 |
3.4 |
| Rice |
0.01 |
113.1 ± 6.3 |
5.6 |
| 0.05 |
107.4 ± 2.8 |
2.6 |
| 0.5 |
85.3 ± 3.5 |
4.1 |
| Pear |
0.01 |
93.3 ± 3.2 |
3.4 |
| 0.05 |
95.6 ± 1.5 |
1.6 |
| 0.5 |
100.1 ± 2.4 |
2.4 |
| Tomato |
0.01 |
115.6 ± 7.9 |
6.8 |
| 0.05 |
109.8 ± 5.5 |
5.0 |
| 0.5 |
83.4 ± 3.2 |
3.8 |
3.8. Analysis of authentic samples
Table 3 shows the results from the established ELISA for benzothiostrobin-positive soil and pear samples in Nanjing, China. According to the results (from 70 to 1490 μg L−1), the residue in the soil and pear samples progressively declined over time. The results of the immunoassays and HPLC for authentic samples exhibited good correlations (R2 = 0.9894, y = 1.0867x + 0.0318) (Fig. 3). Therefore, the developed immunoassay was reliable and accurate.
Table 3 Comparison of benzothiostrobin residues between the ELISA and HPLC in authentic samples (n = 3)
| Sample |
ELISA |
HPLC |
| Mean (mg kg−1) |
RSD (%) |
Mean (mg kg−1) |
RSD (%) |
| Soil |
1 day |
1.49 |
3.1 |
1.37 |
2.5 |
| 3 days |
1.12 |
2.9 |
1.01 |
2.6 |
| 7 days |
0.78 |
3.3 |
0.67 |
2.7 |
| 14 days |
0.64 |
3.2 |
0.57 |
2.6 |
| Pear |
1 day |
0.81 |
5.1 |
0.70 |
4.2 |
| 3 days |
0.63 |
6.1 |
0.55 |
4.5 |
| 7 days |
0.45 |
4.8 |
0.31 |
5.0 |
| 14 days |
0.07 |
5.4 |
0.11 |
4.1 |
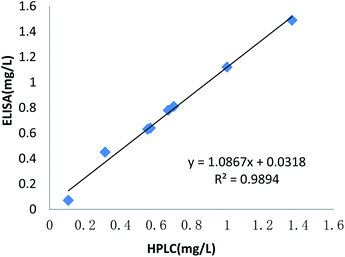 |
| | Fig. 3 Correlation between ELISA and HPLC for authentic samples. | |
4. Conclusions
We reported an ic-ELISA method for detecting benzothiostrobin in environmental and agricultural samples. The quantitative data indicated that the specificity and accuracy of ic-ELISA were ideal and in good agreement with the HPLC measurements. These results indicate that the established ic-ELISA is a potentially useful tool for detecting benzothiostrobin in environmental and agricultural samples. Based on this study, more detection methods, such as chemiluminescence immunoassay, fluorescence immunoassay, and immunochromatographic assay, could be developed for detecting benzothiostrobin.
Acknowledgements
This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (no. 201203022) and the Youth Innovation Fund in Science & Technology of Nanjing Agricultural University (KJ2013008).
References
- P. L. Zhao, F. Wang, W. Huan, Q. Chen and Z. M. Liu, Chin. J. Org. Chem., 2010, 30, 1567 CAS.
- Y. L. Yuan and M. H. Wang, Bioact. Heterocycl. Compd. Cl., 2013, 52, 347 CAS.
- L. M. Hernández, M. A. Fernández, E. Hoyas, M. J. González and J. F. Garcia, Bull. Environ. Contam. Toxicol., 1993, 50, 308 Search PubMed.
- K. A. Bucholski, J. Begerow, G. Winneke and L. Dunemann, J. Chromatogr. A, 1996, 754, 479 CrossRef CAS.
- S. C. Pei, W. J. Lee, G. P. Zhang, X. F. Hu, S. A. Eremin and L. J. Zhang, Food Control, 2013, 31, 65 CrossRef CAS PubMed.
- Q. F. Cai, X. C. Wang, G. M. Liu, L. Zhang, M. M. Ruan, Y. Liu and M. J. Cao, Food Control, 2013, 29, 241 CrossRef CAS PubMed.
- S. Wanatabe, S. Ito, Y. Kamata, N. Omoda, T. Yamazaki, H. Munakata, T. Kaneko and Y. Yuasa, Anal. Chim. Acta, 2001, 427, 211 CrossRef CAS.
- D. L. D. Lima, R. J. Schneider and V. I. Esteves, Environ. Sci. Pollut. Res., 2013, 20, 3157 CrossRef CAS PubMed.
- S. Fang, B. Zhang, K. W. Ren, M. M. Cao, H. Y. Shi and M. H. Wang, J. Agric. Food Chem., 2011, 59, 1594 CrossRef CAS PubMed.
- Y. Y. Xia, Q. X. Li, S. J. Gong, Y. Li, Y. S. Cao, X. L. Liu and J. Q. Li, Food Chem., 2010, 120, 1178 CrossRef CAS PubMed.
- Z. J. Liu, M. Li, H. Y. Shi and M. H. Wang, Food Anal. Methods, 2013, 6, 691 CrossRef.
- J. V. Mercader, C. Suárez-Pantaleón, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, J. Agric. Food Chem., 2008, 56, 7682 CrossRef CAS PubMed.
- J. V. Mercader, C. Suárez-Pantaleón, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, J. Agric. Food Chem., 2008, 56, 2581 CrossRef CAS PubMed.
- J. V. Mercader, C. Suárez-Pantaleón, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, J. Agric. Food Chem., 2008, 56, 1545 CrossRef CAS PubMed.
- F. A. Esteve-Turrillas, J. Parra, A. Abad-Fuentes, C. Agulló, A. Abad-Somovilla and J. V. Mercader, Anal. Chim. Acta, 2010, 682, 93 CrossRef CAS PubMed.
- F. A. Esteve-Turrillas, J. V. Mercader, C. Agulló, A. Abad-Somovilla and A. Abad-Fuentes, J. Chromatogr. A, 2011, 1218, 4902 CrossRef CAS PubMed.
- M. Kondo, K. Tsuzuki and H. Hamada, J. Agric. Food Chem., 2012, 60, 904 CrossRef CAS PubMed.
- V. S. Morozova, A. I. Levashova and S. A. Eremin, J. Anal. Chem., 2005, 60, 202 CrossRef CAS PubMed.
- H. J. Bao, S. Fang, Z. J. Liu, H. Y. Shi, Y. H. Ye and M. H. Wang, J. Agric. Food Chem., 2010, 58, 8167 CrossRef CAS PubMed.
- Q. Zhang, Y. R. Wu, L. M. Wang, B. S. Hu, P. W. Li and F. Q. Liu, Anal. Chim. Acta, 2008, 625, 87 CrossRef CAS PubMed.
- L. G. Yang, S. C. Hu, P. H. Wei and A. Z. Guo, Enzyme linked immunosorbent assays detection technology, Naniing University press, Naniing, 1998, pp. 279–280, Chinese Search PubMed.
- V. M. Josep, S. Celia, A. Consuelo, A. Antonio and A. Antonio, J. Agric. Food Chem., 2008, 56, 7682 CrossRef PubMed.
- Y. Kishiro, M. Kagawa, I. Naito and Y. Sado, Cell Struct. Funct., 1995, 20, 151 CrossRef CAS.
- R. C. Nowinski, M. E. Lostrom, M. R. Tam, M. R. Stone and W. N. Burnette, Virology, 1979, 93, 113 CrossRef.
- G. M. Shan, I. Wengatz, D. W. Stoutamire, S. J. Gee and B. D. Hammock, Chem. Res. Toxicol., 1999, 12, 1033 CrossRef CAS PubMed.
- G. L. Qian, L. M. Wang, Y. R. Wu, Q. Zhang, Q. Sun, Y. Liu and F. Q. Liu, Food Chem., 2009, 117, 364 CrossRef CAS PubMed.
- E. Watanabe, H. Eun, K. Baba, T. Arao, Y. Ishii, S. Endo and M. Ueji, J. Agric. Food Chem., 2004, 52, 2756 CrossRef CAS PubMed.
- F. Malhat, E. Kamel, A. Saber, E. Hassa, A. Youssef, M. Almaz, A. Hassan and A. E. Fayz, Food Chem., 2013, 140, 371 CrossRef CAS PubMed.
- M. Li, E. Z. Sheng, L. J. Cong and M. H. Wang, J. Agric. Food Chem., 2013, 61, 3619 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01845a |
| ‡ Both authors contributed equally to this paper. |
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) and 200 mL of methanol–dichloromethane (1
50 v/v) and 200 mL of methanol–dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v).19 The second fraction was collected and concentrated to obtain hapten, which was characterized by ESI-MS and NMR: ESI-MS, m/z: 346 [M + H+] and 368 [M + Na+]; 1H NMR (400 MHz, DMSO) δ: 3.79 (s, 2H, CH2COO), 3.86 (s, 3H, OCH3), 4.67 (s, 2H, SCH2), 7.01 (dd, 1H, ArH), 7.32–7.22 (m, 3H, ArH), 7.47 (d, 1H, ArH), 7.52 (m, 1H, ArH), 7.88 (m, 1H, ArH), 12.52 (s, 1H, COOH).
30 v/v).19 The second fraction was collected and concentrated to obtain hapten, which was characterized by ESI-MS and NMR: ESI-MS, m/z: 346 [M + H+] and 368 [M + Na+]; 1H NMR (400 MHz, DMSO) δ: 3.79 (s, 2H, CH2COO), 3.86 (s, 3H, OCH3), 4.67 (s, 2H, SCH2), 7.01 (dd, 1H, ArH), 7.32–7.22 (m, 3H, ArH), 7.47 (d, 1H, ArH), 7.52 (m, 1H, ArH), 7.88 (m, 1H, ArH), 12.52 (s, 1H, COOH).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The fused cells were cultured in a hypoxanthine–aminopterin–thymidine (HAT) medium at 37 °C in an atmosphere of 5% CO2. Half of the media in the wells were replaced by fresh HAT media every third day. Fourteen days after cell fusion, the HAT media were changed to HT media. Culture supernatants were screened for their ability to recognize benzothiostrobin, and hybridoma cells in the ELISA positive wells were cloned using the limiting dilution method. Stable antibody-producing clones were expanded. Ascites obtained from BALB/c mice were purified using a protein A column and stored at −20 °C after freeze-drying. The subtyping of MAb was identified by “mouse monoclonal antibody isotyping reagents” (Sigma).
1 ratio. The fused cells were cultured in a hypoxanthine–aminopterin–thymidine (HAT) medium at 37 °C in an atmosphere of 5% CO2. Half of the media in the wells were replaced by fresh HAT media every third day. Fourteen days after cell fusion, the HAT media were changed to HT media. Culture supernatants were screened for their ability to recognize benzothiostrobin, and hybridoma cells in the ELISA positive wells were cloned using the limiting dilution method. Stable antibody-producing clones were expanded. Ascites obtained from BALB/c mice were purified using a protein A column and stored at −20 °C after freeze-drying. The subtyping of MAb was identified by “mouse monoclonal antibody isotyping reagents” (Sigma).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in PBS, 100 μL per well) was dispensed into each well and incubated for 1 h at 37 °C. Then, the plates were washed again, and the substrate solution was added (100 μL per well). The reaction was terminated with sulfuric acid (2 mol L−1, 50 μL per well) after 15 min of incubation. The absorbance was measured at 490 nm.
000 in PBS, 100 μL per well) was dispensed into each well and incubated for 1 h at 37 °C. Then, the plates were washed again, and the substrate solution was added (100 μL per well). The reaction was terminated with sulfuric acid (2 mol L−1, 50 μL per well) after 15 min of incubation. The absorbance was measured at 490 nm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v) was used as the mobile phase at a flow rate of 0.8 mL min−1.2
35, v/v) was used as the mobile phase at a flow rate of 0.8 mL min−1.2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 8
1 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the immunogen and coating antigen, respectively.
1 for the immunogen and coating antigen, respectively.











