Open Access Article
Open Access ArticleNanogel-based composites for bacterial antibiofilm activity: advances, challenges, and prospects
Amaal Abdulraqeb Alia,
Rouba D. Al Bostamib and
Amani Al-Othman *ac
*ac
aDepartment of Chemical and Biological Engineering, American University of Sharjah, P. O. Box 26666, Sharjah, United Arab Emirates. E-mail: aalothman@aus.edu
bBiomedical Engineering Graduate Program, American University of Sharjah, P. O. Box 26666, Sharjah, United Arab Emirates
cEnergy, Water and Sustainable Environment Research Center, American University of Sharjah, P. O. Box 26666, Sharjah, United Arab Emirates
First published on 2nd April 2024
Abstract
Nano-based approaches, particularly nanogels, have recently emerged as a potential strategy for combating biofilm-related infections. Their exceptional characteristics including biocompatibility, biodegradability, stability, high water content, stimuli-responsiveness, and their nano size (which enables their penetration into biofilms) make nanogels a promising technology in the biomedical field. However, exploring nanogels for biofilm treatment remains in its early stages. This review examined the status of nanogels application for the treatment of bacterial biofilms. Recent investigations studied nanogels derived from natural polymers like chitosan (CS), hyaluronic acid (HA), and alginate, among others, for eliminating and inhibiting biofilms. These nanogels were utilized as carriers for diverse antibiofilm agents, encompassing antibiotics, antimicrobial peptides, natural extracts, and nanoparticles. Utilizing mechanisms like conventional antibody-mediated pathways, photodynamic therapy, photothermal therapy, chemodynamic therapy, and EPS degradation, these nanogels effectively administered antibiofilm drugs, exhibiting efficacy across several bacterial strains, notably Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), and Escherichia coli (E. coli), among others. Despite showing promise, nanogels remain relatively underexplored in biofilm treatment. This review concludes that research gaps are still present in biofilm treatment processes including (i) a better understanding of the stimuli-responsive behaviors of nanogels, (ii) active targeting strategies, and (iii) the narrow spectrum of antibiofilm agents loaded into nanogels. Hence, future studies could be directed towards the following elements: the exploration of multi-strain biofilms rather than single-strain biofilms, other endogenous and exogenous stimuli to trigger drug release, active targeting mechanisms, a broader range of antibiofilm agents when employing nanogels, and fostering more comprehensive and reliable biofilm treatment strategies. This review found that there are currently several research gaps as well in the use of nanogels for biofilm therapy, and these include: (i) very limited exogenous and endogenous stimuli were explored to trigger drug release from nanogels, (ii) the active targeting strategies were not explored, (iii) a very narrow spectrum of antibiofilm agents was loaded into nanogels, and (iv) only biofilms of single strains were investigated.
1. Introduction
To adapt to their surrounding environment, many microorganisms have developed the ability to evolve a range of survival strategies. One strategy utilized by microorganisms to survive harsh surrounding conditions such as immune responses and treatment with antimicrobial therapeutics is the development of biofilms. Biofilms are aggregates of microorganisms formed within a self-generated matrix called the extracellular polymeric substance (EPS). Unlike their freely suspended planktonic counterparts, biofilms form on surfaces that can be of living or non-living nature. Such surfaces onto which biofilms can attach and form include dental and implantable device surfaces, tissues, and wounds. Furthermore, biofilm-associated microorganisms differ from planktonic cells in their altered metabolic activity, genetic evolution, and even communication between microorganisms. Importantly, biofilms are typically resistant to antimicrobial therapies due to the biofilm blocking drugs and host immune responses. This, in turn, makes annihilating infections associated with biofilms more complicated and challenging compared to planktonic bacteria and reduces the chances of survival, and increases possible relapse post-treatment.1,2 Overall, biofilm-forming microorganisms account for approximately 65% of clinically encountered microbial infections mainly due to their antimicrobial resistance and evading the immune system.3 It is also expected that by 2050, infections caused by microorganisms resistant to antibiotics will become the primary reason for mortality with biofilms being responsible for most of the long-lasting infections in humans.2The challenge of biofilm treatment stems from several factors of which one of the most important is their self-produced, polysaccharide-based EPS. In addition to polysaccharides, the EPS matrix is also composed of proteins, extracellular DNA, and lipids. The EPS, also defined as “the house of biofilm cells”, accounts for 90% of the biofilm matrix serving as a shelter for the remaining 10% of microbial cells found within the biofilm. The EPS plays a vital role in protecting the biofilm-residing microorganisms from damage by acting as a chemical and physical barrier. Furthermore, in addition to sheltering the microorganisms, the EPS also provides the biofilm with mechanical and structural stability while also keeping the microorganisms close to each other hence facilitating communication between them. Moreover, the EPS also aids in the spread of the microorganisms within the biofilm by enabling oxygen to diffuse and releasing extracellular enzymes to obtain nutrition.4,5 When it comes to treating biofilms, the EPS plays an important role in protecting the biofilm from damage such as that induced by drugs. Due to its small pores, the EPS restricts the penetration of drugs, such as antibiotics, thereby blocking their access to the inside of the biofilm.2 Additionally, the EPS can mitigate the effect of antibiotics by neutralizing them or restricting their diffusion with the aid of extracellular polysaccharides.5 Such limiting of the ability of a drug to penetrate the EPS and reach the embedded bacterial cells increases the dose sufficient to yield an antimicrobial response by up to 1000 times compared to that required for planktonic cells.2 Importantly, although a higher dose can have the desired curative effect, it also increases the risk of drug toxicity and resistance as well as the cost of the treatment.6 Moreover, even if it penetrates the biofilm, the drug could be enzymatically inactivated within the biofilm. Furthermore, microbial cells within the EPS usually exist in a dormant state due to the low levels of oxygen and nutrients present. This can negatively affect drugs that rely on the active growth of microorganisms to exert their effects. These factors, in turn, complicate the annihilation of biofilm-residing microorganisms and increase the risks of re-infection post-treatment.2
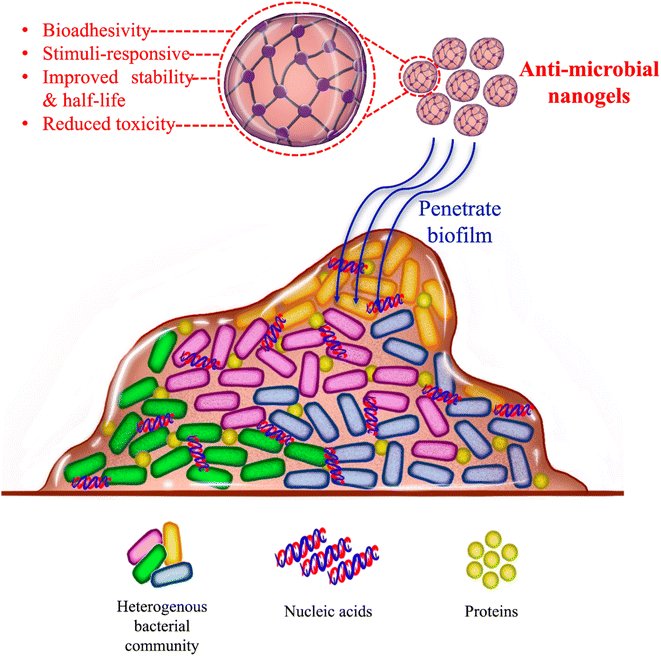
One type of microbial biofilm posing a major clinical challenge is bacterial biofilms, shown in Fig. 1. Almost all types of bacterial strains can develop biofilms that can be of a single bacterial strain (monospecies biofilm) or different strains (multispecies biofilm).4,5 Importantly, once mature, biofilms break releasing mobile bacteria that can move on to colonize new surfaces.4 Although the discovery and introduction of antibiotics represent a major medical breakthrough, controlling infections and saving millions of lives, it has also increased the development of bacteria that are antimicrobial resistant (AMR). Importantly, these AMR bacterial strains can develop biofilms.7 Typically, bacteria residing with biofilms are AMR bacteria possessing between 10 and 1000 times more resistance to antibiotics than their planktonic equivalents. This further expands the challenges inherently associated with the treatment of biofilms as it necessitates finding alternative antimicrobial therapeutics to replace antibiotics.8 Therefore, finding innovative and efficient treatments for bacterial biofilms has become a topic of increased research interest. One field receiving significant research attention for the treatment of numerous medical conditions including biofilm-associated infections is nanotechnology. Nanotechnology involves the use of nanomaterials (particles with at least one dimension in the nanoscale) for various purposes including medical therapeutics and diagnostics.1 Numerous nanomaterials have been studied to combat biofilms whether by therapy, imaging, or dual imaging and therapy.9–13 Nanomaterials can exert their antibiofilm effects either via their intrinsic abilities such as heat generated from nanoparticles like iron oxide nanoparticles14 or by acting as carriers for antibiofilm agents.15 Due to their very small size, nano-sized therapeutic agents are especially advantageous to penetrate the biofilm's EPS and kill the biofilm-residing cells.1 One type of nano-sized material recently being investigated as a promising candidate for the treatment of bacterial biofilms is a types of hydrogels called nanogels.11,12,16,17
Hydrogels are soft networks of hydrophilic polymer chains. Due to their hydrophilicity, hydrogels contain high water contents which contributes to their characteristic biocompatibility and softness. Due to their benefits, hydrogels have reached the clinic in several forms such as contact lenses, dermal fillers, and cancer drug delivery vehicles. However, hydrogels are macroscopic and hence unsuitable for applications where effects at the cellular level, such as bacterial cells for instance, are needed. Therefore, for such applications, nano-sized hydrogels (nanogels) are preferable to interact with cells and possibly be internalized by them.18 Nanogels are hydrogels typically smaller than 100 nm in size that combine the advantageous features of hydrogels with those of the small nano size.19,20 Like hydrogels, nanogels are usually soft, biodegradable, have a high surface area, biocompatible due to their high content of water, and porous which allows them to carry and release materials such as drugs. Furthermore, due to their softness, biocompatibility, deformability, and small size, nanogels usually have good circulation and penetration features.18,21 When it comes to delivering drugs, nanogels have the ability to carry and deliver both hydrophilic and hydrophobic drugs and can be internalized by cells for intracellular drug delivery. Moreover, unlike hydrogels which are macro-sized, nanogels are stimuli-responsive responding rapidly to their surrounding changes.21 Hence, nanogels are considered “smart” biomaterials responding to both endogenous and exogenous stimuli.22,23 Furthermore, in the area of drug delivery, nanogels are more advantageous than hydrogels due to their high drug-loading capacity and stability as well as site-specific targeting.6,16,21,24 Due to their many favorable features, nanogels have very recently been explored for biofilm therapy.
Nanogel's stimuli-responsiveness has been especially beneficial for pathologies having unique microenvironments such as cancer.21 As with cancer, biofilms have a distinctive microenvironment due to their encapsulation within the EPS, with features different from those present outside the biofilm. One of the important features of biofilms is their acidic pH. Due to the blockage of nutrients and oxygen (hypoxia) by EPS, bacteria within the biofilm are forced to undergo anaerobic metabolism thereby resulting in acidic metabolites and making the biofilm microenvironment acidic. Other features unique to the biofilm microenvironment include high H2O2, enzyme overexpression, and redox conditions. Due to their absence in normal tissues, these biofilm-specific attributes can be utilized to achieve a biofilm-specific therapeutic strategy.9,25 Of the endogenous biofilm-specific stimuli, low pH has been the most utilized by nanogels for biofilm treatment.11,13,26 Furthermore, in addition to endogenous stimuli like low pH, exogenous stimuli can be used to improve the specificity of drug delivery and hence the treatment. Exogenous stimuli can include triggers such as near-infrared (NIR) light, magnetic field, ultrasound, and temperature.21,27 As with endogenous stimuli, the exogenous stimuli studied for biofilm therapy using nanogels have been limited to NIR light.13,28–30
Despite the attractive features of nanogels for biofilm therapy, nanogels have only been very briefly and recently studied for the treatment of biofilms. This is in contrast with other medical applications such as cancer theragnostic where nanogels have been more investigated and their potential has been clearly highlighted. To the knowledge of the authors, there are currently no review articles in the literature highlighting the role and status of nanogels in biofilm treatment. Therefore, to address this void in the literature, this review critically summarizes the status of nanogels in the treatment of biofilms, particularly bacterial biofilms. Based on the search of the authors, the earliest study utilizing nanogels for biofilm treatment was reported in 2018 indicating the very recent interest and exploration of nanogels as potential biofilm therapeutic strategies. Moreover, based on our search, three natural polymers have been commonly explored as materials to synthesize antibiofilm nanogels. These polymers are chitosan, hyaluronic acid, and alginate. Other polymers were also briefly investigated for biofilm eradication. Therefore, this review discusses the recent progress in the use of chitosan-, hyaluronic acid-, and alginate-based nanogels as well as other nanogels for biofilm inhibition and eradication. Particularly, it critically analyzes and compares the performance of these nanogels for biofilm treatment in terms of: (i) the ability to eliminate pre-formed biofilms and stop the growth of forming biofilms, (ii) the ability to respond to internal and/or external stimuli, (iii) the different antibiofilm agents and mechanisms used to treat the biofilm, and (iv) and the ability of the nanogel to target the biofilm. From this point on, the manuscript proceeds by first discussing the different types of nanogels used for biofilm therapy, critically analyzing them in terms of the type of nanogel and loaded drug, the antibiofilm activity (killing formed or inhibiting forming biofilm), and the mechanism used by the nanogel to exert its antibiofilm effects. Following that, some gaps in the literature are discussed and potential prospects for nanogels in biofilm treatment are proposed.
2. Nanogels for biofilm therapy
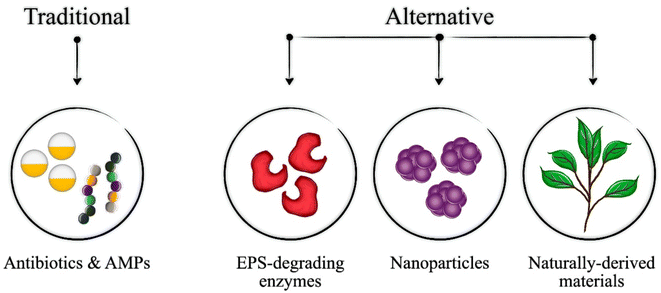
To combat biofilms, nanogels based on natural polymers such as chitosan, hyaluronic acid, and alginate alongside other polymers have been developed and studied as antibiofilm agents. The nanogels have been loaded with a range of biofilm-killing agents (Fig. 2) utilizing different mechanisms to efficiently eradicate formed biofilms and inhibit the growth of forming biofilms. The different nanogels loaded with antibiofilm drugs and mechanisms utilized against bacterial biofilms are discussed in the sections below and summarized in Table 1.| Incorporated molecules | Eradication mechanism | Bacterial strain | Ref. |
|---|---|---|---|
| a The exact mechanism needs further studies. | |||
| Chitosan-based nanogels | |||
| AgNPs | Slow release of Ag+ ions | S. aureus | 11 |
| Chlorogenic acid | Disruption of the bacterial cell membrane due to the nanogel-membrane electrostatic interaction | S. aureus, K. pneumoniae, and P. aeruginosa | 17 |
| Thymol | Reduction of OmpA and PgaB biofilm gene expression | Staphylococcus, Acinetobacter, and Pseudomonas | 44 |
| Mentha piperita essential oils | Down-regulation and inhibition of some glycosyltransferase genes (gtfB, C and D) | S. mutans | 55 |
| Gymnema sylvestre essential oils | Down-regulation of hypha-specific gene ALS3 expression | C. albicans | 56 |
| Tanshinone IIA | Penetration of the biofilm barriers with negatively charged surfaces | S. mutans | 26 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Hyaluronic-based nanogels | |||
| SNAP and AMP | NO and AMP combination therapy to eradicate mature biofilm | MRSA and P. aeruginosa | 16 |
| Azithromycin | Azithromycin-induced prevention of biofilm formation and eradication of pre-formed biofilm | P. aeruginosa | 12 |
| Ab-Cath | Ab-Cath-mediated antimicrobial activity | Biofilm-residing AMR S. aureus and A. baumannii | 8 |
| SAAP-148 | SAAP-148-mediated antimicrobial activity | Biofilm-residing S. aureus and A. baumannii | 65 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Alginate-based nanogels | |||
| Fe3+, and tannic acid | Dual PTT and enhanced CDT | S. aureus and E.coli | 13 |
| Enrofloxacin | Enrofloxacin-induced antimicrobial activity | S. aureus small colony variants | 6 |
| S-Benzyl-L-cysteine | Inhibition of bacterial growth by destruction of their cell walls | P. aeruginosa | 76 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Other nanogels | |||
| Clindamycin | Adhering to the bacterial cells and direct administration of the antibiotic onto the bacterial biofilm cell walls | S. aureus | 77 |
| Cy3-AMP | Gelatinase degrades GNPs to release Cy3-AMP which destroys bacterial cells | S. aureus | 30 |
| Ciprofloxacin | Disruption of the bacterial biofilms EPS matrix by protease Alcalase 2.4 L FG | S. aureus, P. aeruginosa, S. epidermidis, Klebsiella pneumoniae, E. coli, and Enterococcus faecalis | 78 |
| Tetracycline (Tc) | Direct administration of the antibiotic onto the bacterial biofilm cell walls | S. epidermidis | 79 |
| Copper sulfide (CuS) nanoclusters | Suppression of the production of pro-inflammatory cytokines and modulation of the expression of anti-inflammatory factors | S. aureus | 29 |
| Triclosan | Disruption of the bacterial cell membrane and direct administration of the antibiotic onto the bacterial biofilm | S. aureus | 80 |
| Luliconazole | —a | Candida albicans | 82 |
| Peptidomimetic (A lysine-based α-peptide/β-peptoid hybrid) | —a | P. aeruginosa | 81 |
| Indocyanine green (ICG) and manganese pentacarbonyl bromide (MnBr(CO)5) | Penetration and ablation of the biofilm by combined CO, PTT, and PDT | S. aureus and MRSA | 28 |
| ICS-Ag nanocomposite | Damage to the bacterial cytomembrane and the death of the bacterium after the release of its intracellular contents | S. aureus, E. coli, and P. aeruginosa | 83 |
| S-Benzyl-L-cysteine | Inhibition of bacterial growth by destruction of their cell walls | P. aeruginosa | 76 |
2.1 Chitosan-based nanogels for biofilm therapy
Until now, natural polymers have exhibited remarkably efficient performances in advancing biomedical fields, particularly drug delivery, tissue engineering, and wound healing.31–33 To date, natural polymers' effectiveness and significant potential have been validated in producing various nanostructures such as micelles, polymersomes, and nanogels.34 Among the various natural polymers, chitosan (CS) stands out as a cationic natural linear polyaminosaccharide acquired through the deacetylation process of chitin, which is derived from the exoskeleton of crustaceans like shrimp. CS is comprised of β-(1,4)-D-glucosamine and N-acetyl-D-glucosamine units and possesses distinctive characteristics such as non-toxicity, biocompatibility, and biodegradability, along with antibacterial, antifungal, mucoadhesive, and analgesic properties.31,35,36 Due to its resemblance to glycosaminoglycans (GAGs), CS is extensively employed in tissue engineering and stands as one of the most utilized natural biopolymers in biomedical applications.35,37,38A rising trend in antibacterial research involves exploring natural antimicrobial compounds, like CS, as substitutes for synthetic antibiotics. These studies aim to address critical issues, notably the escalating emergence of antibiotic-resistant bacterial infections, largely attributed to the excessive use of antibiotics.18,39 Recent advancements have involved incorporating nanogels to facilitate the transportation and conservation of these natural substances, aiming to enhance their efficacy.40–42 This approach presents a promising potential in tackling multidrug-resistant bacteria.18,43 Therefore, CS nanogels have been recently studied for the treatment of bacterial biofilms.11,17,44
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, utilized CGA due to its known antibacterial properties and potential to disrupt bacterial cell membranes.47 This structure, with negatively charged phosphate ions and positively charged CS, neutralized bacterial growth with both CS and CGA demonstrating the ability to disrupt bacterial cell membranes thereby hindering biofilm formation. The CaPNP@CS@CGA nanogel exhibited a significant 68% increase in biofilm degradation compared to the untreated group. Furthermore, the positively charged nanogel interacted effectively with bacterial cell membranes, disrupting their integrity. Moreover, toxicity studies revealed that CaPNP@CS@CGA nanogels remained non-toxic up to 40 μg mL−1 concentrations for HaCaT cells (immortalized human keratinocytes). These findings highlight the potential of CaPNP@CS@CGA nanogels as a viable solution for biofilm degradation, suggesting its applicability as a restorative dental material.17
1 ratio, utilized CGA due to its known antibacterial properties and potential to disrupt bacterial cell membranes.47 This structure, with negatively charged phosphate ions and positively charged CS, neutralized bacterial growth with both CS and CGA demonstrating the ability to disrupt bacterial cell membranes thereby hindering biofilm formation. The CaPNP@CS@CGA nanogel exhibited a significant 68% increase in biofilm degradation compared to the untreated group. Furthermore, the positively charged nanogel interacted effectively with bacterial cell membranes, disrupting their integrity. Moreover, toxicity studies revealed that CaPNP@CS@CGA nanogels remained non-toxic up to 40 μg mL−1 concentrations for HaCaT cells (immortalized human keratinocytes). These findings highlight the potential of CaPNP@CS@CGA nanogels as a viable solution for biofilm degradation, suggesting its applicability as a restorative dental material.17Additionally, Fan et al.11 aimed to leverage electrostatic interactions between sulfonated chitosan (SCS), silver ions (Ag+), and chitosan (CS) and created a versatile antibacterial CS-based nanogel, AgNPs@CS/SCS. The developed nanogel demonstrated several key properties, such as stability in physiological conditions, slow and sustained release of Ag+ due to the pH-dependent behavior of silver nanoparticles (AgNPs), and remarkable short- and long-term antibacterial efficacy against S. aureus and E. coli, particularly in addressing implant infections.11 AgNPs exhibit antibacterial activities through a sequential cascade of actions contributing to the eventual elimination of bacteria. Initially, AgNPs can dissolve and release Ag+ ions; subsequently, the released Ag+ ions enhance the permeability of the bacterial cell membrane and disrupt bacterial DNA, ultimately leading to the eradication of the bacteria. This multi-faceted approach highlights the efficacy of AgNPs in combating bacterial infections by targeting crucial cellular structures and functions, thereby hindering bacterial growth and survival.11,48 The effectiveness of AgNPs@CS/SCS against S. aureus and E. coli was evaluated for short-term antibacterial activity. The findings reveal that the survival of E. coli and S. aureus relied on the concentration of AgNPs@CS/SCS and the duration of incubation. Higher concentrations and longer incubation periods resulted in stronger antibacterial effects. However, the effectiveness of AgNPs@CS/SCS against E. coli surpassed its impact on S. aureus. While nearly all E. coli were eliminated by AgNPs@CS/SCS, eradicating all S. aureus required more than double the incubation time and concentration compared to E. coli. Furthermore, AgNPs@CS/SCS displayed long-term antibacterial and inhibitory effects on the growth of both E. coli and S. aureus. Importantly, AgNPs@CS/SCS displayed excellent biofilm ablation abilities while maintaining good biocompatibility, hence, showing promise for the effective clinical treatment of implant-related biofilm infections.11
As previously discussed, the intricate and diverse nature of EPS, which encapsulates biofilms, presents significant challenges for therapies aimed at targeting EPS and eradicating biofilms. Within biofilms, an acidic microenvironment can develop, often decreasing below a pH of 4. This acidity primarily results from by-products generated during bacterial carbohydrate metabolism, including acetic and lactic acids, alongside extracellular DNA (eDNA) found within the EPS.49–51 Consequently, these unique characteristics of EPS present formidable obstacles to biofilm-based therapies. Therefore, Wang et al.26 focused on developing CS-based nanogels as carriers for Tanshinone IIA (TA) to heighten their effectiveness against Streptococcus mutans (S. mutans) in terms of antibacterial and antibiofilm activities. The developed nanogels (TA@CS) exhibited remarkable features, including high encapsulation efficiency and stability, even under challenging conditions such as exposure to light and other harsh environments. Notably, TA@CS demonstrated a pH-responsive behavior, allowing the selective release of higher TA amounts in acidic conditions, which could be beneficial for a more targeted, biofilm-specific delivery. Moreover, owing to their positive charge, TA@CS displayed an affinity for negatively charged biofilm surfaces, facilitating their efficient penetration through biofilm barriers and exhibiting promising antibiofilm activity. Crucially, the encapsulation of TA within CS nanogels notably boosted its antibacterial efficacy by at least fourfold. Simultaneously, TA@CS effectively inhibited 72% of biofilm formation. These outcomes highlighted the synergistic enhancement of antibacterial and antibiofilm properties when TA is encapsulated within CS-based nanogels. These advancements hold considerable promise for various applications in pharmaceuticals, food industries, and other relevant domains.26
Therefore, CS-based nanogels have remarkable potential in various biomedical applications including antibiofilm therapeutics. This is due to CS nanogels' unique characteristics and wide-range properties, including antibacterial, antifungal, and mucoadhesive traits. Currently, the emerging research is focusing on utilizing natural antimicrobial compounds, like CS, to reduce rising antibiotic resistance, leveraging recent advancements in nanogel technologies to enhance their efficacy against multidrug-resistant bacteria and bacterial biofilms.
2.2 Hyaluronic acid-based nanogels for biofilm therapy
Like CS, hyaluronic acid (HA), a vital component of cartilage, is another naturally available polysaccharide that has been explored for biomedical purposes including drug delivery.60 HA is highly advantageous for drug delivery applications due to its biocompatibility, biodegradability, and presence of multifunctional groups.61 These benefits make HA an attractive biocompatible and easily functionalized starting material for the synthesis of drug delivery vehicles like nanogels.62 Moreover, in the area of microbiology, HA has been shown to possess bactericidal activity against bacterial biofilms thereby further adding to its suitability as a nanogel material for antibiofilm applications.12 Additionally, the FDA approval of HA gels makes them even more promising candidates to be explored for biofilm treatment.16 Therefore, HA-based nanogels have recently been investigated to eliminate and inhibit the growth of bacterial biofilms. HA-based nanogels studied for bacterial biofilm treatment are discussed below.HA-based nanogels have also been investigated as carriers for several other AMPs such as the synthetic antimicrobial and antibiofilm peptide (SAAP)-148 (ref. 7 and 65), the snake cathelicidin Ab-Cath,7,8 and DJK-5.62 Peptides with antimicrobial and antibiofilm activities are promising alternatives to antibiotics for the treatment of AMR bacterial infections. However, despite their advantageous activity, these peptides are limited by their in vivo toxicity. The cytotoxic effects of these peptides can be reduced by encapsulating them within biocompatible and biodegradable drug delivery systems such as nanogels. Therefore, several studies explored the encapsulation of antimicrobial and antibiofilm peptides within HA-based nanogels for biofilm treatment.7,8,62 For instance, a HA-based nanogel exploited to deliver the AMP Ab-Cath achieved a slow and sustained release of Ab-Cath and exhibited an efficient antimicrobial activity against the AMR S. aureus and A. baumannii strains residing within biofilms. Importantly, the Ab-Cath-loaded nanogel reduced the toxicity typically associated with free Ab-Cath while still preserving the peptide's antimicrobial activity.8 In a similar manner, HA nanogels loaded with SAAP-148 achieved a sustained release of SAAP-148 from the nanogel while also retaining an antimicrobial activity similar to that of free SAAP-148 against the biofilm-inhabiting AMR S. aureus and A. baumannii. The encapsulation within the nanogel also prevented the typical unwanted SAAP-associated toxicity.65 While the nanogels demonstrated impressive results in in vitro models, the proteolytic stability of SAAP-148 post-encapsulation in HA nanogels still needs to be examined with proteases. A more comprehensive evaluation using in vivo models could provide a deeper understanding of the pharmacokinetic and pharmacodynamic properties of the drug delivery system. Furthermore, creating a larger scale delivery system, like a gel, cream, or ointment, that contains the SAAP-148-loaded OSA-HA nanogels could aid in evaluating the potential of this nanoscale delivery system for clinical use as an antimicrobial treatment.65 Another study by Van Gent et al.7 encapsulated both Ab-Cath and SAAP-148 within HA-based nanogels and assessed their antimicrobial activity against AMR S. aureus and A. baumannii. Although this study showed reduced peptide-associated toxicity and maintained peptide-associated antimicrobial activity in AMR S. aureus and A. baumannii, this study did evaluate the performance of the loaded nanogels on the AMR strains residing within biofilms.7 Likewise, HA-based nanogels delivered the potent antibiofilm peptide DJK-5 showing its maintained antimicrobial activity against P. aeruginosa and reduced toxicity typically associated with the peptide in vivo. However, this study also did not relate the results in any way to biofilms.62 Nevertheless, the obtained results could motivate future testing of this peptide-loaded nanogel for biofilm residing bacteria since DJK-5 is one of the most potent antibiofilm peptides available.
HA-based nanogels have also been explored to improve the performance of some antibiotics such as Azithromycin. Although effective against some resistant strains such as P. aeruginosa, Azithromycin is limited by its in vivo widespread tissue distribution which reduces its concentration at the target site. This, in turn, necessitates the use of a suitable delivery vehicle to achieve a targeted delivery of the antibiotic Azithromycin.12 HA-based nanogels have been reported by Kłodzińska et al.12 for the delivery of Azithromycin to P. aeruginosa biofilms for improved antibiofilm activity. Azithromycin-loaded HA nanogels which were found to have a lower minimum inhibitory concentration than bare Azithromycin, effectively penetrated P. aeruginosa biofilms in a time-dependent manner while also significantly attenuating the formation of P. aeruginosa biofilms and eradicating its pre-formed (2 day old) biofilms. Importantly, these effects of the Azithromycin-loaded HA nanogels against forming and pre-formed P. aeruginosa biofilms were significantly greater than those explored with bare Azithromycin treatments. Moreover, bare nanogels had no effect on the biofilms indicating that the observed antibiofilm activity originated from the delivery of Azithromycin by the HA nanogels. In terms of safety, the Azithromycin HA nanogels showed no toxicity to normal cells in vitro.12
Overall, despite not being heavily explored, the promise of HA-based nanogels for biofilm treatment is evident through the delivery of antibiofilm agents such as NO, antibiotics, and antimicrobial peptides. Further progress with HA-based nanogels for biofilm therapy could include the encapsulation of other biofilm-eradicating agents such as iron oxide nanoparticles,69 silver nanoparticles,70 and copper sulfide nanoparticles.67 These nanoparticles can eliminate biofilms via mechanisms different from those exploited by antibiotics and AMPs. For instance, SPIONs can annihilate biofilms via heat generation (hyperthermia) while copper sulfide nanoparticles can eradicate biofilms via photothermal and photodynamic therapies.14,67 Furthermore, HA nanogels can be targeted to biofilms and triggered via stimuli to release their cargo for more efficient elimination and inhibition of biofilms. These modifications could potentially further enhance the performance of drug-loaded HA-based nanogels for biofilm therapy.
2.3 Alginate-based nanogels for biofilm therapy
Another less explored yet very promising polymer explored as a nanogel material for biofilm treatment is alginate. Alginate is a natural polymer known for its favorable features including accessibility, biocompatibility, biodegradability, non-toxicity, and low cost, all of which make it a promising candidate for a range of applications including the medical field. Particularly, the bio-adhesive nature of alginates makes them especially interesting for biomedical and pharmaceutical applications. Furthermore, alginate can be easily functionalized due to its available free hydroxyl and carboxyl functional groups. Sodium alginate (SA), the salt form of alginate, is usually used in literature due to its pH-induced gel-forming ability. Further adding to its advantages, alginate received its FDA approval for use in the medical field as materials for wound dressings and as additives to food.71,72A new generation of antibiotics studied includes the combination of antibacterial positively charged peptides with antibacterial aromatic amino acids. However, the positive charge of the antibacterial composite could exert some cytotoxicity to mammalian cells. The amino acid cysteine and some of its derivatives such as S-benzyl-L-cysteine have been reported to enhance antimicrobial activity. However, some cytotoxicity can still result from positively charged molecules. Therefore, S-benzyl-L-cysteine was crosslinked with the negatively charged alginate to inhibit the formation of P. aeruginosa biofilms. The S-benzyl-L-cysteine-modified alginate-based nanogels effectively inhibited the formation of P. aeruginosa biofilms by destroying the walls of bacteria and inhibiting their growth. Moreover, the bare and S-benzyl-L-cysteine nanogels were safe to normal organoids.76
To the knowledge of the authors, no more studies using alginate-based nanogels were reported for the purpose of biofilm treatment. However, alginate nanogels were more studied for the treatment of microbial infections that did not form biofilms.74 Moreover, alginate has been extensively studied as a highly promising drug delivery material due to its advantageous properties. Therefore, although only very briefly studied for the ablation and inhibition of bacterial biofilms, the promise of alginate-based nanogels for this application is evident. Hence, further study is required to explore the full potential of alginate-based nanogels in this field. As with HA-based nanogels, alginate nanogels were not targeted to biofilms. However, they did benefit from stimuli-responsiveness.
2.4 Other nanogels for biofilm therapy
In addition to CS, HA, and alginate-based nanogels, nanogels based on other polymers have been reported for biofilm treatment although to a much lower extent. These studies collectively showcase the efficacy of various other nanogel formulations in combating bacterial biofilms, which pose significant challenges in wound treatments due to their protective EPS matrix. The use of surface-functionalized nanogel particles in several studies proved promising for eradicating biofilms and overcoming antibacterial resistance mechanisms. For instance, Weldrick et al.77 demonstrated the development of a clindamycin-loaded nanogel functionalized with Alcalase 2.4 L FG, targeting biofilms of Gram-positive bacteria, such as S. aureus. The Alcalase-coated clindamycin-loaded acrylic copolymer nanogels effectively broke down the EPS matrix of biofilms, adhered to bacterial cells, and delivered the antibiotic directly to their cell walls. Notably, these functionalized nanogels exhibited superior biofilm mass reduction against S. aureus compared to conventional antibacterial agents.77 A similar study proposed the same Alcalase-coated nanogel carriers encapsulating ciprofloxacin to be used to disrupt the EPS matrix of biofilms and deliver antibiotics to the embedded bacteria. These nanogels demonstrated effectiveness against multiple biofilm-forming bacteria, including S. aureus, P. aeruginosa, Staphylococcus epidermidis (S. epidermidis), Klebsiella pneumoniae, Escherichia coli, and Enterococcus faecalis, resulting in reduced biofilm mass and bacterial cell density. Co-treatment with ciprofloxacin-loaded Alcalase-coated nanogels showed a significant reduction in viable biofilm-forming cells compared to ciprofloxacin alone, displaying a potential approach for treating chronically infected wounds with biofilm-forming bacteria.78 On the other hand, another study conducted by Li et al.30 focused on treating S. aureus bacterial biofilms using an AMP release nanogel loaded with sulfo-cyanine3 carboxylic acid (Cy3) (cypate-GNPs@Cy3-AMP, CGCA), constructed with gelatinase nanoparticles (GNPs)—the nanogel aimed to control toxicity and facilitate bacterial clearance. Upon degradation of GNPs by gelatinase in the infection site, Cy3-AMP was released to destroy bacterial cells. Adding cypate on GNPs, combined with AMPs and NIR laser irradiation, induced irreversible damage to bacteria, effectively addressing toxicity concerns.30Several other studies showcased innovative approaches using nanogels for eradicating biofilms, combating antimicrobial resistance, and advancing medical treatments. Each investigation employed distinct strategies and various nanogel formulations, demonstrating the versatile applications of these nanoparticles in addressing diverse microbial challenges, from specific biofilm eradication to enhancing antimicrobial efficacy and reducing cytotoxicity. One study targeted Staphylococcus epidermidis biofilms using enzyme-coated tetracycline (Tc)-loaded poly(acrylic acid) copolymer nanogel particles. These formulations effectively penetrated biofilms, delivering antibiotics to bacterial cells and outperforming free antibiotics.79 The study examined the effects of nanogel formulations loaded with Tc and their individual components on the HeLa cell line, a human cell model, in an in vitro setting. The group found that while free Tc could significantly disrupt the biofilm, its effectivity was reduced against the bacteria within the biofilm. This suggested that the necessary therapeutic concentration of free Tc needed to kill the bacteria within the biofilm was not achieved, resulting in a lower killing ability compared to Tc delivered by the nanocarrier. The enzymes on the surface of the nanogel broke down the components of the EPS matrix, enabling them to penetrate deeper into the EPS and effectively deliver and release the encapsulated Tc to the bacteria within the biofilm. When an equivalent concentration of Tc was encapsulated in Carbopol nanogel particles and separately coated with the enzymes, there was a greater disruption of the biofilm and killing of the bacteria within it. The nano-formulated antibiotic overcame the EPS matrix barrier by effectively breaking down its components through enzymatic digestion. This allowed the enzyme-coated nanogel particle to deliver the required therapeutic dose of Tc, effectively killing the bacteria within the biofilm. This study highlights the potential of nanotechnology as a powerful tool for controlling pathogenic bacterial biofilms, with possible applications in treating chronic wounds and other hospital-acquired infections. The study also indicates the potential for conducting in vivo studies with pig skin and other more advanced animal models to fully assess the impact of these nano-formulations in a wound environment.79 Another research focused on developing antimicrobial coatings for medical implants, utilizing non-quaternized poly(N-isopropylacrylamide-co-N-[3(dimethylamino)propyl]methacrylamide)(P(NIPAM-co-DMAPMA) nanogels modified with quaternary ammonium compounds and triclosan. This approach showcased strong antibacterial properties against S. aureus biofilms while maintaining antifouling behavior.80 Additionally, quercetin-based carbonized nanogels embedded with copper sulfide nanoclusters displayed NIR responsiveness and effectively eradicated MRSA biofilms in diabetic wounds, demonstrating anti-inflammatory properties that promoted wound healing.29 Another significant advancement involved a carbon monoxide (CO)-enhanced multi-mode antibacterial nanoplatform. The combination of CO, photothermal therapy (PTT), and photodynamic therapy (PDT) exhibited a notable efficacy in biofilm penetration, antibacterial action, and anti-inflammatory effects.28 Additionally, biopolymer nanogels incorporating antibacterial peptidomimetics reduced cytotoxicity while maintaining antibacterial efficacy against P. aeruginosa.81 DNA was also explored as a nanogel material for antibiofilm activity. S-Benzyl-L-cysteine was crosslinked with the negatively charged DNA to inhibit P. aeruginosa biofilms. The S-benzyl-L-cysteine-modified DNA-based nanogel was reported to inhibit the formation of P. aeruginosa biofilms by destroying the walls of bacteria and inhibiting their growth. Importantly, the S-benzyl-L-cysteine-modified DNA nanogel was found to be safe to normal organoids.76
Furthermore, as with CS, other nanogels were also explored for fungi biofilms. In addressing vulvovaginal candidiasis, a nanostructured lipid carrier-based transvaginal gel loaded with luliconazole showed promising results against Candida albicans biofilms.82 Moreover, biofunctionalized nanosilver (ICS-Ag) using itaconyl-chondroitin sulfate nanogel (ICSNG) effectively combated microbial infections on medical devices. ICS-Ag demonstrated that it damaged the bacterial cell membrane, resulting in the release of internal cell contents and causing bacterial death. Hence, this nanosilver formulation exhibited excellent antibacterial and antifungal properties alongside high biocompatibility.83
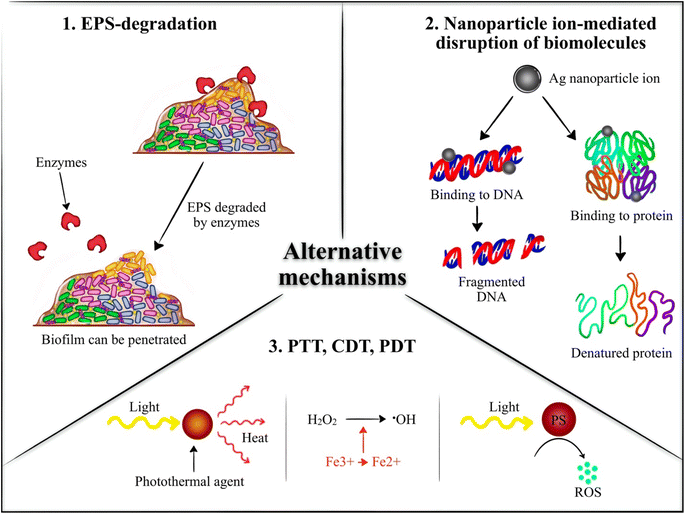
Therefore, although CS, HA, and alginate-based nanogels are the most commonly studied for biofilm elimination and growth inhibition, several other nanogels formulations hold promise for this application. However, additional investigation is needed on these formulations to explore their full potential in the treatment of bacterial biofilms. Table 1 summarizes the types of nanogels investigated for biofilm annihilation and inhibition along with their mechanisms of action and target bacterial strains. The various mechanisms utilized by the nanogels are summarized in Fig. 3.
In this regard, it is interesting and noteworthy to report here that the literature seemed to be greatly lacking in the area of utilization of stimuli responsiveness and targeting agents. The obtained results for all the studies report similar improved antibiofilm activity of nanogel encapsulated antimicrobial agent compared to unencapsulated antimicrobial agents. Stimuli-responsiveness and targeted therapy are concepts that have become increasingly investigated in recent years for several diseases such as cancer. Specifically, nanogels have been utilized to respond to various stimuli such as pH and NIR and targeting agents such as aptamers and homotypic targeting.84–87 Therefore, despite the promising results that are in line with each other, several more investigations are yet to be conducted such as equipping the nanogels with stimuli responsiveness and targeting.
3. Future directions
Although relatively limited, the immense potential of nanogel-based composites as antibiofilm agents for advancements in combating complex biofilm infections is evident in the available literature. However, despite their promising antibiofilm activity, several key aspects are required for further exploration to effectively treat biofilms. The first aspect includes the need to transition from single-strain biofilm studies to complex multi-bacterial strains co-cultured within a single dish. Presently, the literature predominantly examines biofilms with a single bacterial strain. Yet, real-life biofilms often encompass diverse bacterial species, forming poly-microbial biofilms with combinations of Gram-positive, Gram-negative bacteria, and even fungal species sometimes. Understanding and treating such polymicrobial biofilms presents a critical challenge, demanding antimicrobial agents effective against all pathogenic microorganisms present within these biofilms.88 Therefore, it is not guaranteed that promising results obtained with single-strain biofilms will be duplicated in multi-strain biofilms. Hence, antibiofilm nanogels should be investigated against multi-strain biofilms mimicking real-life biofilms to ensure the effectiveness of the nanogels in antibiofilm therapy. A further path for future exploration involves diversifying the types of antimicrobial components carried by the nanogels. This could include loading nanoparticles that can exert antibiofilm effects into nanogels. Such nanoparticles could include iron oxide nanoparticles,69 silver nanoparticles,70 and copper sulfide nanoparticles67 which can eliminate biofilms via mechanisms different from the conventional ones exploited by antibiotics. For instance, superparamagnetic iron oxide nanoparticles can kill biofilms via hyperthermia while copper sulfide nanoparticles can annihilate biofilms via photothermal and photodynamic therapies.14,67 Furthermore, the co-delivery of antimicrobial drugs such as antibiotics or nanoparticles could be explored to benefit from the synergistic effect of the drugs for better therapeutic effects against biofilm infections.89Another crucial aspect for future research involves using targeting moieties to target nanogels specifically to biofilms. However, currently, there is insufficient knowledge about overexpressed proteins on bacterial surfaces within biofilms. Therefore, it is essential to explore and identify novel moieties to achieve targeted interventions against biofilms. Moreover, leveraging the enhanced permeation and retention effect (EPR) observed in biofilm-infected sites, similar to that seen in tumors, can enable passive targeting of nanoparticles within bacteria-infected regions. This passive accumulation can be achieved due to the permeable nature of blood vessels in inflammatory areas, aiding the efficient accumulation of polymeric therapeutic nanoparticles within the biofilm microenvironment.90 Additionally, stimuli-responsive nanogels represent another promising avenue. While some studies have employed stimuli, such as pH and NIR, the exploration of various other endogenous and exogenous stimuli remains largely untapped. Investigating additional stimuli, both endogenous and exogenous, holds the potential for enhancing the specificity and efficacy of nanogel-based treatments for biofilms.21
Furthermore, the range of bacterial strains studied with nanogel-based composites remains limited. While strains like E. coli and S. aureus have received the most attention, other Gram-positive and Gram-negative bacterial strains need exploration. This includes less prominent strains like Acinetobacter baumannii, Klebsiella, Salmonella, and Streptococcus strains, among others. Diversifying the study to encompass a broader spectrum of bacterial strains will provide a more comprehensive understanding of the efficacy and potential limitations of nanogel-based interventions against a wider array of biofilm infections. Therefore, focusing on more bacterial strains from both Gram-positive and Gram-negative categories will allow for a more nuanced exploration, shedding light on potential strain-specific responses and aiding in the development of more universally effective treatments against biofilms.
In conclusion, despite the promising results on antibiofilm nanogels, the available data remains limited in several aspects. Therefore, further research is imperative in the field of biofilm treatment using nanogels-based composites. While existing studies have provided valuable insights, the need persists for more comprehensive investigations involving multi-strain biofilms, targeting agents, diverse stimuli, and a wider spectrum of bacterial strains. Multiple formulations have demonstrated immense potential in exhibiting robust antibiofilm activity, emphasizing the promising trajectory of nanogel-based interventions in combating complex biofilm infections and motivating further studies to explore their full potential.
4. Conclusions
The treatment of bacterial infections has become significantly complicated by the emergence of biofilms to which conventional antibiotics are ineffective. Consequently, several nano-based strategies have been developed and studied to combat biofilm-associated infections. Among these strategies, nanogels have shown promise as a therapeutic strategy for a range of medical conditions including biofilms. This is due to nanogels' small size which enables their penetration into biofilms, along with their biocompatibility, biodegradability, good circulation and stability, and their stimuli-responsiveness. However, despite their promising features, nanogels have only very recently begun to be investigated for biofilm treatment.Nanogels derived from natural polymers such as CS, HA, and alginate, alongside other polymers, have been recently studied for biofilm eradication and growth inhibition. These nanogels delivered diverse antibiofilm agents ranging from antibiotics and antimicrobial peptides to natural extracts and nanoparticles. Employing various mechanisms namely conventional antibody-mediated mechanisms, alternative photodynamic therapy, photothermal therapy, chemodynamic therapy, and EPS degradation, the studied nanogels effectively delivered the carried antibiofilm drugs and exerted their antibiofilm effects in several bacterial strains including S. aureus, P. aeruginosa, S. epidermidis, Klebsiella pneumoniae, E. coli, and Enterococcus faecalis, and A. baumannii. However, the most investigated strains were S. aureus, P. aeruginosa, and E. coli.
This review concluded that nanogel encapsulation of antimicrobial agents has shown to be more effective than free antimicrobials. The encapsulation exhibited excellent antibacterial and anti-biofilm activities, as well as controlled release. These results, demonstrating improved treatment of various biofilm infections and combating resistant bacteria, are of great significance. The strategy of combining nanogels with various antibacterial agents could be recommended as a novel approach for applications to treat the hazardous biofilm infection. A current challenge nanogels face is the formulation of compositions and surface coatings used to possess antibiofilm and antibacterial properties, in both in vitro and in vivo conditions. To tackle this issue, the integration of various strategies, such as the loading of AMPs, antibiotics, essential oils, and nanomaterials into nanogels, serves as a crucial example of potential ideas that could progress the development of efficient nanoparticles to inhibit infections.
Despite their promising performance, nanogels remain relatively underexplored for biofilm treatment. Limited studies have focused on the stimuli-responsiveness of nanogels for biofilm treatment with the studied stimuli restricted to only pH and NIR. Moreover, active targeting strategies to specifically target nanogels to the biofilm have not been explored yet. Additionally, limited antibiofilm agents were loaded into nanogels. Several other antibiofilm agents such as nanomaterials like iron oxide nanoparticles can be investigated with nanogels for better antibiofilm treatment. Most importantly, biofilms typically contain two or more bacterial strains. However, the currently available literature investigates single-strain biofilms neglecting multi-strain ones. This presents a major limitation on the reliability of the antibiofilm activity observed with the studied nanogels.
Conflicts of interest
There are no conflics to declare.References
- K. Zhang, X. Li, C. Yu and Y. Wang, Front. Cell. Infect. Microbiol., 2020, 10, 359 CrossRef CAS PubMed
.
- Y. Wang, J. Appl. Microbiol., 2021, 131, 2626–2639 CrossRef CAS PubMed
.
- N. B. S. Silva, L. A. Marques and D. D. B. Röder, J. Appl. Microbiol., 2021, 131, 2148–2160 CrossRef CAS PubMed
.
- K. U. Mahto, Vandana, M. Priyadarshanee, D. P. Samantaray and S. Das, J. Clean. Product., 2022, 379, 134759 CrossRef CAS
.
- A. Zhao, J. Sun and Y. Liu, Front. Cell. Infect. Microbiol., 2023, 13, 1137947 CrossRef CAS PubMed
.
- W. Luo, J. Liu, S. A. Algharib and W. Chen, J. Vet. Sci., 2022, 23, e48 CrossRef PubMed
.
- M. E. Van Gent, S. N. Klodzinska, J. W. Drijfhout, H. M. Nielsen and P. H. Nibbering, Eur. J. Pharm. Biopharm., 2023, 193, 254–261 CrossRef CAS PubMed
.
- M. E. Van Gent, S. N. Kłodzińska, M. Severin, M. Ali, B. R. Van Doodewaerd, E. Bos, R. I. Koning, J. W. Drijfhout, H. M. Nielsen and P. H. Nibbering, Nanomed. Nanotechnol. Biol. Med., 2023, 52, 102694 CrossRef CAS PubMed
.
- W. Xiu, S. Gan, Q. Wen, Q. Qiu, S. Dai, H. Dong, Q. Li, L. Yuwen, L. Weng, Z. Teng, Y. Mou and L. Wang, Research, 2020, 9426453 CAS
.
- A. Gupta, R. Das, G. Yesilbag Tonga, T. Mizuhara and V. M. Rotello, ACS Nano, 2018, 12, 89–94 CrossRef CAS PubMed
.
- M. Fan, J. Si, X. Xu, L. Chen, J. Chen, C. Yang, J. Zhu, L. Wu, J. Tian, X. Chen, X. Mou and X. Cai, Carbohydr. Polym., 2021, 257, 117636 CrossRef CAS PubMed
.
- S. N. Kłodzińska, F. Wan, H. Jumaa, C. Sternberg, T. Rades and H. M. Nielsen, J. Colloid Interface Sci., 2019, 555, 595–606 CrossRef PubMed
.
- S. Zhao, Y. Xia, Q. Lan, Q. Wu, X. Feng and Y. Liu, ACS Appl. Nano Mater., 2023, 6, 8643–8654 CrossRef CAS
.
- T.-K. Nguyen, H. T. T. Duong, R. Selvanayagam, C. Boyer and N. Barraud, Sci. Rep., 2015, 5, 18385 CrossRef CAS PubMed
.
- E. Natsaridis, F. Gkartziou, S. Mourtas, M. C. A. Stuart, F. Kolonitsiou, P. Klepetsanis, I. Spiliopoulou and S. G. Antimisiaris, Pharmaceutics, 2022, 14, 370 CrossRef CAS PubMed
.
- V. O. Fasiku, C. A. Omolo, L. W. Kiruri, N. Devnarain, M. Faya, C. Mocktar and T. Govender, Int. J. Biol. Macromol., 2022, 206, 381–397 CrossRef CAS PubMed
.
- S. Palaniraj, R. Murugesan and S. Narayan, Int. J. Biochem. Cell Biol., 2019, 114, 105566 CrossRef CAS PubMed
.
- D. Keskin, G. Zu, A. M. Forson, L. Tromp, J. Sjollema and P. Van Rijn, Bioact. Mater., 2021, 6, 3634–3657 CAS
.
- P. Eslami, F. Rossi and S. Fedeli, Pharmaceutics, 2019, 11, 71 CrossRef CAS PubMed
.
- R. T. Chacko, J. Ventura, J. Zhuang and S. Thayumanavan, Adv. Drug Delivery Rev., 2012, 64, 836–851 CrossRef CAS PubMed
.
- A. A. Ali, A. Al-Othman and M. H. Al-Sayah, J. Controlled Release, 2022, 351, 476–503 CrossRef CAS PubMed
.
- C. S. A. D. Lima, T. S. Balogh, J. P. R. O. Varca, G. H. C. Varca, A. B. Lugão, L. A. Camacho-Cruz, E. Bucio and S. S. Kadlubowski, Pharmaceutics, 2020, 12, 970 CrossRef PubMed
.
- M. Karg, A. Pich, T. Hellweg, T. Hoare, L. A. Lyon, J. J. Crassous, D. Suzuki, R. A. Gumerov, S. Schneider, I. I. Potemkin and W. Richtering, Langmuir, 2019, 35, 6231–6255 CrossRef CAS PubMed
.
- T. Wang, F. Rong, Y. Tang, M. Li, T. Feng, Q. Zhou, P. Li and W. Huang, Prog. Polym. Sci., 2021, 116, 101389 CrossRef CAS
.
- Y. Hu, X. Ruan, X. Lv, Y. Xu, W. Wang, Y. Cai, M. Ding, H. Dong, J. Shao, D. Yang and X. Dong, Nano Today, 2022, 46, 101602 CrossRef CAS
.
- M. Wang, T. Muhammad, H. Gao, J. Liu and H. Liang, Int. J. Biol. Macromol., 2023, 237, 124177 CrossRef CAS PubMed
.
- A. A. Ali, W. H. Abuwatfa, M. H. Al-Sayah and G. A. Husseini, Nanomaterials, 2022, 12, 3706 CrossRef CAS PubMed
.
- X. Cai, J. Tian, J. Zhu, J. Chen, L. Li, C. Yang, J. Chen and D. Chen, Chem. Eng. J., 2021, 426, 131919 CrossRef CAS
.
- A. Nain, Y.-T. Tseng, A. Gupta, Y.-F. Lin, S. Arumugam, Y.-F. Huang, C.-C. Huang and H.-T. Chang, Nanoscale Horiz., 2023, 8, 1652–1664 RSC
.
- M. Li, X. Wang, C. Wang, L. Qiu, Y. Xuan, X. Lei, P. Jiang, H. Shi and J. Wang, ACS Biomater. Sci. Eng., 2022, 8, 3463–3472 CrossRef CAS PubMed
.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. A. M. Aliabadi, S. Sukhtezari, B. Tahmasebi, A. Maleki and H. Madanchi, Sci. Rep., 2021, 11, 650 CrossRef CAS PubMed
.
- C. Vasile, D. Pamfil, E. Stoleru and M. Baican, Molecules, 2020, 25, 1539 CrossRef CAS PubMed
.
- B. Sarker, D. G. Papageorgiou, R. Silva, T. Zehnder, F. Gul-E-Noor, M. Bertmer, J. Kaschta, K. Chrissafis, R. Detsch and A. R. Boccaccini, J. Mater. Chem. B, 2014, 2, 1470 RSC
.
- Z. Tang, C. He, H. Tian, J. Ding, B. S. Hsiao, B. Chu and X. Chen, Prog. Polym. Sci., 2016, 60, 86–128 CrossRef CAS
.
- B. R. Rizeq, N. N. Younes, K. Rasool and G. K. Nasrallah, Int. J. Mol. Sci., 2019, 20, 5776 CrossRef CAS PubMed
.
- D.-Q. Lu, D. Liu, J. Liu, W.-X. Li, Y. Ai, J. Wang and D. Guan, Int. J. Biol. Macromol., 2022, 218, 335–345 CrossRef CAS PubMed
.
- S. Manivong, A. Garcia Ac, S. Patten, J. Fernandes, M. Benderdour, X. Banquy, F. Moldovan and V. Roullin, Nanomaterials, 2022, 12, 1337 CrossRef CAS PubMed
.
- C.-H. Kim, S. J. Park, D. H. Yang and H. J. Chun, in Novel Biomaterials for Regenerative Medicine, ed. H. J. Chun, K. Park, C.-H. Kim and G. Khang, Springer Singapore, Singapore, 2018, vol. 1077, pp. 475–485 Search PubMed
.
- S. J. Baker, D. J. Payne, R. Rappuoli and E. De Gregorio, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 12887–12895 CrossRef CAS PubMed
.
- Z. Chen, X. Lv, M. Zhao, P. Zhang, X. Ren and X. Mei, Colloids Surf., B, 2018, 170, 648–655 CrossRef CAS PubMed
.
- Y. Zhao, W. Sun and M. D. A. Saldaña, J. Polym. Res., 2018, 25, 253 CrossRef
.
- L. Li, L. Fu, X. Ai, J. Zhang and J. Zhou, Chem.–Eur. J., 2017, 23, 18088 CrossRef CAS
.
- M. Molina, M. Asadian-Birjand, J. Balach, J. Bergueiro, E. Miceli and M. Calderón, Chem. Soc. Rev., 2015, 44, 6161–6186 RSC
.
- T. P. Gharaghie, S. Beiranvand, N. J. Shirin, Y. Elahianfar, S. Ghahari and S. Ghahari, Thymol-based chitosan nanogels have strong antibacterial and anti-biofilm effects on multidrug-resistant pathogens, arXiv, preprint, 2021, DOI:10.21203/rs.3.rs-128664/v3.
- T. J. Silhavy, D. Kahne and S. Walker, Cold Spring Harbor Perspect. Biol., 2010, 2, a000414 Search PubMed
.
- S. Petti and C. Scully, J. Dent., 2009, 37, 413–423 CrossRef CAS PubMed
.
- Z. Lou, H. Wang, S. Zhu, C. Ma and Z. Wang, J. Food Sci., 2011, 76(6), M398–M403, DOI:10.1111/j.1750-3841.2011.02213.x
.
- T. Bruna, F. Maldonado-Bravo, P. Jara and N. Caro, Int. J. Mol. Sci., 2021, 22, 7202 CrossRef CAS PubMed
.
- K. R. Sims, Y. Liu, G. Hwang, H. I. Jung, H. Koo and D. S. W. Benoit, Nanoscale, 2019, 11, 219–236 RSC
.
- F. J. Geissel, V. Platania, A. Gogos, I. K. Herrmann, G. N. Belibasakis, M. Chatzinikolaidou and G. A. Sotiriou, J. Colloid Interface Sci., 2022, 608, 3141–3150 CrossRef CAS PubMed
.
- Y. Wang, J. Zhou, L. Yuan, F. Wu, L. Xie, X. Yan, H. Li, Y. Li, L. Shi, R. Hu and Y. Liu, Small, 2023, 19, 2206657 CrossRef CAS PubMed
.
- B. Salehi, A. P. Mishra, I. Shukla, M. Sharifi-Rad, M. D. M. Contreras, A. Segura-Carretero, H. Fathi, N. N. Nasrabadi, F. Kobarfard and J. Sharifi-Rad, Phytother. Res., 2018, 32, 1688–1706 CrossRef PubMed
.
- W. Yuan and H.-G. Yuk, Appl. Environ. Microbiol., 2019, 85, e002711–e002719 CrossRef PubMed
.
- S. Chouhan, K. Sharma and S. Guleria, Medicines, 2017, 4, 58 CrossRef PubMed
.
- B. Ashrafi, M. Rashidipour, A. Marzban, S. Soroush, M. Azadpour, S. Delfani and P. Ramak, Carbohydr. Polym., 2019, 212, 142–149 CrossRef CAS PubMed
.
- S. Akbari, M. Bayat, S. Roudbarmohammadi and J. Hashemi, Period. Polytech. Chem. Eng., 2019, 63(4) DOI:10.3311/PPch.12519
.
- S. Zhaveh, A. Mohsenifar, M. Beiki, S. T. Khalili, A. Abdollahi, T. Rahmani-Cherati and M. Tabatabaei, Ind. Crops Prod., 2015, 69, 251–256 CrossRef CAS
.
- M. Beyki, S. Zhaveh, S. T. Khalili, T. Rahmani-Cherati, A. Abollahi, M. Bayat, M. Tabatabaei and A. Mohsenifar, Ind. Crops Prod., 2014, 54, 310–319 CrossRef CAS
.
- S. T. Khalili, A. Mohsenifar, M. Beyki, S. Zhaveh, T. Rahmani-Cherati, A. Abdollahi, M. Bayat and M. Tabatabaei, LWT–Food Sci. Technol., 2015, 60, 502–508 CrossRef CAS
.
- S. Manivong, A. Garcia Ac, S. Patten, J. Fernandes, M. Benderdour, X. Banquy, F. Moldovan and V. Roullin, Nanomaterials, 2022, 12, 1337 CrossRef CAS PubMed
.
- S. Luan, Y. Zhu, X. Wu, Y. Wang, F. Liang and S. Song, ACS Biomater. Sci. Eng., 2017, 3, 2410–2419 CrossRef CAS PubMed
.
- S. N. Kłodzińska, D. Pletzer, N. Rahanjam, T. Rades, R. E. W. Hancock and H. M. Nielsen, Nanomed. Nanotechnol. Biol. Med., 2019, 20, 102022 CrossRef PubMed
.
- M. Piechota, B. Kot, A. Frankowska-Maciejewska, A. Grużewska and A. Woźniak-Kosek, BioMed Res. Int., 2018, 2018, 1–7 CrossRef PubMed
.
- A. J. Kunz Coyne, A. El Ghali, D. Holger, N. Rebold and M. J. Rybak, J. Infect. Dis. Ther., 2022, 11, 661–682 CrossRef PubMed
.
- M. E. Van Gent, T. Van Baaren, S. N. Kłodzińska, M. Ali, N. Dolezal, B. R. Van Doodewaerd, E. Bos, A. M. De Waal, R. I. Koning, J. W. Drijfhout, H. M. Nielsen and P. H. Nibbering, Pharmaceutics, 2023, 15, 429 CrossRef CAS PubMed
.
- D. S. Benoit and H. Koo, Nanomedicine, 2016, 11, 873–879 CrossRef CAS PubMed
.
- X. Dai, J. Ma, N. Chen, Y. Cai, Y. He, X. Li and F. Gao, ACS Appl. Bio Mater., 2021, 4, 2810–2820 CrossRef CAS PubMed
.
- L. Sun, W. Jiang, H. Zhang, Y. Guo, W. Chen, Y. Jin, H. Chen, K. Du, H. Dai, J. Ji and B. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 2302–2316 CrossRef CAS PubMed
.
- E. N. Taylor, K. M. Kummer, N. G. Durmus, K. Leuba, K. M. Tarquinio and T. J. Webster, Small, 2012, 8, 3016–3027 CrossRef CAS PubMed
.
- K. Kalishwaralal, S. BarathManiKanth, S. R. K. Pandian, V. Deepak and S. Gurunathan, Colloids Surf., B, 2010, 79, 340–344 CrossRef CAS PubMed
.
- D. Jain and D. Bar-Shalom, Drug Dev. Ind. Pharm., 2014, 40, 1576–1584 CrossRef CAS PubMed
.
- D. M. Hariyadi and N. Islam, Adv. Pharmacol. Pharm. Sci., 2020, 2020, 1–16 CrossRef PubMed
.
- S. Iravani and R. S. Varma, Mar. Drugs, 2022, 20, 598 CrossRef CAS PubMed
.
- V. Hegde, U. T. Uthappa, T. Altalhi, H.-Y. Jung, S. S. Han and M. D. Kurkuri, Mater. Today Commun., 2022, 33, 104813 CrossRef CAS
.
- X. Jiang, Z. Du, X. Zhang, F. Zaman, Z. Song, Y. Guan, T. Yu and Y. Huang, Front. Bioeng. Biotechnol., 2023, 11, 1158749 CrossRef PubMed
.
- F.-Y. Chung, C.-R. Huang, C.-S. Chen and Y.-F. Chen, Biomater. Adv., 2023, 153, 213551 CrossRef CAS PubMed
.
- P. J. Weldrick, S. San and V. N. Paunov, ACS Appl. Nano Mater., 2021, 4, 1187–1201 CrossRef CAS
.
- P. J. Weldrick, M. J. Hardman and V. N. Paunov, ACS Appl. Mater. Interfaces, 2019, 11, 43902–43919 CrossRef CAS PubMed
.
- E. O. Asare, A. Seidakhanova, D. Amangeldinova, E. Marsili and V. N. Paunov, ACS Appl. Nano Mater., 2023, 22792–22806 CrossRef CAS
.
- D. Keskin, L. Tromp, O. Mergel, G. Zu, E. Warszawik, H. C. Van Der Mei and P. Van Rijn, ACS Appl. Mater. Interfaces, 2020, 12, 57721–57731 CrossRef CAS PubMed
.
- S. N. Kłodzińska, N. Molchanova, H. Franzyk, P. R. Hansen, P. Damborg and H. M. Nielsen, Eur. J. Pharm. Biopharm., 2018, 128, 1–9 CrossRef PubMed
.
- N. Hassan, U. Farooq, A. K. Das, K. Sharma, M. A. Mirza, S. Fatima, O. Singh, M. J. Ansari, A. Ali and Z. Iqbal, ACS Omega, 2023, 8, 6918–6930 CrossRef CAS PubMed
.
- R. Yahya and N. M. Alharbi, Int. J. Biol. Macromol., 2023, 253, 127080 CrossRef CAS PubMed
.
- W. Wu, T. Zhou, A. Berliner, P. Banerjee and S. Zhou, Chem. Mater., 2010, 22, 1966–1976 CrossRef CAS
.
- F. Howaili, E. Özliseli, B. Küçüktürkmen, S. M. Razavi, M. Sadeghizadeh and J. M. Rosenholm, Front. Chem., 2021, 8, 602941 CrossRef PubMed
.
- A. A. Attama, P. O. Nnamani, O. B. Onokala, A. A. Ugwu and A. L. Onugwu, Front. Pharmacol., 2022, 13, 874510 CrossRef CAS PubMed
.
- J. Gao, F. Wang, S. Wang, L. Liu, K. Liu, Y. Ye, Z. Wang, H. Wang, B. Chen, J. Jiang, J. Ou, J. C. M. Van Hest, F. Peng and Y. Tu, Adv. Sci., 2020, 7, 1903642 CrossRef CAS PubMed
.
- R. Ruhal and R. Kataria, Microbiol. Res., 2021, 251, 126829 CrossRef CAS PubMed
.
- S. Liang, L. Xiao, Y. Fang, T. Chen, Y. Xie, Z. Peng, M. Wu, Y. Liu, J. Xie, Y. Nie, X. Zhao, Y. Deng, C. Zhao and Y. Mai, Int. J. Pharm., 2024, 649, 123638 CrossRef CAS PubMed
.
- B. Cao, X. Lyu, C. Wang, S. Lu, D. Xing and X. Hu, Biomaterials, 2020, 262, 120341 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |