Advances in circularly polarized luminescence materials based on helical polymers
Shi-Yi
Li
a,
Lei
Xu
d,
Run-Tan
Gao
a,
Zheng
Chen
 *c,
Na
Liu
*b and
Zong-Quan
Wu
*c,
Na
Liu
*b and
Zong-Quan
Wu
 *a
*a
aState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China. E-mail: zqwu@jlu.edu.cn
bThe School of Pharmaceutical Sciences, Jilin University, 1266 Fujin Road, Changchun, Jilin 130021, P. R. China. E-mail: liuna606@jlu.edu.cn
cNational and Local Joint Engineering Laboratory for Synthetic Technology of High Performance Polymer, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China. E-mail: chenzheng2013@jlu.edu.cn
dKey Laboratory of Green and Precise Synthetic Chemistry and Applications, Ministry of Education, Huaibei Normal University, Huaibei, Anhui 235000, P. R. China
First published on 21st December 2022
Abstract
Helical polymers are widely used in chiral recognition, chiral response, asymmetric catalysis, photoelectric functional materials, and other fields due to their unique helical structure and optical activity. In recent years, chiral helical structure and excellent processing properties have made helical polymers a new favorite in the field of circularly polarized luminescence (CPL) materials. In this review, CPL materials are briefly introduced, and then the advantages and classification of CPL materials based on helical polymers are discussed. According to the classification, the research progress of CPL materials of helical polymers is summarized. It is hoped that this brief review can stimulate the interest in helical polymers and promote the further development of helical polymers in the field of CPL.
Introduction
Helical structures widely exist in nature, such as DNAs and proteins in the microscopic world and the cosmic galaxies in the macroscopic world.1,2 The helical structure is chiral, and the left-handed and right-handed helices are mirror images of each other. Inspired by this, people began to explore the ways of artificial synthetic helical polymers in the polymer material field. In the past decades, a variety of helical polymers have been designed and developed, including polyamides (PAIs), polyisonitriles (PINs), and polyacetylenes (PAEs). Due to their unique helical structures, these polymers can be widely used in chiral recognition, asymmetric catalysis, chiral separation, photoelectric functional materials, and other fields.3–9Circularly polarized luminescence (CPL) has gradually become a new research hotspot in the photoelectric functional material field because of its potential in applications of various new areas, such as display technology, data storage processing, spin information communication, and other fields.10–12 CPL refers to the phenomenon of left or right circular polarized light emitted by the chiral polarized materials, which can intuitively reflect the excited state structural information of chiral luminescence materials.13–15 Fluorescence quantum yield (ΦF) and dissymmetry factor (glum) are two important parameters for characterizing the properties of CPL materials.16–20 The ΦF refers to the ratio of the emitted photon number to the absorbed photon number, which is used to describe the emitting light ability of the material. glum refers to the difference ratio of left- and right-handed circularly polarized light to the total average luminous intensity, which characterizes the luminous intensity of the material. In earlier studies, the research works of CPL materials mainly focused on chiral transition metal complexes. The research found that the chiral lanthanide CPL complexes have high asymmetry factors, but their quantum efficiencies are generally low due to the existence of heavy atom effects.21–23 In recent years, chiral organic small molecules and polymers have gradually become the new choices for CPL materials due to their advantages of easy modification and high quantum efficiency.24–26 Although small organic molecules have high quantum efficiency, their glum values have been low. Compared with the CPL of small molecules, the CPL polymers can always exhibit a higher quantum efficiency and dissymmetry factor. The reason is CPL polymers possess higher molecular weight and more repetitive units than the CPL molecules, which can amplify the luminescence properties and chiral signals.28–30 In addition, CPL polymers also have excellent photoelectric properties and good processability. As a result, CPL polymers have attracted more and more attention in recent years.
Helical polymers with CPL activity (CPL helical polymers, CHPs) combine chirality and chromophores. These two outstanding characteristics of CHPs make it a classical CPL material. According to the construction ways, CHPs can be divided into four categories: (1) CPL based on optically active helical polymers: CHPs are obtained from CPL small molecules by homo-polymerization or copolymerization and using covalent bonds for chiral transfer. (2) CPL based on chiral induced helical polymers: under the condition of external chiral environment induction, a chiral transfer is carried out through non-covalent interactions to obtain chiral helical polymer containing chromophore. (3) CPL based on self-assembled helical polymers: in the absence of an external chiral environment, molecules self-assemble through non-covalent interactions as the driving force to form stable chiral helical conformation and obtain CPL ability. (4) CPL based on composite helical polymers: no external chiral environment and any covalent bonds or non-covalent bonds, the chiral unit and fluorescent unit are directly combined, and then, the chiral structure of the helical polymer is used to select and absorb the light emitted from fluorescence source to obtain helical polymer composite material with CPL ability.
CPL based on optically active helical polymers
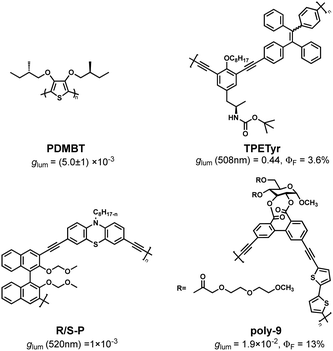
The most direct and effective way to construct CPL materials is to transfer chirality through covalent bonds by homopolymerization or copolymerization to obtain chiral helical macromolecules containing chromophores.13–31According to the report, the first CHP is PDMBT (Fig. 1), a polythiophene derivative based on chiral side chain modification with glum up to (5.0 ± 1.0) × 10−3. It was developed by Meijer et al. in 1996.32
In 2013, Cheng et al. designed and synthesized a chiral copolymer TPETyr (Fig. 1) containing TPE units by Sonogashira coupling reaction, which exhibited a high asymmetry factor in THF solution (glum = 0.44, ΦF = 3.6%). (Fig. 2).33 Two years later, the CPL copolymer R/S-P (Fig. 1) was reported by the same team, and it was prepared through copolymerization of luminescent chromophore phenothiazine and chiral 1,1′-naphthalenyl-2,2′-diol (BINOL). The test results show that its glum can achieve 1 × 10−3.34 Based on these works, Cheng's group further synthesized a class of four-component helical polymers in 2017, and a deep red CPL signal (glum up to 2 × 10−3) can be tested due to intramolecular Förster resonance energy transfer (FRET).35 Subsequently, a series of related research works were expanded by this team for demonstrating that dinaphthalene based chiral polymers are one of the best choices for constructing CHPs.36–40
In 2014, a quinoline-based helical polymer with chiral side chains was prepared by Suginome et al. It was found that the CPL reversed with the replacement of the solvent from trichloromethane to 1,1,1-trichloroethane. They attributed this finding to the change of the M-helix to the P-helix structure.41 Subsequently, they also developed quinoline-based copolymers containing different copolymerization units and achieved colour modulation. These works demonstrate the potential application of such helical polymers in the CPL field.42,43
In 2016, inspired by the one-hand excess helical structure of ellagitannin, Ikai et al. designed and synthesized optically active polythiophene (poly-9, Fig. 1). It is worth noting that poly-9 shows a strong CPL emission property (glum = 1.9 × 10−2, ΦF = 13%) because the biphenyl structure units linked single-handed excess axial deformation glucose units are introduced into its backbone.44 In 2017, they synthesized a series of cellulose-derived polymers that also possess CPL activity and have a glum of 3.0 × 10−3.45 Subsequently, a series of chiral polymers with glucose groups, also possessing CPL activity, was developed on the basis of these works.46–49 These results show that chiral biomass resources have the potential to be developed into novel CHPs materials.
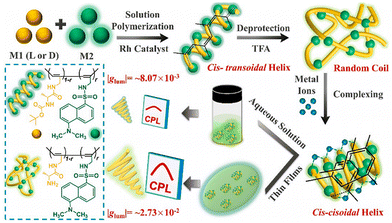
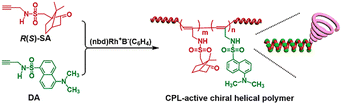
In 2018, a CPL-active chiral helical polymer was (Fig. 3) reported by Deng et al.50 This polymer was prepared by copolymerizing achiral acetylenic fluorescent unit and chiral unit and exhibited excellent optical activity and CPL capability with glum of 10−1 (Fig. 4). The single helical structure R(S)-SA of the copolymer plays a dual role in achieving the CPL process: one provides helical chirality for the CPL materials, and the other effectively suppresses the aggregation-caused quenching (ACQ) effect of the fluorescent groups. Subsequently, in 2020, Deng et al. prepared a CPL copolymer consisting of chiral units and achiral alkyne units containing tetraphenylene (TPE) and found that the polymer can exhibit a high glum value of CPL emission in solid films, while no CPL emission was detected in the dispersed state.51
 | ||
| Fig. 3 Schematic illustration for preparing fluorescent chiral helical monosubstituted polyacetylenes with CPL performance. Copyright 2018, American Chemical Society. | ||
Wan et al. reported the first example of luminescent monosubstituted polyacetylenes (mono-PAs) based on a contracted cis-cisoid polyene backbone. It has an excellent CPL performance and has a glum of 10−1.52 They developed a series of mono-PAs that can recognize structurally diverse achiral amines. The achievement of this function relies on the cis-transoid to the cis-cisoid helical transition of the polyphenylacetylene backbone.53
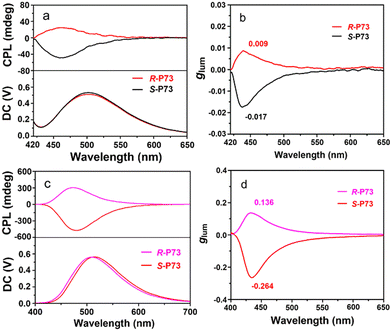
In 2021, using a chiral donor–acceptor copolymerization strategy, Chen et al. developed a series of chiral non-conjugated polymers (R, R)-/(S, S)-pTpAcDPS, (R, R)-/(S, S)-pTpAcBP, S–P and R–P with excellent circularly polarized electroluminescence (CP-EL) capability and have a gEL of 10−3.54,55 Cheng et al. introduced a chiral functionalized bis-benzoxanethonyl unit by intramolecular chiral transfer mechanism to prepare three conjugated polymer backbones (S-/R-BP, S-/R-WP1, and S-/R-WP2) to construct full-colour CP-EL.56
In addition, Chen et al. synthesized a pair of linear axially chiral conjugated polymers by incorporating rigid axially chiral chromophores into the polymer backbones.57 The twisted biphenyl skeleton effectively reduces the energy gap between the S1 and T, which greatly facilitates the intersystem crossing (ISC) process. The unique structural design makes it have low-temperature UOP (ultralong organic phosphorescence) and CPL properties.
In general, the construction of intrinsic CPL-helical polymers by homopolymerization and copolymerization is one of the most common and simplest methods.
CPL based on chiral induced helical polymers
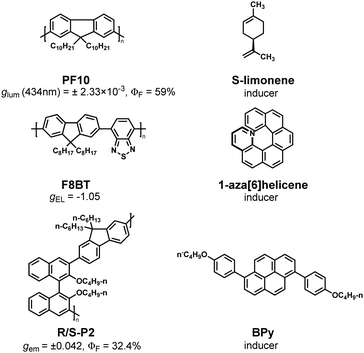
In fact, the chiral transfer not only can be achieved by the covalent bonds but also by the non-covalent bonds when some polymers in a chiral environment, such as chiral solvents, small chiral molecules, and circularly polarized light.58–64 Therefore, the chiral structural character can be transferred to polymers’ chromophores by non-covalent interactions under a chiral environment; as a result, the polymers can exhibit CPL ability.In 2010, Fujiki et al. found that the achiral conjugated polymer PF10 can produce asymmetric aggregation in a chiral mixed solvent. The chiral solvent consists of a large amount of R/S-limonene (the main solvent), a small amount of chloroform (the good solvent), and a small amount of methanol (the poor solvent) (Fig. 5).65 Under this chiral environment, the backbone of polymer PF10 was arranged in a single helical direction to realize the chiral transfer successfully so that PF10 not only acquired chirality but also possessed CPL ability (glum = ±2.33 × 10−3, ΦF = 59%). In subsequent studies, the chiral transfer of more achiral conjugated polymers in chiral solvents was explored, and these research results laid a foundation for further exploring solvent-induced CPL-helical polymer materials.66–69
In 2012, Akagi et al. used liquid crystalline polyacetylene (diLCPA) derivatives bearing 4-nonyloxy phenyl groups and chiral dopants to obtain a chiral nematic liquid crystal material with excellent CPL capability with an asymmetry factor of 10−1.70
Using the chiral solvent introduction method, some achiral branched polymers were also investigated by Zhang et al. By the chiral solvent introduction, a series of achiral polymers with azo side chains and achiral polyfluorene polymers successfully obtained the CPL activity. These results further enriched the research work on solvent-induced CPL-helical polymer materials.61–76 In addition, they also proposed a co-gel method for realizing the chiral induction based on achiral polymer PCz8 and chiral D/L-BG gels.77 In the mixed system, the gel agent BG is the chiral matrix material, which can induce the polymer PCz8 to form a helical conformation through the entangling of alkyl chains during the formation of the co-gel. After removing the chiral gel matrix, the induced chirality of the polymer PCz8 can be maintained, resulting in a chiral helical polymer with CPL ability (Fig. 7).
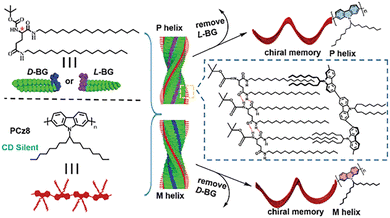 | ||
| Fig. 7 Illustration of chiral transfer from gelator BG to achiral polymer and chiral memory effect. Copyright 2016, Royal Society of Chemistry. | ||
Fuchter et al. doped the chiral aza[6]helicene molecules into the copolymer of dioctylfluorene and benzothiadiazole (F8BT).78,79 Under the π–π interaction, the backbone of polymer F8BT was induced to form a helical conformation, which made the composite system F8BT/aza exhibit obvious CPL characteristics with gEL = −1.05 (Fig. 5). In 2020, Cheng et al. used another chiral doped small molecule, R-/S-3, to induce F8BT polymer and also obtained the helical polymer with CPL characteristics.80 Fuchter et al. and Ji-Seon Kim et al. successfully prepared materials with CP-EL capability using F8T2 and F8BT after chiral aza[6]helicene molecule induction. They have a gEL of −0.65 and 0.3, respectively.81,82 These results indicate that F8BT with chiral helical structure has great application potential in CP-OLED.78–80,82
In 2020, Zou et al. found that after the irradiation of left-handed or right-handed CPL light, the CPL signal can be measured from achiral luminescent polymer F6BT.83 The intensity and direction of the generating CPL signal are strongly influenced by the polarization direction and irradiation wavelength of the chiral source CPL light.
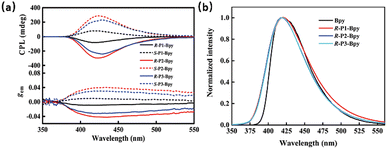
In 2021, Cheng et al. used the chiral non-fluorescent polymer R/S-P (1-3) to induce small fluorescent molecule BPy through π–π interaction during thermal annealing, and this system also showed obvious CPL characteristics and had a gem of ±0.042 (ΦF = 32.4%) (Fig. 5 and 6).84 In 2022, Cheng et al. used two chiral binaphthyl polymers as chiral inducers to construct co-assemblies with achiral pyrene-naphthalimide dye (NPy) and obtained an excellent CP-EL performance (gEL = 4.8 × 10−2).85 Subsequently, they used three achiral conjugated pyrene-based dyes (BP, w-WP, and c-WP) doped with chiral binaphthyl-based enantiomers (S-/R-M) and successfully obtained nanofibers with regular helical structure by chiral co-assembly under the effect of thermal annealing.86 This material has excellent CP-EL capability with a gEL of 6.2 × 10−2. In subsequent work, they chose three achiral liquid crystal polymers (LC-P1/P2/P3) and chiral dinaphthyl inducers (R/S-M) with anchored dihedral angles to construct chiral co-assembled liquid crystal polymers.87 It was found that regular helical nanofibers with CPL activity signals could be generated after thermal annealing.
In 2022, Li et al. exploited the synergistic effect of hydrogen bonds and metal coordination in an aqueous solution and successfully induced a single chiral helical polymer with CPL activity (Fig. 8).88
In the chiral environment, non-covalent interactions, such as molecular π–π interactions, hydrogen bonds, and chiral light sources, can be used to carry out the chiral transfer and obtain CPL active helical polymers, which is also a common method to obtain CPL-helical polymers.
CPL based on self-assembled helical polymers
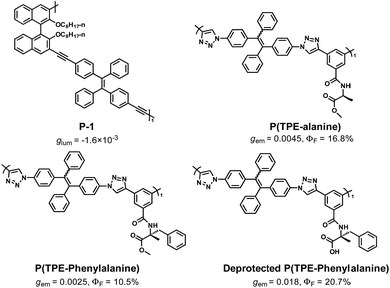
In addition, only relying on the interaction between molecules themselves, the ordered helical chiral structure can also be obtained by self-assembly behaviour without a chiral environment. Therefore, the chiral polymer with CPL ability also can be obtained in this way.In 2015, Cheng et al. synthesized a chiral conjugated polymer P-1 (Fig. 9) containing (R)-1,1′-binaphthyl and tetraphenylethene moieties. The CPL signal was not found in the THF solution of P-1 but was detected in its THF/H2O mixed solution. That is because P-1 can induce self-assembly behaviour into helical nanofibers by π–π interactions in its THF/H2O solution. The helical nanofibers of P-1 have CPL ability (glum = −1.6 × 10−3).89
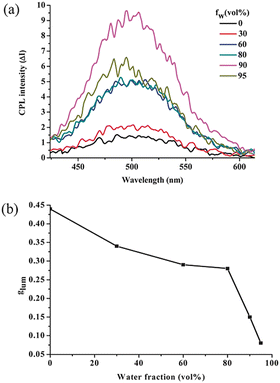
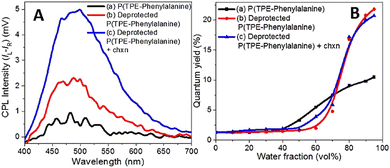
Tang et al. introduced TPE into the conjugated polymer backbone, and copolymer P(TPE-alanine) was obtained, which had excellent AIE and CPL performance (gem = 0.0045, ΦF = 16.8%).90 More interestingly, the copolymer self-assembled in the THF/H2O system. With the increase in water content, its self-assembled form changed from microspheres to fibres. The self-assembled polymers exhibited CD and CPL properties, and the glum reached 0.045. Subsequently, they constructed a polymer with a similar structure, P(TPE-phenylanine), and studied the self-assembly behaviour of P(TPE-phenylanine) under the condition of deprotection and adding chiral additives (Fig. 9).91 With the improvement of hydrogen bonding, the self-assembly behaviour also changed, the helical nanofibers were gradually formed, and the corresponding CPL ability was gradually improved (gem = 0.018, ΦF = 20.7%) (Fig. 10).
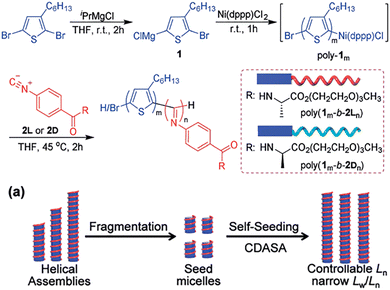
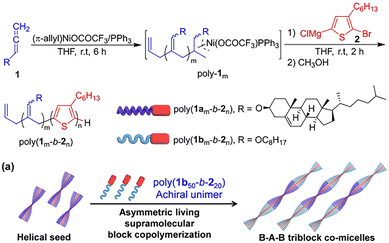
In 2020, our group designed and synthesized the P3HT-b-PPI block copolymer modified with chiral alkyl side chains.92 Using the excellent crystallinity of P3HT and the chirality of PPI, this block copolymer can realize the crystallization-driven asymmetric self-assembly (CDSA) to form a single helical chiral fibre with controllable length, narrow dispersion, and clear handedness. In the process of self-assembly, the chiral property is transferred from PPI to supramolecular assembly, which makes the copolymer assembly have good chiral optical activity. The helical assembly formed by the chiral block copolymer exhibits white light emission and excellent CPL performance (Fig. 11). Then, we reported the controlled synthesis of π-conjugated poly(phenyl isocyanide)-b-poly(phenyleneethylene) (PPI-b-PPE) copolymers via chain extension of ethynyl 4-iodobenzene initiated by Pd(II)-terminated helical poly(phenyl isocyanide) (PPI).93 The polymerization induced chiral self-assembly (PISA) of the resulting block copolymers, resulting in one-handed helical nanofibers with defined helicity and controllable size. Interestingly, the helical chirality of PPI is transferred to the supramolecular structure, resulting in high optical activity of the supramolecular nanostructure and the clear emission of CPL (Fig. 12).
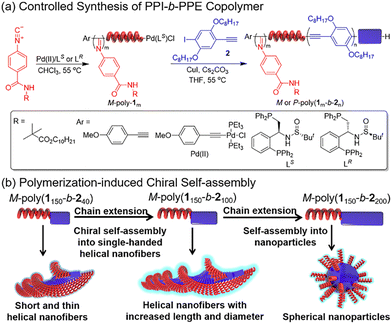 | ||
| Fig. 12 (a) Controlled synthesis of the PPI-b-PPE copolymer; (b) schematic illumination of polymerization-Induced chiral self-assembly. Copyright 2021, American Chemical Society. | ||
Recently, we synthesized a series of copolymers, poly(cholesterol allene)-b-poly(3-hexylthiophene) (PCA-b-P3HT) containing helical PCA and poly(alkoxy allene)-b-poly(3-hexylthiophene) (PAA-b-P3HT) containing achiral PAA segments by using a nickel catalyst.94 Crystallization of P3HT and helicity of PCA drove PCA-b-P3HT self-assemble into spherical nanoparticles that gradually transformed into one-handed helical nanofibers by CDSA. The chirality of PCA was transferred to the supramolecular structure, which induced high optical activity of P3HT. The chiral seed micelles of PCA-b-P3HT were found to induce asymmetric block copolymerization of achiral PAA-b-P3HT copolymers, which led to one-handed helical supramolecular block copolymers. Although PAA-b-P3HT is achiral, the block copolymerization proceeded in a helix selective manner, giving the supramolecular block copolymers controlled helicity. Consequently, the supramolecular block copolymers showed interesting white-light emission and CPL (Fig. 13).
In summary, due to the complex structural design and control of self-assembly, few self-assembled CPL-helical polymer materials have been reported so far. It is hoped that this review will give researchers new enlightenment and research ideas.
CPL based on composite helical polymers
In addition to covalent and non-covalent bonds, a new method to construct CPL-helical polymer materials has emerged in recent years. The chiral polymer materials with CPL ability were obtained by combining the chiral polymer and fluorescent molecules and using the chiral structure of the chiral polymer to screen the light emitted by the fluorescence source.Deng et al. established a strategy to prepare CPL-helical polymers based on chiral helical polyalkyne and achiral fluorescent units.95,96 Based on this strategy, they proposed the “matching rule”, which means that when the CD signal wavelength of the chiral helical polyalkyne partially matches the fluorescence emission wavelength of the fluorescence unit, the CPL signal can still be generated in the separated state even if they have no chemical interaction. This is mainly attributed to the helical polymer acting as a filter to screen the light emitted by the fluorescent source to obtain circularly polarized light with single handedness (Fig. 14). Based on this strategy, they constructed CPL materials of full colour and fabricated CPL devices with switching responsiveness using binary and ternary chiral fluorescence films, with a maximum glum of +0.323. Then, they constructed a composite of chiral helical polymers with perovskite materials, which also had CPL activity.97 A series of CPL helical polymer composites with different colours were obtained by electrospinning.98
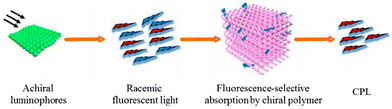 | ||
| Fig. 14 Schematic illustration of the fluorescent selective absorption mechanism. Copyright 2019, American Chemical Society. | ||
Conclusions and outlook
This review describes the research progress of CPL materials constructed by different methods based on helical polymers. Firstly, helical polymers and circularly polarized luminescence are briefly introduced. According to the different ways of constructing circularly polarized luminescence materials, helical polymers are divided into four categories. Then, according to these classifications, the research trends of helical polymers in the field of CPL in recent years are described. It can be found that researchers have developed various spiral polymers with CPL activity through different methods, but few of the CPL helical polymer materials can break the glum of 1. Therefore, we believe that the future development direction and difficulty of CPL-helical polymers lie in how to improve the asymmetry factor through reasonable structural design or other means, such as doping. Moreover, the mechanical properties and stability of the polymer material, as well as the availability of mass production, will be the main factors affecting the development of CPL-helical polymers if practical applications are considered. In conclusion, challenges and opportunities exist, and it can be expected that the exploration of CPL-helical polymer materials has great significance and broad application prospects.Author contributions
S.-Y. L, L. X., and R.-T. G. wrote and edited the paper. Z. C., N. L., and Z.-Q. W. conceived the idea and corrected the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (NSFC, Grant No. 22071041, Z.W.; 21971052, N.L.; 21871073, Z.W.; and 52273006 N.L.), the Fundamental Research Funds for the Central Universities of China, and the Cross Discipline Training Program for Young Teachers and Students of Jilin University (No. 415010300062).Notes and references
- L. Pauling, R. B. Corey and H. R. Branson, Proc. Natl. Acad. Sci. U. S. A., 1951, 37, 205–211 CrossRef PubMed
.
- J. D. Watson and F. H. Crick, Nature, 1953, 171, 737–738 CrossRef PubMed
.
- H. Zhong and J. Deng, Macromol. Rapid Commun., 2021, 42, 2100341 CrossRef PubMed
.
- Y. X. Xue, Y. Y. Zhu, L. M. Gao, X. Y. He, N. Liu, W. Y. Zhang, J. Yin, Y. Ding, H. Zhou and Z. Q. Wu, J. Am. Chem. Soc., 2014, 136, 4706–4713 CrossRef CAS PubMed
.
- T. Ikai, T. Kurake, S. Okuda, K. Maeda and E. Yashima, Angew. Chem., Int. Ed., 2021, 60, 4625–4632 CrossRef CAS PubMed
.
- E. Yashima and K. Maeda, Macromolecules, 2008, 41, 3–12 CrossRef CAS
.
- M. Reggelin, M. Schultz and M. Holbach, Angew. Chem., Int. Ed., 2002, 41, 1614–1617 CrossRef CAS
.
- C. Zhang, H. Wang, Q. Geng, T. Yang, L. Liu, R. Sakai, T. Satoh, T. Kakuchi and Y. Okamoto, Macromolecules, 2013, 46, 8406–8415 CrossRef CAS
.
- R. Cheerla and M. Krishnan, Macromolecules, 2016, 49, 700–707 CrossRef CAS
.
- F. Zinna, M. Pasini, F. Galeotti, C. Botta, L. Di Bari and U. Giovanella, Adv. Funct. Mater., 2017, 27, 1603719 CrossRef
.
- M. Li, W.-B. Lin, L. Fang and C.-F. Chen, Acta Chim. Sin., 2017, 75, 1150–1163 CrossRef
.
- R. Farshchi, M. Ramsteiner, J. Herfort, A. Tahraoui and H. T. Grahn, Appl. Phys. Lett., 2011, 98, 162508 CrossRef
.
- H. Hayasaka, T. Miyashita, K. Tamura and K. Akagi, Adv. Funct. Mater., 2010, 20, 1243–1250 CrossRef CAS
.
- S. Fukao and M. Fujiki, Macromolecules, 2009, 42, 8062–8067 CrossRef CAS
.
- J. M. Yu, T. Sakamoto, K. Watanabe, S. Furumi, N. Tamaoki, Y. Chen and T. Nakano, Chem. Commun., 2011, 47, 3799–3801 RSC
.
- X. Jiang, X. Liu, Y. Jiang, Y. Quan, Y. Cheng and C. Zhu, Macromol. Chem. Phys., 2014, 215, 358–364 CrossRef CAS
.
- F. Li, Y. Wang, Z. Wang, Y. Cheng and C. Zhu, Polym. Chem., 2015, 6, 6802–6805 RSC
.
- J. Li, X. Peng, C. Huang, Q. Qi, W.-Y. Lai and W. Huang, Polym. Chem., 2018, 9, 5278–5285 RSC
.
- T. Ikai, T. Yoshida, S. Awata, Y. Wada, K. Maeda, M. Mizuno and T. M. Swager, ACS Macro Lett., 2018, 7, 364–369 CrossRef CAS
.
- C. Zhang, M. Li, H. Y. Lu and C. F. Chen, RSC Adv., 2018, 8, 1014–1021 RSC
.
- L. Gu, W. Ye, X. Liang, A. Lv, H. Ma, M. Singh, W. Jia, Z. Shen, Y. Guo and Y. Gao, J. Am. Chem. Soc., 2021, 143, 18527–18535 CrossRef CAS
.
- R. Liu, B. Ding, D. Liu and X. Ma, Chem. Eng. J., 2021, 421, 129732 CrossRef CAS
.
- S. Wang, D. Hu, X. Guan, S. Cai, G. Shi, Z. Shuai, J. Zhang, Q. Peng and X. Wan, Angew. Chem., Int. Ed., 2021, 60, 21918–21926 CrossRef CAS PubMed
.
- Y. F. Wang, M. Li, J. M. Teng, H. Y. Zhou, W. L. Zhao and C. F. Chen, Angew. Chem., Int. Ed., 2021, 60, 23619–23624 CrossRef CAS PubMed
.
- W. Zheng, T. Ikai and E. Yashima, Angew. Chem., Int. Ed., 2021, 60, 11294–11299 CrossRef
.
- O. El-Zubir, P. R. Martinez, G. Dura, L. L. G. Al-Mahamad, T. Pope, T. J. Penfold, L. E. Mackenzie, R. Pal, J. Mosely and F. Cucinotta, J. Mater. Chem. C, 2022, 10, 7329–7335 RSC
.
- J. M. Teng, D. W. Zhang, Y. F. Wang and C. F. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 1578–1586 CrossRef PubMed
.
- K. Dhbaibi, C. Shen, M. Jean, N. Vanthuyne, T. Roisnel, M. Górecki, B. Jamoussi, L. Favereau and J. Crassous, Front. Chem., 2020, 8, 237 CrossRef
.
- H. Yu, K. Pan and J. Deng, Macromolecules, 2018, 51, 5656–5664 CrossRef
.
- X.-J. Zhang, R.-T. Gao, S.-M. Kang, X.-J. Wang, R.-J. Jiang, G.-W. Li, L. Zhou, N. Liu and Z.-Q. Wu, Polymer, 2022, 245, 124712 CrossRef
.
- M. Pan, R. Zhao, B. Zhao and J. Deng, Macromolecules, 2021, 54, 5043–5052 CrossRef CAS
.
- B. M. W. Langeveld-Voss, R. A. J. Janssen, M. P. T. Christiaans, S. C. J. Meskers, H. P. J. M. Dekkers and E. W. Meijer, J. Am. Chem. Soc., 1996, 118, 4908–4909 CrossRef CAS
.
- X. Liu, J. Jiao, X. Jiang, J. Li, Y. Cheng and C. Zhu, J. Mater. Chem. C, 2013, 1, 4713–4719 RSC
.
- F. Li, Y. Wang, Y. Sheng, G. Wei, Y. Cheng and C. Zhu, RSC Adv., 2015, 5, 105851–105854 RSC
.
- Z. Wang, Y. Fang, X. Tao, Y. Wang, Y. Quan, S. Zhang and Y. Cheng, Polymer, 2017, 130, 61–67 CrossRef CAS
.
- Z. Wang, S. Liu, Y. Wang, Y. Quan and Y. Cheng, Macromol. Rapid Commun., 2017, 38, 1700150 CrossRef
.
- J. Ma, Y. Wang, X. Li, L. Yang, Y. Quan and Y. Cheng, Polymer, 2018, 143, 184–189 CrossRef CAS
.
- F. Meng, F. Li, L. Yang, Y. Wang, Y. Quan and Y. Cheng, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 1282–1288 CrossRef CAS
.
- Y. Wang, X. Li, L. Yang, W.-Y. Sun, C. Zhu and Y. Cheng, Mater. Chem. Front., 2018, 2, 554–558 RSC
.
- L. Yang, Y. Zhang, X. Zhang, N. Li, Y. Quan and Y. Cheng, Chem. Commun., 2018, 54, 9663–9666 RSC
.
- Y. Nagata, T. Nishikawa and M. Suginome, Chem. Commun., 2014, 50, 9951–9953 RSC
.
- T. Nishikawa, Y. Nagata and M. Suginome, ACS Macro Lett., 2017, 6, 431–435 CrossRef CAS PubMed
.
- S. Kuriyama, Y. Nagata and M. Suginome, ACS Macro Lett., 2019, 8, 479–485 CrossRef CAS PubMed
.
- T. Ikai, S. Shimizu, S. Awata, T. Kudo, T. Yamada, K. Maeda and S. Kanoh, Polym. Chem., 2016, 7, 7522–7529 RSC
.
- T. Ikai, Y. Kojima, K.-i Shinohara, K. Maeda and S. Kanoh, Polymer, 2017, 117, 220–224 CrossRef CAS
.
- T. Ikai, S. Awata and K.-i Shinohara, Polym. Chem., 2018, 9, 1541–1546 RSC
.
- T. Ikai, S. Minami, S. Awata, S. Shimizu, T. Yoshida, M. Okubo and K.-i Shinohara, Polym. Chem., 2018, 9, 5504–5510 RSC
.
- T. Ikai, S. Shimizu, S. Awata and K.-i Shinohara, Macromolecules, 2018, 51, 2328–2334 CrossRef CAS
.
- T. Ikai, K. Takayama, Y. Wada, S. Minami, C. Apiboon and K. I. Shinohara, Chem. Sci., 2019, 10, 4890–4895 RSC
.
- B. Zhao, K. Pan and J. Deng, Macromolecules, 2018, 51, 7104–7111 CrossRef CAS
.
- N. Lu, X. Gao, M. Pan, B. Zhao and J. Deng, Macromolecules, 2020, 53, 8041–8049 CrossRef CAS
.
- S. Wang, D. Hu, X. Guan, S. Cai, G. Shi, Z. Shuai, J. Zhang, Q. Peng and X. Wan, Angew. Chem., Int. Ed., 2021, 60, 21918–21926 CrossRef CAS PubMed
.
- S. Wang, S. Xie, H. Zeng, H. Du, J. Zhang and X. Wan, Angew. Chem., Int. Ed., 2022, 61, e202202268 CAS
.
- Y. F. Wang, M. Li, J. M. Teng, H. Y. Zhou, W. L. Zhao and C. F. Chen, Angew. Chem., Int. Ed., 2021, 60, 23619–23624 CrossRef CAS PubMed
.
- J. M. Teng, D. W. Zhang, Y. F. Wang and C. F. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 1578–1586 CrossRef CAS
.
- Y. Zhang, T. Jing, Y. Quan, S. Ye and Y. Cheng, Adv. Optical. Mater., 2022, 10, 2200915 CrossRef CAS
.
- D. W. Zhang, M. Li and C. F. Chen, Angew. Chem., Int. Ed., 2022, e202213130 CAS
.
- S. Guo, H. Kamite, N. Suzuki, L. Wang, A. Ohkubo and M. Fujiki, Biomacromolecules, 2018, 19, 449–459 CrossRef CAS
.
- K. Liu, Y. Shen, X. Li, Y. Zhang, Y. Quan and Y. Cheng, Chem. Commun., 2020, 56, 12829–12832 RSC
.
- K. Yang, S. Ma, Y. Zhang, B. Zhao and J. Deng, Macromol. Rapid Commun., 2022, 43, 2200111 CrossRef CAS
.
- C. Zou, D. Qu, H. Jiang, D. Lu, X. Ma, Z. Zhao and Y. Xu, Molecules, 2019, 24, 1008 CrossRef CAS PubMed
.
- Q. Li, J. Yuan, H. Liang, F. Zheng, X. Lu, C. Yu and Q. Lu, ACS Nano, 2020, 14, 8939–8948 CrossRef
.
- J. Hong, S. Kim, G. Park, Y. Lee, H. Kim, S. Kim, T. W. Lee, C. Kim and Y. You, Chem. Sci., 2021, 12, 8668–8681 RSC
.
- X. Gao, J. Wang, K. Yang, B. Zhao and J. Deng, Chem. Mater., 2022, 34, 6116–6128 CrossRef
.
- Y. Nakano, Y. Liu and M. Fujiki, Polym. Chem., 2010, 1, 460–469 RSC
.
- Y. Kawagoe, M. Fujiki and Y. Nakano, New J. Chem., 2010, 34, 637–647 RSC
.
- D. Lee, Y.-J. Jin, H. Kim, N. Suzuki, M. Fujiki, T. Sakaguchi, S. K. Kim, W.-E. Lee and G. Kwak, Macromolecules, 2012, 45, 5379–5386 CrossRef
.
- L. Wang, N. Suzuki, J. Liu, T. Matsuda, N. A. A. Rahim, W. Zhang, M. Fujiki, Z. Zhang, N. Zhou and X. Zhu, Polym. Chem., 2014, 5, 5920–5927 RSC
.
- M. Fujiki, K. Yoshida, N. Suzuki, N. A. A. Rahim and J. A. Jalil, J. Photochem. Photobiol., A, 2016, 331, 120–129 CrossRef
.
- B. A. San Jose, S. Matsushita and K. Akagi, J. Am. Chem. Soc., 2012, 134, 19795–19807 CrossRef
.
- J. Liu, J. Zhang, S. Zhang, N. Suzuki, M. Fujiki, L. Wang, L. Li, W. Zhang, N. Zhou and X. Zhu, Polym. Chem., 2014, 5, 784–791 RSC
.
- S. Jiang, Y. Zhao, L. Wang, L. Yin, Z. Zhang, J. Zhu, W. Zhang and X. Zhu, Polym. Chem., 2015, 6, 4230–4239 RSC
.
- L. Yin, Y. Zhao, S. Jiang, L. Wang, Z. Zhang, J. Zhu, W. Zhang and X. Zhu, Polym. Chem., 2015, 6, 7045–7052 RSC
.
- T. Miao, L. Yin, X. Cheng, Y. Zhao, W. Hou, W. Zhang and X. Zhu, Polymers, 2018, 10, 612 CrossRef PubMed
.
- Y. Zhao, N. A. Abdul Rahim, Y. Xia, M. Fujiki, B. Song, Z. Zhang, W. Zhang and X. Zhu, Macromolecules, 2016, 49, 3214–3221 CrossRef
.
- Y. Zhao, H. Chen, L. Yin, X. Cheng, W. Zhang and X. Zhu, Polym. Chem., 2018, 9, 2295–2301 RSC
.
- D. Yang, Y. Zhao, K. Lv, X. Wang, W. Zhang, L. Zhang and M. Liu, Soft Matter, 2016, 12, 1170–1175 RSC
.
- Y. Yang, R. C. da Costa, D. M. Smilgies and A. J. Campbell, Adv. Mater., 2013, 25, 2624–2628 CrossRef PubMed
.
- L. Wan, J. Wade, F. Salerno, O. Arteaga, B. Laidlaw, X. Wang, T. Penfold, M. J. Fuchter and A. J. Campbell, ACS Nano, 2019, 13, 8099–8105 CrossRef
.
- X. Zhang, Z. Xu, Y. Zhang, Y. Quan and Y. Cheng, J. Mater. Chem. C, 2020, 8, 15669–15676 RSC
.
- L. Wan, J. Wade, X. Wang, A. J. Campbell and M. J. Fuchter, J. Mater. Chem. C., 2022, 10, 5168–5172 RSC
.
- H. Yan, J. Wade, L. Wan, S. Kwon, M. J. Fuchter, A. J. Campbell and J.-S. Kim, J. Mater. Chem. C., 2022, 10, 9512–9520 RSC
.
- J. Cheng, F. Ge, Y. Xiang, H. Zhang, Y. Kuai, P. Hou, D. Zhang, L. Qiu, Q. Zhang and G. Zou, J. Mater. Chem. C., 2020, 8, 6521–6527 RSC
.
- Z. Geng, Y. Zhang, Y. Zhang, Y. Li, Y. Quan and Y. Cheng, J. Mater. Chem. C., 2021, 9, 12141–12147 RSC
.
- Z. Geng, Y. Zhang, Y. Zhang, Y. Quan and Y. Cheng, Angew. Chem., Int. Ed., 2022, 61, e202202718 Search PubMed
.
- Y. Zhang, Y. Li, Y. Quan, S. Ye and Y. Cheng, Angew. Chem., Int. Ed., 2022, e202214424 Search PubMed
.
- Y. Zhang, H. Li, Z. Geng, W. H. Zheng, Y. Quan and Y. Cheng, ACS Nano, 2022, 16, 3173–3181 CrossRef
.
- H. Duan, C. Zhu, D. Qi and J. Li, Polymer, 2022, 255, 125123 CrossRef
.
- S. Zhang, Y. Sheng, G. Wei, Y. Quan, Y. Cheng and C. Zhu, Polym. Chem., 2015, 6, 2416–2422 RSC
.
- Q. Liu, Q. Xia, S. Wang, B. S. Li and B. Z. Tang, J. Mater. Chem. C, 2018, 6, 4807–4816 RSC
.
- Q. Liu, Q. Xia, Y. Xiong, B. S. Li and B. Z. Tang, Macromolecules, 2020, 53, 6288–6298 CrossRef
.
- L. Xu, C. Wang, Y. X. Li, X. H. Xu, L. Zhou, N. Liu and Z.-Q. Wu, Angew. Chem., Int. Ed., 2020, 59, 16675–16682 CrossRef
.
- X. H. Xu, Z. Q. Jiang, L. Xu, L. Zhou, N. Liu and Z.-Q. Wu, ACS Appl. Bio Mater., 2021, 4, 7213–7221 CrossRef PubMed
.
- C. Wang, L. Xu, L. Zhou, N. Liu and Z.-Q. Wu, Angew. Chem., Int. Ed., 2022, 61, e202207028 Search PubMed
.
- B. Zhao, K. Pan and J. Deng, Macromolecules, 2018, 52, 376–384 CrossRef
.
- B. Zhao, H. Yu, K. Pan, Z. Tan and J. Deng, ACS Nano, 2020, 14, 3208–3218 CrossRef
.
- B. Zhao, X. Gao, K. Pan and J. Deng, ACS Nano, 2021, 15, 7463–7471 CrossRef CAS PubMed
.
- P. Li, X. Gao, B. Zhao, K. Pan and J. Deng, Adv. Fiber Mater., 2022, 4, 1632–1644 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2023 |