Hemin-functionalized MoS2 nanosheets: enhanced peroxidase-like catalytic activity with a steady state in aqueous solution†
Bang Lin Lia,
Hong Qun Luoa,
Jing Lei Lei*b and
Nian Bing Li*a
aKey Laboratory of Eco-environments in Three Gorges Reservoir Region (Ministry of Education), School of Chemistry and Chemical Engineering, SouthwestUniversity, Chongqing 400715, P.R. China. E-mail: linb@swu.edu.cn; Fax: +86-23-68253237; Tel: +86-23-68253237
bSchool of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400044, P.R. China. E-mail: jllei@cqu.edu.cn
First published on 13th May 2014
Abstract
Hemin-functionalized MoS2 nanosheets (hemin/MoS2-NSs) are first obtained via van der Waals interactions between few-layered MoS2 nanosheets (MoS2-NSs) and hemin molecules. It is demonstrated that a portion of MoS2-NSs undergoes a phase transition from semiconducting to metallic phase under the influence of hemin, which shows the coexistence of semiconducting and metallic phases in the crystal structure of hemin/MoS2-NSs. MoS2-NSs prepared from sonication-induced exfoliation of bulk MoS2 crystals in aqueous surfactant solution exhibit intrinsic peroxidase-like activity for the oxidation of 3,3,5,5-tetramethylbenzidine in the presence of H2O2, which is further improved by the functionalization of hemin. Significantly, MoS2-NSs are presented as a new support of hemin, and when compared to MoS2-NSs, hemin/MoS2-NSs exhibit better dispersity in aqueous solution, which is used in the development of H2O2 sensor based on the enhanced peroxidase-like activity.
1. Introduction
Hydrogen peroxide is a product of enzymatic reactions and a chemical threat to the environment, which makes its determination important in various fields including environmental, food, clinic, and pharmaceutical analysis.1 Up to now, a number of H2O2 sensors have been reported based on electrochemical, chemiluminescence, fluorometry, and colorimetry techniques.1–4 Among these analytical methods, colorimetric measurement using the substrate 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of artificial enzymes is considered as a low-cost, simple, fast, sensitive, and reliable technique to construct H2O2 sensors.4 Even though artificial enzymes have been applied in various fields, their deficiencies such as instability, high cost, and critical operating conditions may pose limitations to their applications. Hence, developing artificial enzymes, which are stable, can be easily prepared, and are cost-efficient still remains a central challenge.With the discovery of striking properties and rapid development of graphene, two-dimensional (2D) layered materials such as hexagonal boron nitride (h-BN), metal chalcogenides, transition metal oxides, and transition metal dichalcogenides (TMDs) have attracted a significant amount of interest, which have been demonstrated to play a crucial role in developing advanced functional materials by exploiting their excellent properties.5–8 Due to the specific 2D confinement of electron motion and the absence of interlayer perturbation, MoS2 nanosheets (MoS2-NSs) possess a direct band gap and show some remarkable properties, which offer new opportunities in various areas.9–11 Recently, MoS2-NSs have been fabricated into transistors,12 integrated circuits,13 nanometer thick photovoltaics,14 sensors,15 and battery materials.16,17 Furthermore, MoS2-NSs have also been considered as an excellent catalytic material for hydrogen evolution reaction, which is induced by the active edge sites.18,19 However, the catalytic ability of MoS2-NSs in hydrogen evolution is significantly limited by their low conductivity and lack of active edge sites.18–21 In order to expand the application and achieve the potential values of MoS2-NSs, a number of MoS2-based nanohybrids, which combine Au nanoparticles (NPs),22 iron oxide NPs,23 graphene,24 and carbon nanotubes,25 have been synthesized. So far, the exfoliation of bulk MoS2 crystals was considered as a direct route for obtaining MoS2-NSs. It is known that bulk MoS2 can be exfoliated by lithium intercalation but this method is sensitive to environmental conditions and results in structural deformations.10,26 Alternatively, the sonication-assisted exfoliation of MoS2 powder in aqueous surfactant solution has been considered as an efficient and environmentally friendly method, resulting in the preparation of small but high-quality exfoliated nanosheets, which can then be fabricated into composite materials.26,27
Hemin, a natural metalloporphyrin and the active center of the heme-protein family, is well-known for its properties of catalyzing a variety of oxidation reactions. Nevertheless, the direct application of hemin is challenging because of its molecular aggregation in aqueous solution to form catalytically inactive dimers and oxidative self-destruction in the oxidizing media.24 Therefore, the development of novel materials as hemin supports to achieve biomimetic catalysts with enzyme-like activity is highly desired. Recently, it was reported that hemin could be adsorbed on graphene,28,29 single-walled carbon nanotubes,30 metal–organic framework,31 polypyrrole,32 and TiO2 nanowires33 to form composite nanomaterials, exhibiting good properties in various applications. Significantly, studies, which show that MoS2-NSs can efficiently adsorb ssDNA molecules because of strong van der Waals interaction to achieve the construction of biosensors have been reported.34,35 On the basis of the aforementioned works, we thought that a MoS2 nanosheet possessing a similar 2D structure with graphene might serve as an available platform, which could be used to form composite nanomaterials with some plane-like molecules. Due to the important properties and potential applications of hemin, MoS2-NSs were considered as a new support to form hemin/MoS2-NSs. The properties and applications of MoS2-NSs and hemin/MoS2-NSs, which are used as artificial enzymes have never been explored before.
Herein, few-layered MoS2-NSs were obtained from the exfoliation of MoS2 powder in aqueous surfactant solution under sonication, which was followed by the interaction with hemin in methanol solution, leading to the formation of hemin/MoS2-NSs. The catalytic activities of MoS2-NSs and hemin/MoS2-NSs in the reduction of H2O2 in the presence of 3,3,5,5-tetramethylbenzidine (TMB) were explored. It was found that when compared to MoS2-NSs, the hemin/MoS2-NSs composite exhibited higher peroxidase-like activity with greater dispersity in aqueous solution, which could have potential applications in various significant areas.
2. Experimental section
2.1. Reagents and chemicals
Hemin (>98%), molybdenum(IV) sulfide (MoS2 crystalline powder, <2 μm, 99%), and horseradish peroxidase (HRP) were obtained from Sigma-Aldrich Co., USA. Sodium cholate and TMB (98%) were purchased from Aladdin Chemistry Co., Ltd., Shanghai, China. Other chemicals employed in this work were of analytical reagent grade, and were purchased from Kelong Chemical Reagent Co., Ltd., Chengdu, China. All the reagents were used as received. Doubly distilled water was used throughout the experiments.2.2. Apparatus
All electrochemical processes were conducted on a CHI 660B electrochemical workstation (Chenhua Instruments Co., China). A three-electrode system consisted of a modified glassy carbon working electrode with a diameter of 3 mm, a saturated Ag/AgCl reference electrode, and an auxiliary electrode made of platinum. All potentials were given with respect to the Ag/AgCl electrode. The KQ-250B ultrasonic bath (250 W, Kun Shan Ultrasonic Instruments Co., Ltd, China) was used to exfoliate MoS2 powder and prepare hemin/MoS2-NSs. Transmission electron microscopy (TEM) measurements were performed on a Tecnai G2 F20 S-TWIN transmission electron microscope (FEI, USA) operated at 200 kV. Scanning electron microscopy (SEM) images were obtained using a Hitachi S-4800 field emission scanning electron microscope (Japan). Atomic force microscopy (AFM) data were obtained in a Dimension Icon atomic force microscope (Bruker, Germany). X-ray diffraction (XRD) patterns were obtained with a D8 DISCOVER X-ray diffractometer (Bruker, Germany). X-ray photoelectron spectra (XPS) analyses were conducted using ESCALAB 250Xi X-ray photoelectron spectroscopy (Thermo Electron, USA). UV-visible spectra were recorded on a UV-2450 UV-vis spectrophotometer (Shimadzu, Japan). Centrifugation was carried out in a TGL-16M high-speed refrigerated centrifuge (Xiangyi, China).2.3. Sonication-induced exfoliation of bulk MoS2
MoS2 nanosheets were prepared through sonicated-exfoliation of MoS2 crystals in an aqueous surfactant solution.26 The mixed water solution, containing 5 mg mL−1 MoS2 powder and 1.5 mg mL−1 sodium cholate, was sonicated at room temperature (25 °C) for 10 h, which resulted in the dispersion of black MoS2 nanosheets. To remove bulk MoS2, the dispersed nanosheets were centrifuged at 3000 rpm for 30 min followed by the collection of a yellow-green supernatant. The separated supernatant was then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min, and the MoS2 nanosheets were collected. Exfoliated MoS2 nanosheets were further dispersed in ultrapure water. Similarly, the regenerated dispersed nanosheets were centrifuged at 12
000 rpm for 30 min, and the MoS2 nanosheets were collected. Exfoliated MoS2 nanosheets were further dispersed in ultrapure water. Similarly, the regenerated dispersed nanosheets were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min, accompanied by the collection of sediments to complete the washing process. The washing process was repeated two times to completely remove the surfactant sodium cholate. Ultimately, the sediments were dispersed in ultrapure water or methanol to prepare uniform exfoliated MoS2 nanosheets aqueous or methanol solution, respectively.
000 rpm for 30 min, accompanied by the collection of sediments to complete the washing process. The washing process was repeated two times to completely remove the surfactant sodium cholate. Ultimately, the sediments were dispersed in ultrapure water or methanol to prepare uniform exfoliated MoS2 nanosheets aqueous or methanol solution, respectively.
2.4. Synthesis of hemin-functionalized MoS2 nanosheets
The synthesis of hemin/MoS2-NSs was carried out using methanol as the solvent. Initially, a mixed methanol solution containing 0.5 mg mL−1 hemin and MoS2-NSs was prepared and then sonicated (250 W) at room temperature (25 °C) for 2 h. The sonicated solution was then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and black sediments, hemin/MoS2-NSs, were obtained. Furthermore, the as-prepared hemin/MoS2-NSs were dispersed into methanol and the regenerated dispersed nanosheets were centrifuged at 12
000 rpm for 30 min and black sediments, hemin/MoS2-NSs, were obtained. Furthermore, the as-prepared hemin/MoS2-NSs were dispersed into methanol and the regenerated dispersed nanosheets were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min followed by the collection of sediments for completing the washing process. Finally, the sediments were dispersed into water to prepare the hemin/MoS2-NSs aqueous solution under sonication.
000 rpm for 30 min followed by the collection of sediments for completing the washing process. Finally, the sediments were dispersed into water to prepare the hemin/MoS2-NSs aqueous solution under sonication.
2.5. Preparation of MoS2-NSs and hemin/MoS2-NSs modified glassy carbon electrode
Prior to modification, the glassy carbon electrode (GCE, 3 mm) was polished with 0.3 and 0.05 μm alumina slurry to obtain a mirror finish, rinsed and sonicated in doubly distilled water for 5 min. The MoS2-NSs modified GCE and the hemin/MoS2-NSs modified GCE were obtained by dropping 5 μL of 50 μg mL−1 MoS2-NSs or hemin/MoS2-NSs aqueous suspension on the surface of the well-polished GCE, which were then dried in air.3. Results and discussion
In our experiments, MoS2 nanosheets were prepared by the exfoliation of bulk MoS2 crystals in an aqueous surfactant solution under sonication according to a previously reported method.26 As we know, ultrasonic waves generate cavitation bubbles that collapse into high-energy jets, breaking up the MoS2 crystallites and producing MoS2 nanosheets. Meanwhile, the presence of surfactant molecules stabilized MoS2 nanosheets in mixed solutions, avoiding the aggregation of nanosheets, which is induced by the large surface energy due to their van der Waals binding to the exfoliated sheets and the subsequent electrostatic stabilization.26 The characterization of as-exfoliated MoS2 nanosheets was carried out using SEM, TEM, and AFM techniques. As we can see from the SEM image (Fig. 1A), the exfoliated MoS2 nanosheets exhibited a sheet structure, showing that the plane size of MoS2-NSs was about 100 nm. The result of SEM measurement was further confirmed using TEM (Fig. 1B). As seen from the AFM image, the planar 2D structures of the as-prepared MoS2-NSs with multiple monolayers are confirmed (Fig. 1C) and the sample with a thickness of about 3.4 nm corresponded to 4 and 5 monolayers of MoS2 (inset of Fig. 1C).14,36 A statistical analysis based on AFM measurements indicates that the MoS2-NSs obtained from the sonication-assisted exfoliation have various thicknesses with the majority in the range of 3 to 5 monolayers (Fig. 1D). The average thickness of MoS2-NSs was calculated to be 3.15 nm with a relative standard deviation (RSD) of 10.5%.Because of the insolubility of hemin in neutral aqueous solution, the synthesis of hemin/MoS2-NSs was carried out using methanol as the solvent (Fig. 2A). Initially, the mixed methanol solution containing 0.5 mg mL−1 hemin and MoS2-NSs was prepared followed by sonication at room temperature for 2 h. The sonicated solution was then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and the black sediments, hemin/MoS2-NSs, were obtained (b1, Fig. 2A). According to the Lambert–Beer theory, the concentration of hemin in supernatant was detected to be 0.38 mg mL−1. Therefore, the mass fraction of hemin in hemin/MoS2-NSs was calculated to be 19.4%. To conduct the characterizations and explore the properties of hemin/MoS2-NSs, the sediments were dispersed into water to prepare the hemin/MoS2-NSs aqueous solution.
000 rpm for 30 min and the black sediments, hemin/MoS2-NSs, were obtained (b1, Fig. 2A). According to the Lambert–Beer theory, the concentration of hemin in supernatant was detected to be 0.38 mg mL−1. Therefore, the mass fraction of hemin in hemin/MoS2-NSs was calculated to be 19.4%. To conduct the characterizations and explore the properties of hemin/MoS2-NSs, the sediments were dispersed into water to prepare the hemin/MoS2-NSs aqueous solution.
The UV-vis absorption spectra of MoS2-NSs and as-prepared hemin/MoS2-NSs were obtained, which are shown in Fig. 2B. As we know, bulk MoS2 is a semiconductor with an indirect band gap of about 1.29 eV where no characteristic absorption peaks could be observed in the UV-vis absorption spectrum. However, it was demonstrated that an indirect to direct band gap transition occurred in the d-electron system when the bulk MoS2 is thinned to a single layer, while exfoliated MoS2-NSs, consisting of one to several layers, possess two characteristic absorption peaks at approximately 610 and 670 nm,6,37 which were observed in the as-prepared MoS2-NSs aqueous solution (curve (1), Fig. 2B). The hemin/MoS2-NSs aqueous dispersion exhibited two absorption peaks at around 610 and 670 nm, corresponding to the characteristic peaks of MoS2-NSs, and a broad absorption peak was observed at around 400 nm (curve (2), Fig. 2B), close to the Soret band of hemin. Furthermore, because hemin is insoluble in water, the control experiment of free hemin was carried out in methanol solution, which exhibited an absorption peak at around 400 nm (curve (3), Fig. 2B). It is clearly demonstrated that MoS2-NSs adsorbed hemin molecules at the surface of nanosheets through van der Waals interactions, which allowed them to be well dispersed in aqueous solution where a characteristic absorption of hemin was observed from complex nanosheets. The attachment of hemin onto the MoS2-NSs surface was also characterized by an electrochemical method. Fig. 2C shows the cyclic voltammograms recorded at the bare GCE (a), the MoS2-NSs modified GCE (b), and the hemin/MoS2-NSs modified GCE in phosphate buffer solution (PBS). As expected, redox peaks were not observed on the bare GCE and MoS2-NSs modified GCE, whereas a pair of well-defined redox peaks located at around −0.3 V were clearly seen on the hemin/MoS2-NSs modified GCE. The redox peaks could be ascribed to the constitution of hemin, which has the characteristic of a single electron transfer process of iron at the core of hemin for the heminox/heminred pair.29 As is well-known, the strong van der Waals interactions between few-layered MoS2-NSs lead to the aggregation of MoS2-NSs in aqueous solution without stabilizers, which limits the application of MoS2-NSs. However, compared to MoS2-NSs, the hemin/MoS2-NSs exhibited better dispersity in aqueous solution, which can be well dispersed without aggregation for at least 72 h (Fig. 3A). The electronegative hemin molecules adsorbed on the surface of MoS2-NSs can prevent the aggregation of composite nanosheets due to the effect of electrostatic stabilization. The SEM and AFM images of the as-prepared hemin/MoS2-NSs are shown in Fig. 3B and C, respectively, which were used to confirm the nanosheet structure of hemin/MoS2-NSs, indicating that hemin molecules adsorbed on the surface of MoS2-NSs would not break their original nanosheet morphology.
The crystal structures of exfoliated MoS2-NSs and hemin/MoS2-NSs were investigated using the X-ray diffraction system with bulk MoS2 powder used as the reference. From the XRD patterns shown in Fig. 4A, it can be seen that both exfoliated MoS2-NSs and hemin/MoS2-NSs are mainly identified as 2H MoS2, which have a dominant peak appearing at 14.4°, representing the (002) plane (ICDD card no. 77-1716).38 In order to confirm the chemical composition, the XPS survey of MoS2-NSs and as-prepared hemin/MoS2-NSs were recorded (Fig. 4B). From Fig. 4B, it can be seen that the relative intensities of C 1s and O 1s binding energy at hemin/MoS2-NSs increase significantly when compared to that of MoS2-NSs. Meanwhile, a weak peak related to Fe 2p binding energy appears, indicating the formation of hemin/MoS2-NSs. As is well-know, MoS2-NSs have two different types of phases, i.e. stable hexagonal semiconducting phase (2H phase) and metastable metallic phase (1T), where it has been demonstrated that a transition from 2H to 1T phase occurs after the semiconducting MoS2 was intercalated with Li+, K+, Na+, and H+.36,38 To explore the phase changes of MoS2-NSs under the influence of hemin molecules, high resolution Mo 3d and S 2p spectra of MoS2-NSs and hemin/MoS2-NSs were recorded. According to the XPS results of exfoliated MoS2-NSs (Fig. 4C and D), Mo 3d3/2, Mo 3d5/2, S 2s, S 2p1/2, and S 2p3/2 peaks were observed at 232.6, 229.4, 226.5, 163.5, and 162.3 eV, respectively, showing the dominant 2H phase in MoS2-NSs, which were obtained from the sonication-assisted exfoliation of MoS2 powder. However, after MoS2-NSs interacted with hemin, new peaks at 231.4 and 228.1 eV for Mo and 161.3 eV for S were observed at lower binding energies when compared to those of the 2H phase peaks (Fig. 4E and F). These new peaks were identified as 1T phase peaks, and it is suggested that a small portion of MoS2-NSs undergoes a phase transition from semiconducting to metallic phase under the influence of hemin.38 Meanwhile, the peaks representing the 2H phase can still be observed, indicating the coexistence of 1T and 2H phases in the crystal structure of hemin/MoS2-NSs.
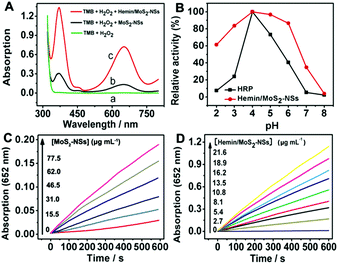
As a new support of hemin, MoS2-NSs were presented to make hemin/MoS2-NSs, which were expected to be great biomimetic catalysts based on the effect of hemin functionalization. The catalytic activities of MoS2-NSs and hemin/MoS2-NSs were explored using TMB as the substrate, which could be oxidized in the presence of H2O2 to produce a blue oxidation product. When compared to the absorption spectra of blank TMB reaction solution (Fig. 5A), the absorbance of reaction solutions increased in the presence of MoS2-NSs and as-prepared hemin/MoS2-NSs for the same reaction time, indicating that both MoS2-NSs and hemin/MoS2-NSs have peroxidase-like catalytic activities. The catalytic activity of MoS2-NSs in TMB oxidation reaction can be ascribed to the active sites located at the edges of sheets, which exhibit significant catalytic activity for hydrogen evolution reaction.18–21 However, when compared to MoS2-NSs (curve b, Fig. 5A), hemin/MoS2-NSs (curve c, Fig. 5A) show a much stronger catalytic activity because of the contribution of hemin functionalization. Meanwhile, a higher catalytic activity of hemin/MoS2-NSs was observed when compared to that of pure hemin, which is attributed to the support of MoS2-NSs (Fig. S1, ESI†). In comparison to HRP, hemin/MoS2-NSs exhibited significant catalytic activity over a broader pH range (from 2.0 to 6.0), and showed an optimal catalytic activity at the pH of 4.0 (Fig. 5B). The catalytic reaction can be detected by monitoring the change in TMB absorbance at 652 nm, and the time-dependent absorbance changes against the concentrations of MoS2-NSs and hemin/MoS2-NSs were recorded. It can be seen from Fig. 5C that with the addition of the same amount of H2O2, the TMB oxidation rate was increased with increasing MoS2-NSs concentration, indicating that the catalytic activity to H2O2 reduction was related to the concentration of MoS2-NSs. Fig. 5D shows the time-dependent absorption changes against the concentrations of hemin/MoS2-NSs. In the presence of a constant concentration of H2O2, the TMB oxidation reaction rate also increased with increasing hemin/MoS2-NSs concentration. The catalytic activity of hemin/MoS2-NSs was inhibited at high H2O2 concentration. Moreover, the typical Michaelis–Menten curves can be obtained for hemin/MoS2-NSs at a certain range of H2O2 concentrations (Fig. S2A and S2B, ESI†). For further analysis of the catalytic mechanism and the calculation of hemin/MoS2-NSs catalytic activity, the apparent steady-state kinetic parameters for TMB oxidation reaction in the presence of H2O2 and hemin/MoS2-NSs were determined. Meanwhile, the maximum initial velocity (Vmax) and Michaelis–Menten constant (Km) were recorded in Table 1 based on the Lineweaver–Burk plot and the double reciprocal plots of initial velocity against the concentration of one substrate were obtained over a range of concentrations of the second substrate (Fig. S2C and S2D, ESI†). These parallel lines are characteristic of a ping-pong mechanism, which is used to confirm the catalytic mechanism of hemin/MoS2-NSs. The high catalytic activity of hemin/MoS2-NSs is confirmed when compared to previous studies (Table 1).
| Km [mM] | Vm [10−8 M s−1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst | TMB | H2O2 | TMB | H2O2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| a SWCNT: single-walled carbon nanotube; MOF: metal–organic framework (MTL-101(Al)–NH2); GNs: graphene nanosheets. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hemin/MoS2-NSs | 0.119 | 0.15 | 6.52 | 3.21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MoS2-NSs | 3.74 | 7.36 | 1.28 | 0.64 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hemin-SWCNT30 | — | 0.08 | — | 4.79 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hemin@MOF31 | 0.068 | 10.9 | 6.07 | 8.98 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hemin-GNs29 | 5.1 | 2.256 | 4.55 | 5.06 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
On the basis of the intrinsic peroxidase property of hemin/MoS2-NSs, a novel H2O2 sensor was fabricated using spectrophotometry (Fig. 6A). Fig. 6B shows the time-dependent absorption changes of TMB reaction solutions at 652 nm with different concentrations of H2O2 using hemin/MoS2-NSs as the catalyst. It can be seen from Fig. 6B that the absorbance of TMB reaction solutions increases with increasing H2O2 concentration after reacting for 10 min. Because the catalytic activity of hemin/MoS2-NSs is dependent on H2O2 concentration, a H2O2 sensor is constructed. Fig. 6C exhibits a typical H2O2 concentration–response curve under optimal conditions after reacting for 10 min. As we can see from the inset of Fig. 6C, the absorbance of reaction solution has a good linear relationship with the concentration of objective H2O2 in the range of 2.0 × 10−7 to 4.0 × 10−6 M. The detection limit, which is defined as the concentration of the analyte giving signals equivalent to three times the standard deviation of the blank signals (S/N = 3) was calculated to be 4.3 × 10−8 M, which was lower than that reported in most of the previous reports (Table S1, ESI†).
In order to explore the selectivity of the novel H2O2 sensor based on hemin/MoS2-NSs, influences from other impurities such as NaCl, KCl, CaCl2, glucose, urine, ascorbic acid, dopamine, and cysteine were studied (Table S2, ESI†). We found that when the concentrations of inorganic salts were higher than 0.5 M, hemin/MoS2-NSs were observed to aggregate in salt solution because of the charge screening effects, which have been used to account for the decrease in the stability of hemin-graphene in salt solutions.29 Furthermore, the aggregation of hemin/MoS2-NSs reduced numerous active sites, which would disturb the H2O2 detection. It was verified that small biological molecules did not influence the determination of H2O2 when their concentrations were lower than 5 mM. Furthermore, the influences of other common oxidants, such as ClO− and Cr2O72−, were also explored. It was demonstrated that the developed sensor exhibited a great selectivity, which could be used to detect H2O2 in the presence of other oxidants.
4. Conclusion
In summary, few-layered MoS2-NSs prepared from the sonication-induced exfoliation of bulk MoS2 have been demonstrated to possess peroxidase-like activity. Meanwhile, hemin/MoS2-NSs were synthesized through the van der Waals interaction between MoS2-NSs and hemin molecules in mixed methanol solution with the assistance of sonication. The hemin/MoS2-NSs had high dispersity in aqueous solution, and exhibited a high catalytic activity in the oxidation of TMB in the presence of H2O2. As a new support of hemin, MoS2-NSs were presented, showing their strong van der Waals force when interacting with plane-like molecules. On the basis of high catalytic activity of hemin/MoS2-NSs, a novel H2O2 sensor was fabricated using spectrophotometric method. In further research, hemin/MoS2-NSs can be considered a reliable biomimetic catalyst for other oxidation reactions and it can also be taken into consideration as a novel sensing platform for biomolecular detection, raising new potentials concerning MoS2-NSs applications in various significant areas.Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos 20975083 and 21273174) and the Municipal Science Foundation of Chongqing City (no. CSTC-2013jjB00002).Notes and references
- B. L. Li, J. R. Chen, H. Q. Luo and N. B. Li, J. Electroanal. Chem., 2013, 706, 64–68 CrossRef CAS PubMed.
- J. Yuan and A. M. Shiller, Anal. Chem., 1999, 71, 1975–1980 CrossRef CAS.
- T. Wen, F. Qu, N. B. Li and H. Q. Luo, Anal. Chim. Acta, 2012, 749, 56–62 CrossRef CAS PubMed.
- N. Li, Y. Yan, B. Y. Xia, J. Y. Wang and X. Wang, Biosens. Bioelectron., 2014, 54, 521–527 CrossRef CAS PubMed.
- M. Xu, T. Liang, M. Shi and H. Chen, Chem. Rev., 2013, 113, 3766–3798 CrossRef CAS PubMed.
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 6139–6156 CrossRef.
- X. Huang, Z. Zeng and H. Zhang, Chem. Soc. Rev., 2013, 42, 1934–1946 RSC.
- T. Stephenson, Z. Li, B. Olsen and D. Mitlin, Energy Environ. Sci., 2014, 7, 209–231 CAS.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805–136808 CrossRef.
- G. Cunningham, M. Lotya, C. S. Cucinotta, S. Sanvito, S. D. Bergin, R. Menzel, M. S. P. Shaffer and J. N. Coleman, ACS Nano, 2012, 6, 3468–3480 CrossRef CAS PubMed.
- Y. Yan, X. Ge, Z. Liu, J. Y. Wang, J. M. Lee and X. Wang, Nanoscale, 2013, 5, 7768–7771 RSC.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- B. Radisavljevic, M. B. Whitwick and A. Kis, ACS Nano, 2011, 5, 9934–9938 CrossRef CAS PubMed.
- M. Bernardi, M. Palummo and J. C. Grossman, Nano Lett., 2013, 13, 3664–3670 CrossRef CAS PubMed.
- S. Wu, Z. Zeng, Q. He, Z. Wang, S. J. Wang, Y. Du, Z. Yin, X. Sun, W. Chen and H. Zhang, Small, 2012, 8, 2264–2270 CrossRef CAS PubMed.
- Y. Liang, R. Feng, S. Yang, H. Ma, J. Liang and J. Chen, Adv. Mater., 2011, 23, 640–643 CrossRef CAS PubMed.
- J. Xiao, D. Choi, L. Cosimbescu, P. Koech, J. Liu and J. P. Lemmon, Chem. Mater., 2010, 22, 4522–4524 CrossRef CAS.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 17881–17888 CrossRef CAS PubMed.
- D. Voiry, M. Salehi, R. Silva, T. Fujita, M. Chen, T. Asefa, V. B. Shenoy, G. Eda and M. Chhowalla, Nano Lett., 2013, 13, 6222–6227 CrossRef CAS PubMed.
- D. Y. Chung, S. K. Park, Y. H. Chung, S. H. Yu, D. H. Lim, N. Jung, H. C. Ham, H. Y. Park, Y. Piao, S. J. Yoo and Y. E. Sung, Nanoscale, 2014, 6, 2131–2136 RSC.
- J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807–5813 CrossRef CAS PubMed.
- T. S. Sreeprasad, P. Nguyen, N. Kim and V. Berry, Nano Lett., 2013, 13, 4434–4441 CrossRef CAS PubMed.
- Y. A. Kabachii, A. S. Golub, S. Y. Kochev, N. D. Lenenko, S. S. Abramchuk, M. Y. Antipin, P. M. Valetky, B. D. Stein, W. E. Mahmoud, A. A. Al-Ghamdi and L. M. Bronstein, Chem. Mater., 2013, 25, 2434–2440 CrossRef CAS.
- X. Zhou, L. J. Wan and Y. G. Guo, Chem. Commun., 2013, 49, 1838–1840 RSC.
- Y. Yan, X. Ge, Z. Liu, J. Y. Wang, J. M. Lee and X. Wang, Nanoscale, 2013, 5, 7768–7771 RSC.
- R. J. Smith, P. J. King, M. Lotya, C. Wirtz, U. Khan, S. De, A. O'Neill, G. S. Duesberg, J. C. Grunlan, G. Moriarty, J. Chen, J. Wang, A. I. Minett, V. Nicolosi and J. N. Coleman, Adv. Mater., 2011, 23, 3944–3948 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H. Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- T. Xue, S. Jiang, Y. Qu, Q. Su, R. Cheng, S. Dubin, C. Y. Chiu, R. Kaner, Y. Huang and X. Duan, Angew Chem, Int. Ed., 2012, 51, 3822–3825 CrossRef CAS PubMed.
- Y. Guo, L. Deng, J. Li, S. Guo, E. Wang and S. Dong, ACS Nano, 2011, 5, 1282–1290 CrossRef CAS PubMed.
- Y. Zhang, C. Xu and B. Li, RSC Adv., 2013, 3, 6044–6050 RSC.
- F. X. Qin, S. Y. Jia, F. F. Wang, S. H. Wu, J. Song and Y. Liu, Catal. Sci. Technol., 2013, 3, 2761–2768 CAS.
- P. Hu, L. Han and S. Dong, ACS Appl. Mater. Interfaces, 2014, 6, 500–506 CAS.
- J. Tang, B. Kong, Y. Wang, M. Xu, Y. Wang, H. Wu and G. Zheng, Nano Lett., 2013, 13, 5350–5354 CrossRef CAS PubMed.
- C. Zhu, Z. Zeng, H. Li, F. Li, C. Fan and H. Zhang, J. Am. Chem. Soc., 2013, 135, 5998–6001 CrossRef CAS PubMed.
- J. Ge, E. C. Ou, R. Q. Yu and X. Chu, J. Mater. Chem. B, 2014, 2, 625–628 RSC.
- J. Z. Ou, A. F. Chrimes, Y. Wang, S. Tang, M. S. Strano and K. Kalantar-Zadeh, Nano Lett., 2014, 14, 857–863 CrossRef CAS PubMed.
- A. Splendiani, L. Sun, Y. Zhang, T. Li, J. Kim, C. Y. Chim, G. Galli and F. Wang, Nano Lett., 2010, 10, 1271–1275 CrossRef CAS PubMed.
- Y. Wang, J. Z. Ou, S. Balendhran, A. F. Chrimes, M. Mortazavi, D. D. Yao, M. R. Field, K. Latham, V. Bansal, J. R. Friend, S. Zhuiykov, N. V. Medhekar, M. S. Strano and K. Kalantar-Zadeh, ACS Nano, 2013, 7, 10083–10093 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 and S2, Tables S1 and S2. See DOI: 10.1039/c4ra01746c |
| This journal is © The Royal Society of Chemistry 2014 |