First-row transition metal carbonates catalyze the electrochemical oxygen evolution reaction: iron is master of them all†
Iranna
Udachyan
 a,
Jayesh T.
Bhanushali
a,
Jayesh T.
Bhanushali
 a,
Tomer
Zidki
a,
Tomer
Zidki
 a,
Amir
Mizrahi
b and
Dan
Meyerstein
*ac
a,
Amir
Mizrahi
b and
Dan
Meyerstein
*ac
aDepartment of Chemical Sciences, and The Radical Research Center, Ariel University, Ariel, Israel. E-mail: danm@ariel.ac.il
bChemistry Department, Nuclear Research Centre Negev, Beer-Sheva 8419001, Israel
cDepartment of Chemistry, Ben-Gurion University, Beer-Sheva, Israel
First published on 24th May 2024
Abstract
In pursuing green hydrogen fuel, electrochemical water-splitting emerges as the optimal method. A critical challenge in advancing this process is identifying a cost-effective electrocatalyst for oxygen evolution on the anode. Recent research has demonstrated the efficacy of first-row transition metal carbonates as catalysts for various oxidation reactions. In this study, Earth-abundant first-row transition metal carbonates were electrodeposited onto nickel foam (NF) electrodes and evaluated for their performance in the oxygen evolution reaction. The investigation compares the activity of these carbonates on NF electrodes against bare NF electrodes. Notably, Fe2(CO3)3/NF exhibited superior oxygen evolution activity, characterized by low overpotential values, i.e. Iron is master of them all (R. Kipling, Cold Iron, Rewards and Fairies, Macmillan and Co. Ltd., 1910). Comprehensive catalytic stability and durability tests also indicate that these transition metal carbonates maintain stable activity, positioning them as durable and efficient electrocatalysts for the oxygen evolution reaction.
Introduction
The escalating global energy demand, driven by the surge in population, necessitates sustainable solutions, prominently exemplified by the utilization of renewable energy sources such as electrochemical water splitting.1,2 Electrochemically generated hydrogen holds a distinct advantage in its ease of storage compared to other renewable sources, including solar, wind, and hydropower.2,3 Furthermore, hydrogen emerges as a superior alternative to nonrenewable energy sources, offering a clean and green energy generation pathway. In the electrochemical water-splitting process, hydrogen evolution at the cathode is coupled with oxygen evolution at the anode.4,5 The sluggish kinetics of the latter process, involving a four-electron transfer, presents a bottleneck.6To date, noble metal oxides, notably IrO2 and RuO2, have been recognized for their efficiency as catalysts for the oxygen evolution reaction (OER). However, their commercial viability is hindered by the prohibitive cost and limited availability of noble metals.7,8 Consequently, substantial efforts are underway to design catalysts with advanced materials using Earth-abundant transition metals.9–12
Recent studies have identified first-row transition metal carbonates as promising catalysts for electrochemical oxidation processes.13–22 These metal carbonates, widely employed in industrial applications such as plastics, paper, paint, and rubber industries, have shown utility in various electrochemical catalytic reactions.23–26 Metal carbonates are widely used in solid oxide fuel cells, batteries, and supercapacitors.27–29 Notably, the carbonate's role as σ-donor ligand stabilizes high oxidation states of metals like MnIII, FeIV/III, CoV/IV/III, NiIII, and CuIV/III, facilitating the stabilization of metal complexes’ high oxidation states and partial radicalization of the carbonate ligand.16,17,30–32 In the high oxidation states of these complexes, the carbonate functions as an electron donor to the central metal cation, stabilizing the high oxidation states of metal complexes while also inducing partial radicalization of the carbonate ligand.18,19 Additionally, metal complex intermediates in higher oxidation states boost electrochemical reactions.
The report by Chen et al. shows that incorporating Fe in NiFeOx catalysts enhances the water oxidation activity and delivers a stable current density of 100 mA cm−2 in an alkaline media.31 Building on this background, the present study demonstrates a series of first-row transition metal carbonates as effective OER catalysts and identifies the most potent catalyst among them. Electrochemical deposition of the metal carbonate complexes onto Ni foam substrate (NF) was accomplished through anodization in aqueous electrolytes. The synthesized catalysts were comprehensively analyzed for their chemical and physical properties through various spectroscopic techniques. Subsequently, the activity of the deposited metal carbonate complexes on the NF electrode in the electrochemical OER was determined and compared.
The experimental procedures for the electrochemical deposition of metal carbonates, their respective characterization, and electrochemical activity are described in the ESI.†
Results and discussion
All metal carbonates were deposited electrochemically on the NF surface using the standard three-electrode cell setup by chronoamperometry at 1.1 V vs. Ag/AgCl. The electrochemically deposited metal carbonates on the NF surface were subjected to various physical and chemical characterization tools. Firstly, all the metal carbonates on NF and bare NF were subjected to FE-SEM analysis to determine the morphology and particle sizes. The results obtained are presented in Fig. 1. From Fig. 1a, NF exhibited a smooth surface. Mn2(CO3)3/NF showed a random orientation of spherical particles on NF to form a cauliflower-like morphology.33 Similarly, Fe2(CO3)3/NF, Co2(CO3)3/NF, Ni2(CO3)3/NF, and Cu(CO3)/NF also displayed spherical morphologies with randomly orientated microspheres. Moreover, specific flakes of carbonate species were clearly observable in Cu(CO3)/NF.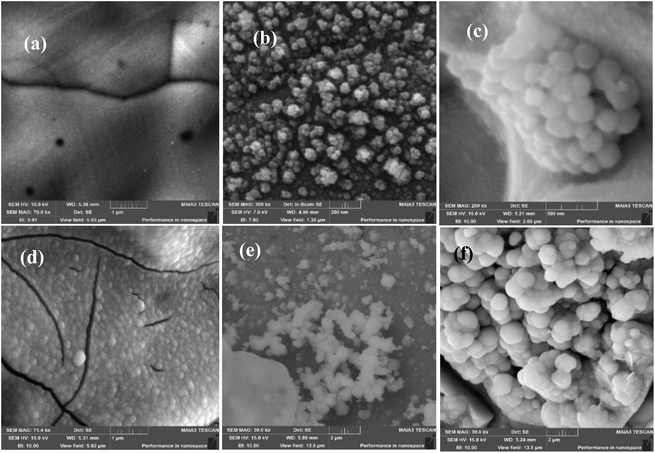 | ||
| Fig. 1 SEM images of NF (a), Mn2(CO3)3/NF (b), Fe2(CO3)3/NF (c), Co2(CO3)3/NF (d), Ni2(CO3)3/NF (e), Cu(CO3)/NF (f). | ||
The interaction type between the metal and carbonate was explored by ATR-IR spectroscopy. The IR spectrum of Mn2(CO3)3/NF is presented in Fig. 2a. The peak at 1713 cm−1 is ascribed to CO stretching. Further, 1410, 864, and 718 cm−1 peaks are characteristic Mn2(CO3)3 peaks,34 corresponding to asymmetric stretching of CO32−, out-of-plane, and in-plane bending vibrations, respectively.35,36 Similarly, the other metal carbonates showed similar IR spectra, which are presented in the ESI (Fig. S1†).
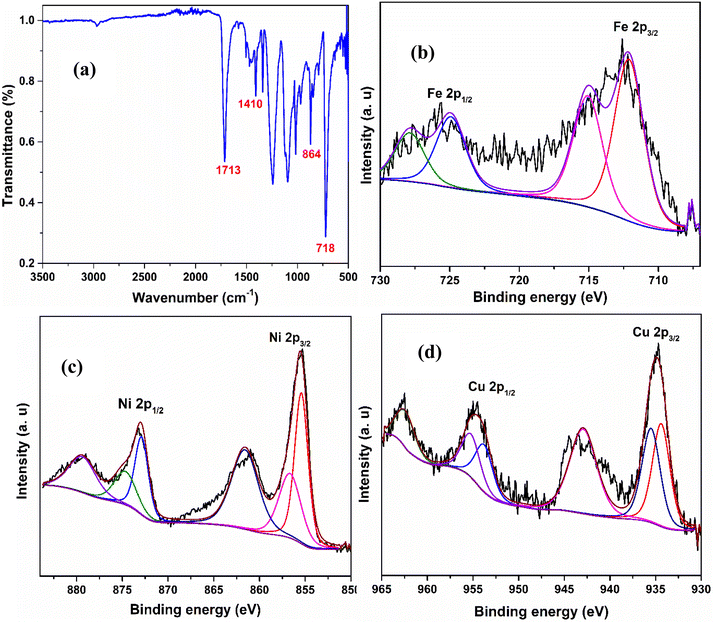 | ||
| Fig. 2 ATR spectrum of Mn2(CO3)3 (a), XPS spectra of Fe 2p for Fe2(CO3)3/NF (b), Ni 2p for Ni2(CO3)3/NF (c) and Cu 2p for Cu(CO3)/NF (d). | ||
XPS analysis of the metal carbonates on the NF electrode was performed to determine the elemental composition and their oxidation states. The obtained XPS spectra are presented in Fig. 2b–d. Fe2(CO3)3/NF showed the presence of Fe in +2 and +3 oxidation states, as noted from the Fe 2p3/2 (712.1 eV) and Fe 2p1/2 (725.0 eV) peaks and their respective satellite peaks (714.9 and 727.8 eV) (Fig. 2b).37 Similarly, Ni2(CO3)3/NF and Cu(CO3)/NF displayed the presence of Ni in +2, +3 oxidation states (Fig. 2c)38 and Cu in +2 oxidation state (Fig. 2d)39 by the Ni 2p3/2 (855.4 eV), Ni 2p1/2 (873.3 eV), Cu 2p3/2 (934.9 eV), and Cu 2p1/2 (954.6) peaks along with their respective satellite peaks (Ni 2p3/2 861.8 eV, Ni 2p1/2 879.2 eV, Cu 2p3/2 943.4 eV and Cu 2p1/2 962.7). The data corresponding to other metal carbonates, namely, Co2(CO3)3/NF and Mn2(CO3)3/NF, are presented in Fig. S2.† In addition, carbon and oxygen signals were detected in the XPS analysis of the metal carbonates, as seen in Fig. S2f–j.†
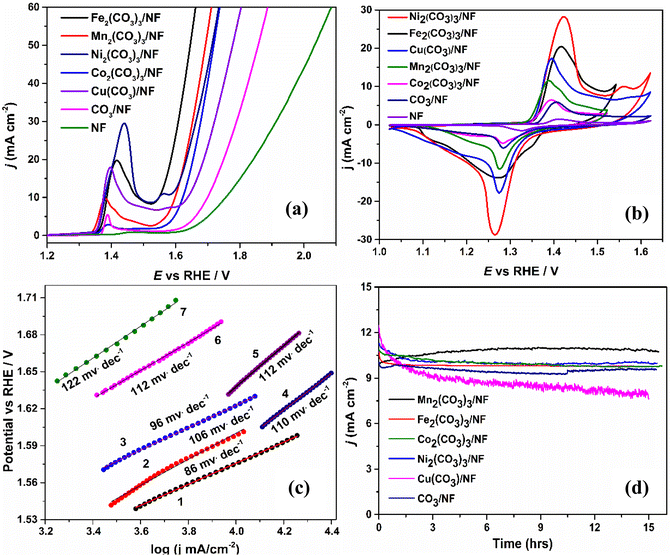
The electrochemically deposited Mn2(CO3)3/NF, Fe2(CO3)3/NF, Co2(CO3)3/NF, Ni2(CO3)3/NF, and Cu(CO3)/NF were investigated for the oxygen evolution reaction in 1.0 M KOH N2-purged solutions using the standard three-electrode system with a scan rate of 20 mV s−1. Fig. 3a shows the LSV curves (without IR correction) for Mn2(CO3)3/NF, Fe2(CO3)3/NF, Co2(CO3)3/NF, Ni2(CO3)3/NF, Cu(CO3)/NF, CO3/NF, and NF. To begin with, the highest electrocatalytic activity for oxygen evolution was observed for the Fe2(CO3)3/NF electrode with 10 mA cm−2 current density at a low overpotential of 310 mV (1.54 V vs. RHE), which is much lower in comparison with the other metal carbonates. Also, the other catalysts achieved 10 mA cm−2 current densities at low over potential values namely, Mn2(CO3)3/NF at 360 mV (1.59 V vs. RHE), Co2(CO3)3/NF at 390 mV (1.62 V vs. RHE), Ni2(CO3)3/NF at 350 mV (1.58 V vs. RHE), Cu(CO3)/NF at 400 mV (1.63 V vs. RHE), CO3/NF at 460 mV (1.69 V vs. RHE), and bare NF at 520 mV (1.75 V vs. RHE), respectively. These results indicate that first-row transition metal carbonates are active electrocatalysts for the OER. Among these metal carbonates, the Fe2(CO3)3/NF showed superior electrocatalytic behavior with the lowest overpotential. Furthermore, all the metal carbonates show multiple oxidation peaks before the sharp catalytic current peak for water oxidation, as was observed from the respective cyclic voltammograms, Fig. 3b. Additional results obtained are explained in the ESI.† Moreover, a comparative study was performed between the metal carbonates and the substrates used for the deposition of these metal carbonates to determine the effect on the OER activity. The results thus obtained are presented in the ESI.†
Additionally, Tafel curves were used to study the kinetics of the OER; the data is provided in Fig. 3c. The corresponding Tafel slope curves were fitted with the Tafel equation (η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a, η = overpotential, j = current density, b = Tafel slope).40,41 From Fig. 3b, Fe2(CO3)3/NF displayed the lowest Tafel slope of 86 mV dec−1 compared to the other metal carbonates. Correspondingly, Mn2(CO3)3/NF, Co2(CO3)3/NF, Ni2(CO3)3/NF, Cu(CO3)/NF, CO3/NF, and NF displayed Tafel slopes of 106 mV dec−1, 96 mV dec−1, 110 mV dec−1, 112 mV dec−1, 112 mV dec−1 and 122 mV dec−1, respectively. The results indicate that metal carbonates show rapid reaction kinetics for oxygen evolution.42,43
j + a, η = overpotential, j = current density, b = Tafel slope).40,41 From Fig. 3b, Fe2(CO3)3/NF displayed the lowest Tafel slope of 86 mV dec−1 compared to the other metal carbonates. Correspondingly, Mn2(CO3)3/NF, Co2(CO3)3/NF, Ni2(CO3)3/NF, Cu(CO3)/NF, CO3/NF, and NF displayed Tafel slopes of 106 mV dec−1, 96 mV dec−1, 110 mV dec−1, 112 mV dec−1, 112 mV dec−1 and 122 mV dec−1, respectively. The results indicate that metal carbonates show rapid reaction kinetics for oxygen evolution.42,43
Moreover, the electron transfer kinetics for the OER were determined by electrochemical impedance spectroscopy (EIS). Fig. S4† shows the EIS-Nyquist plot of the metal carbonates. The experiments relating to EIS were carried out at constant potential for oxygen evolution, as mentioned in the caption of Fig. S4.† All the metal carbonates exhibited smaller charge transfer resistance (Rct) than NF and CO3/NF. Interestingly, Co2(CO3)3/NF showed a bit higher charge transfer resistance; the values are given in Table S1.† These metal carbonates may be considered suitable electron transfer catalysts and exhibit good conductivity towards electrochemical OER.44–46
Further, the key factor for a catalyst to be applied depends on its long-term stability and durability. Therefore, chronoamperometric analysis was carried out in 1.0 M KOH for the metal carbonates/NF with CO3/NF to determine their stability for a duration of 15 h; the results are presented in Fig. 3d. Mn2(CO3)3/NF, Fe2(CO3)3/NF, Co2(CO3)3/NF, Ni2(CO3)3/NF and CO3/NF showed good electrocatalytic stability whereas, Cu(CO3)/NF showed decrement in electrocatalytic stability. The analysis was performed for all catalysts at their respective electrocatalytic oxygen evolution potentials. The results indicate no significant change in catalytic current density after 15 h except for a slight decay in the catalytic current density in the initial stage of the experiment for Fe2(CO3)3/NF, Co2(CO3)3/NF, Ni2(CO3)3/NF and Cu(CO3)/NF. After one h, the current density stabilized for Fe2(CO3)3/NF, whereas Cu(CO3)/NF exhibited slight decay in the catalytic current density throughout the analysis.
Conclusions
In summary, first-row transition metal carbonates were deposited by electrochemical deposition on a nickel foam (NF) electrode surface, and the electrodeposited metal carbonates were physically and chemically characterized using various characterization techniques. The electrochemically deposited metal carbonates with CO3/NF were successfully used for the electrochemical oxygen evolution reaction. Additionally, Mn2(CO3)3/NF, Fe2(CO3)3/NF, Co2(CO3)3/NF, Ni2(CO3)3/NF, Cu(CO3)/NF, CO3/NF displayed overpotentials of 360 mV, 310 mV, 390 mV, 350 mV, 400 mV, 460 mV and 520 mV, respectively. Considering the overpotential values, the study clearly demonstrates that iron, the cheapest transition metal investigated for the OER, is the best catalyst. Tafel curves indicated lower slope value and faster electron transfer reaction followed by the EIS showing lower Rct values, supporting the faster reaction kinetics. The long-term stability and catalytic durability test inferred that first-row transition metal carbonates are stable catalysts for electrochemical oxygen evolution. The plausible roles of transition metal carbonates as catalysts of OER were discussed in ref. 15.Conflicts of interest
The authors declare that there are no conflicts of interest to declare.Acknowledgements
The authors are indebted to the Pazy Foundation (Grant No. RA1700000176) for financial support, and I. U. is thankful to Ariel University for a Ph.D. Fellowship.References
- M. Amin, H. H. Shah, A. G. Fareed, W. U. Khan, E. Chung, A. Zia, Z. U. R. Farooqi and C. Lee, Int. J. Hydrogen Energy, 2022, 47(77), 33112–33134 CrossRef CAS.
- S. Y. Tee, K. Y. Win, W. S. Teo, L. Koh, S. Liu, C. P. Teng and M. Han, Adv. Sci., 2017, 4, 1600337 CrossRef PubMed.
- X. Li, X. Hao, A. Abudula and G. Guan, J. Mater. Chem. A, 2016, 4, 11973–12000 RSC.
- L. Li, P. Wang, Q. Shao and X. Huang, Chem. Soc. Rev., 2020, 49, 3072–3106 RSC.
- J. Wang, W. Cui, Q. Liu, Z. Xing, A. M. Asiri and X. Sun, Adv. Mater., 2016, 28, 215–230 CrossRef CAS PubMed.
- K. Zhang and R. Zou, Small, 2021, 17, 2100129 CrossRef CAS PubMed.
- Z. Tao, T. Wang, X. Wang, J. Zheng and X. Li, ACS Appl. Mater. Interfaces, 2016, 8, 35390–35397 CrossRef CAS PubMed.
- Z. Wu, X. F. Lu, S. Zang and X. W. Lou, Adv. Funct. Mater., 2020, 30, 1910274 CrossRef CAS.
- Y. Zhang, J. Wu, B. Guo, H. Huo, S. Niu, S. Li and P. Xu, Carbon Energy, 2023, 5, e375 CrossRef CAS.
- R. Liang, B. Zhang, Y. Du, X. Han, S. Li and P. Xu, ACS Catal., 2023, 13, 8821–8829 CrossRef CAS.
- B. Guo, Y. Ding, H. Huo, X. Wen, X. Ren, P. Xu and S. Li, Nano-Micro Lett., 2023, 15, 57 CrossRef CAS PubMed.
- J. Zhao, Y. Zhang, Y. Xia, B. Zhang, Y. Du, B. Song, H.-L. Wang, S. Li and P. Xu, Appl. Catal., B, 2023, 328, 122447 CrossRef CAS.
- I. Udachyan, T. Zidki, A. Mizrahi, S. G. Patra and D. Meyerstein, ACS Appl. Energy Mater., 2022, 5, 12261–12271 CrossRef CAS.
- I. Udachyan, J. T. Bhanushali, A. Mizrahi, T. Zidki and D. Meyerstein, ACS Appl. Energy Mater., 2022, 5, 13903–13912 CrossRef CAS.
- S. G. Patra, A. Mizrahi and D. Meyerstein, Acc. Chem. Res., 2020, 53, 2189–2200 CrossRef CAS PubMed.
- A. Mizrahi, E. Maimon, H. Cohen, H. Kornweitz, I. Zilbermann and D. Meyerstein, Chem. – Eur. J., 2018, 24, 1088–1096 CrossRef CAS PubMed.
- A. Burg, Y. Wolfer, D. Shamir, H. Kornweitz, Y. Albo, E. Maimon and D. Meyerstein, Dalton Trans., 2017, 46, 10774–10779 RSC.
- A. Mizrahi and D. Meyerstein, in Adv. Inorg. Chem, Elsevier, 2019, 74, 343–360 Search PubMed.
- S. G. Patra, E. Illés, A. Mizrahi and D. Meyerstein, Chem. – Eur. J., 2020, 26, 711–720 CrossRef CAS PubMed.
- J. Du, Z. Chen, S. Ye, B. J. Wiley and T. J. Meyer, Angew. Chem., Int. Ed., 2015, 54, 2073–2078 CrossRef CAS PubMed.
- T. Nishimoto, T. Shinagawa, T. Naito, K. Harada, M. Yoshida and K. Takanabe, ChemSusChem, 2023, 16, e202201808 CrossRef CAS PubMed.
- M. Maazallahi, R. Bagheri, S. Nandy, K. H. Chae and M. M. Najafpour, ACS Appl. Energy Mater., 2023, 6(10), 5536–5547 CrossRef CAS.
- O. A. Jimoh, K. S. Ariffin, H. Hussin and A. E. Temitope, Carbonates Evaporites, 2018, 33, 331–346 CrossRef CAS.
- S. Eloneva, S. Teir, J. Salminen, C.-J. Fogelholm and R. Zevenhoven, Ind. Eng. Chem. Res., 2008, 47, 7104–7111 CrossRef CAS.
- K. Song, in Progress in Rubber Nanocomposites, Elsevier, 2017, pp. 41–80 Search PubMed.
- F. C. Donnelly, F. Purcell-Milton, V. Framont, O. Cleary, P. W. Dunne and Y. K. Gun'ko, Chem. Commun., 2017, 53, 6657–6660 RSC.
- L. Fan, C. He and B. Zhu, Int. J. Energy Res., 2017, 41, 465–481 CrossRef CAS.
- R. Zhang, Q. Fu, P. Gao, W. Zhou, H. Liu, C. Xu, J.-F. Wu, C. Tu and J. Liu, J. Energy Chem., 2022, 70, 95–120 CrossRef CAS.
- Y. Wang, C. Shi, Y. Chen, D. Li, G. Wu, C. Wang, L. Guo and J. Ma, Electrochim. Acta, 2020, 363, 137236 CrossRef CAS.
- A. Khorobrykh, J. Dasgupta, D. R. J. Kolling, V. Terentyev, V. V. Klimov and G. C. Dismukes, ChemBioChem, 2013, 14, 1725–1731 CrossRef CAS PubMed.
- J. Wang, L. Ji and Z. Chen, ACS Catal., 2016, 6, 6987–6992 CrossRef CAS.
- Z. Zhao, Z. Wang, D. K. Denis, X. Sun, J. Zhang, L. Hou, X. Zhang and C. Yuan, Electrochim. Acta, 2019, 307, 20–29 CrossRef CAS.
- S. Rengaraj, S. Venkataraj, S. H. Jee, Y. Kim, C. Tai, E. Repo, A. Koistinen, A. Ferancova and M. Sillanpaa, Langmuir, 2011, 27, 352–358 CrossRef CAS PubMed.
- L. Wang, F. Tang, K. Ozawa, Z. Chen, A. Mukherj, Y. Zhu, J. Zou, H. Cheng and G. Q. Lu, Angew. Chem., Int. Ed., 2009, 48, 7048–7051 CrossRef CAS PubMed.
- L. Wang, Y. Sun, S. Zeng, C. Cui, H. Li, S. Xu and H. Wang, CrystEngComm, 2016, 18, 8072–8079 RSC.
- Y. Mu, L. Wang, Y. Zhao, M. Liu, W. Zhang, J. Wu, X. Lai, G. Fan, J. Bi and D. Gao, Electrochim. Acta, 2017, 251, 119–128 CrossRef CAS.
- K. O. Moura, R. J. S. Lima, A. A. Coelho, E. A. Souza-Junior, J. G. S. Duque and C. T. Meneses, Nanoscale, 2014, 6, 352–357 RSC.
- Z. Fu, J. Hu, W. Hu, S. Yang and Y. Luo, Appl. Surf. Sci., 2018, 441, 1048–1056 CrossRef CAS.
- Z. Jin, C. Liu, K. Qi and X. Cui, Sci. Rep., 2017, 7, 39695 CrossRef CAS PubMed.
- B. Malik, K. Vijaya Sankar, R. Konar, Y. Tsur and G. D. Nessim, ChemElectroChem, 2021, 8, 517–523 CrossRef CAS.
- K. C. Majhi, P. Karfa, S. De and R. Madhuri, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2019, vol. 577, p. 12076.
- M. Shen, C. Ruan, Y. Chen, C. Jiang, K. Ai and L. Lu, ACS Appl. Mater. Interfaces, 2015, 7, 1207–1218 CrossRef CAS PubMed.
- Y. Wang, Y. Zhang, Z. Liu, C. Xie, S. Feng, D. Liu, M. Shao and S. Wang, Angew. Chem., Int. Ed., 2017, 56, 5867–5871 CrossRef CAS PubMed.
- B.-A. Mei, O. Munteshari, J. Lau, B. Dunn and L. Pilon, J. Phys. Chem. C, 2018, 122, 194–206 CrossRef CAS.
- F.-T. Tsai, Y.-T. Deng, C.-W. Pao, J.-L. Chen, J.-F. Lee, K.-T. Lai and W.-F. Liaw, J. Mater. Chem. A, 2020, 8, 9939–9950 RSC.
- P. T. Babar, A. C. Lokhande, M. G. Gang, B. S. Pawar, S. M. Pawar and J. H. Kim, J. Ind. Eng. Chem., 2018, 60, 493–497 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4dt00708e |
| This journal is © The Royal Society of Chemistry 2024 |