Recent advances in the interfacial engineering of organic–inorganic hybrid perovskite solar cells: a materials perspective
Zhaochen
Guo
a,
Zhongbin
Wu
 a,
Yonghua
Chen
a,
Yonghua
Chen
 b,
Songcan
Wang
b,
Songcan
Wang
 *a and
Wei
Huang
*a
*a and
Wei
Huang
*a
aFrontiers Science Center for Flexible Electronics, Xi’an Institute of Flexible Electronics (IFE), Xi’an Institute of Biomedical Materials & Engineering, Northwestern Polytechnical University, 127 West Youyi Road, Xi’an 710072, China. E-mail: iamscwang@nwpu.edu.cn; iamwhuang@nwpu.edu.cn
bKey Laboratory of Flexible Electronics (KLOFE) & Institution of Advanced Materials (IAM), Nanjing Tech University (NanjingTech), Nanjing, 211816, China
First published on 20th July 2022
Abstract
Research on perovskites, a highly promising type of semiconductor material in the field of photovoltaics, has made amazing progress. The development of perovskite solar cells (PSCs) has so far reached a certified power conversion efficiency (PCE) of 25.7%. Since the interfaces in PSCs are closely connected to the carrier dynamics (charge separation, transport, injection, collection and recombination), interfacial engineering has become a powerful tool in enhancing the device performance and long-term stability. This review focuses on critically discussing the emerging materials and strategies for interfacial engineering at the charge transport layer (CTL)/perovskite and CTL/electrode interfaces in PSCs with both normal and inverted structures. In particular, the underlying mechanisms of various materials for interfacial engineering in maximizing the PCE and the long-term stability of PSCs are systematically demonstrated. Finally, a brief summary of the latest advances and breakthroughs, and a perspective on the future research directions are presented, which are expected to promote the development of this important research field.
1. Introduction
As the global population grows as well as the increasing demand for energy, fossil fuels will gradually face depletion, leading to a non-negligible issue of an energy crisis.1 On the other hand, the requirement to reduce carbon dioxide emissions has been raised due to the increasing greenhouse effect that has been changing the global climate.2,3 On Earth, solar energy is extensively distributed and inexhaustible as a renewable and clean energy source.4,5 The rational use of solar energy to change the current energy mix has been proved to be one of the most effective methods.6With the rapid development of photovoltaic technology, solar energy can be directly converted into electricity to meet the world's energy needs with little environmental effects.7 Currently, solar cells are divided mainly into silicon-based solar cells,8,9 thin-film solar cells,10 and new solar cells from a developmental stage.11,12 Compared with traditional solar cells, new solar cells have the advantages of using a simpler preparation process, having cheaper material costs, being more environmentally friendly, and having considerable efficiency, which signifies their huge development potential.
Organic–inorganic hybrid perovskite solar cells (PSCs) are one of the most important new solar cells that have attracted great attention since their first discovery in 2009.13 The standard formula of the organic–inorganic hybrid perovskite material is ABX3, where A is an organic cation, such as CH3NH3+ (MA+), CH(NH2)2+ (FA+), etc., B is a metal cation, such as Pb2+, Sn2+, etc., and X is a halogen anion, like Cl−, Br−, I−, etc.14 This material is an excellent light-absorbing material that has a special crystal structure and excellent photoelectric properties, including a long carrier lifetime, a large light-absorption coefficient, a high defect-state tolerance, and a high dielectric constant.15 In addition, its simple preparation method, low cost, and compatibility with flexible substrates meet the future development needs for flexible or foldable solar cells; hence, its application to new solar cells shows enormous development potential.16–19 It should be mentioned that the perovskite material was first applied in 2009 as a sensitizer for dye-sensitized solar cells, and obtained a power conversion efficiency (PCE) of only 3.8%, opening up a new era for solar cells.13 In 2012, a new technique using solid organic–inorganic hybrid perovskites was developed after abandoning the liquid-phase conditions,20 resulting in a further breakthrough in the photovoltaic performance. Since then, the speed of research related to PSCs has increased. As of 2021, the PCE of PSCs has reached 25.7%.21
Despite the outstanding photovoltaic performance of PSCs, their stability and lifetime issues are major challenges for their practical application, and even for their gradual replacement of silicon cells in the future.22–24 With the rapid development of PSC technology and the maturation of device-preparation processes, the ability to improve their performance indices by improving the device structure has gradually decreased. Therefore, this necessitates a more in-depth investigation of the interfaces between the device layers and the perovskite material itself during the device design and preparation, as well as optimization to obtain better interfacial properties for achieving devices with a higher photovoltaic performance.25–27 As shown in Fig. 1a, since the photogenerated electron and hole carriers from the perovskite layer will transfer to the electrodes through the charge transport layers (CTLs), the perovskite/CTL and CTL/electrode interfaces are closely connected to the carrier dynamics (i.e., charge separation, transport, injection, collection and recombination) and significantly affect the device performance. Their interfacial defects, interfacial reactions, and non-radiative recombination due to energy-level mismatch have a substantial impact on the performance of the device.28,29 Interfacial engineering is a means of improving the device performance by adding or changing both the materials and structure of the interface, which can optimize the energy-level alignment, improve the film quality, passivate defects, and enhance the device stability (Fig. 1b). Therefore, interfacial engineering has become an important strategy for enhancing the performance of PSCs.
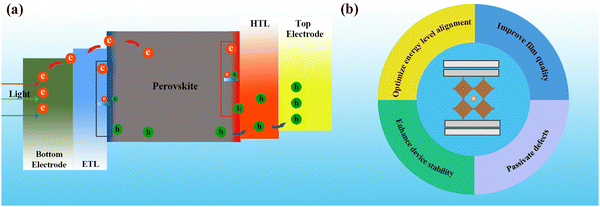 | ||
| Fig. 1 (a) Charge transport diagram of a typical n–i–p PSC. (b) Functions of interfacial engineering in PSCs. | ||
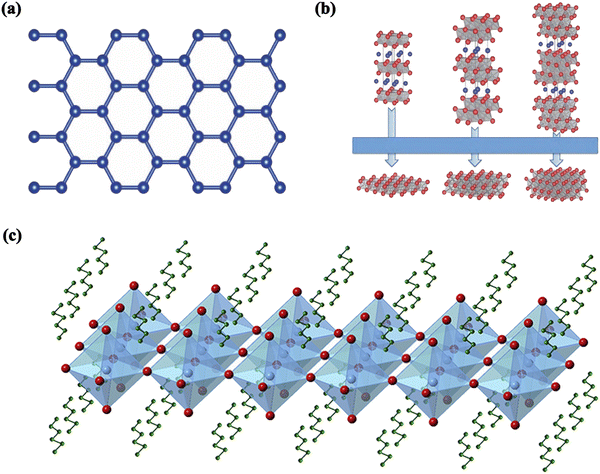
In recent years, there have been some excellent reviews on interfacial engineering, but most of them have focused only on normal-structured PSCs.30–32 Owing to the dynamic development of this important research field, a comprehensive review that focuses on the interfacial engineering of PSCs with both normal and inverted structures remains necessary to provide readers with a better understanding of the state-of-the-art progress in this hot and dynamic research field. Here, recent advances in the modification of different interfaces to improve the performance and stability of PSCs are comprehensively reviewed from the perspective of the materials, which are classified as inorganic salts, organic molecules and polymers, quantum dots (QDs), fullerenes and their derivatives, self-assembled small molecules (SAMs) and two-dimensional (2D) materials. It is worth mentioning that 2D materials include the general 2D materials as well as 2D perovskites. As a typical 2D material, graphene was successfully prepared via a mechanical exfoliation method, initiating the research into 2D materials.33,34 Graphene has attracted much attention due to its excellent physical properties, which include a high carrier mobility, high strength, high flexibility and high optical transparency. Fig. 2a represents the graphene crystal structure. Along with graphene, many other 2D materials have been developed. In the research field of PSCs, 2D materials that include graphene and its derivatives as well as transition metal carbides/nitrides (MXenes) are generally applied as interfacial modification materials (Fig. 2b). In addition to being used as light-absorbing layers, 2D perovskites are often used as interfacial modification layers, with the effect of improving the interlayer energy levels, reducing charge recombination, and effectively inhibiting ion migration (Fig. 2c). In this review article, we primarily discuss the up-dated progress that has been reported over the past few years for interfacial engineering at the electron transport layer (ETL)/perovskite, perovskite/hole transport layer (HTL), and CTL/electrode interfaces for PSCs with both normal and inverted structures. This is because these interfaces are the focus of investigations on interface modification and are directly related to the extraction of photogenerated carriers from the perovskite layer. In particular, the strategies of dual interfacial modification, i.e., the simultaneous modification at different interfacial layers, are also critically discussed. Finally, a brief summary of the current research status is presented, along with the prospects of interfacial engineering for the development of efficient, large-area PSCs for possible commercialization.
2. Roles of interfacial engineering
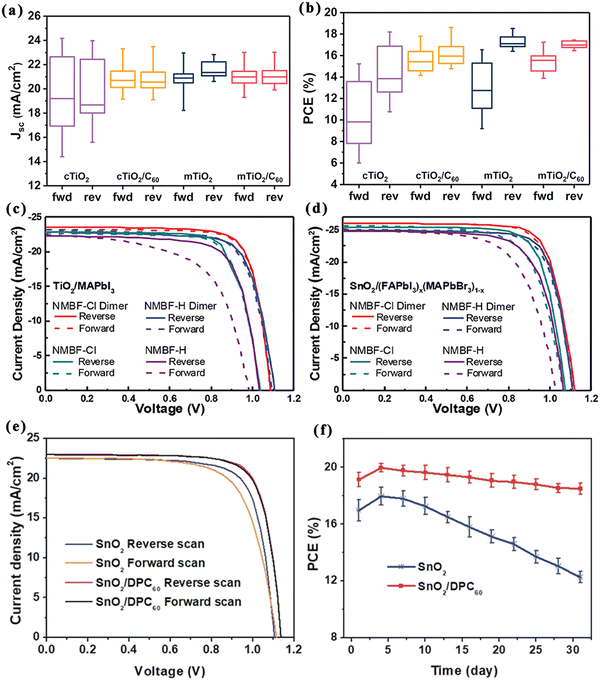
Depending on whether or not the cell structure contains a titanium dioxide (TiO2) mesoporous layer, PSCs can be classified into mesoporous structures and planar heterogeneous structures.35,36 Thus far, most high-efficiency PSCs are processed with mesoporous TiO2 materials at high temperatures (>450 °C), which is not favorable for the development of low-cost and flexible PSCs. By contrast, planar structures not only simplify the device processing but also enable low-temperature processing, while providing the possibility of preparing stacked devices, which can now achieve device efficiencies close to those of mesoporous structures processed at high temperatures. Fig. 3 shows the schematic diagrams of PSCs with different structures, where planar heterostructure solar cells are subdivided into normal (n–i–p) and inverted (p–i–n) configurations according to the direction of photogenerated carrier transport. More specifically, the mesoporous structure shown in Fig. 3a consists of a transparent conducting oxide cathode (FTO/ITO), a dense ETL (TiO2), a perovskite material attached to a mesoporous metal oxide layer (TiO2 or Al2O3), followed by an HTL as well as a metal electrode. This structure is characterized by its ability to enhance charge accumulation by reducing the carrier diffusion length and also effectively reducing carrier recombination. However, the grain confinement of the perovskite in the mesopores enables a large amount of perovskite to be present in the amorphous phase, resulting in lower short-circuit current (Jsc) and open-circuit voltage (Voc) values. In addition, a perovskite with long-lived carriers and long diffusion distances means that it can work even without a mesoporous TiO2 layer, and thus planar structured PSCs emerge.37,38Fig. 3b shows the planar n–i–p structure, where the mesoporous layer is omitted and the perovskite layer is sandwiched between the HTL and the ETL. Compared with mesoporous n–i–p PSCs fabricated using similar materials and methods, the planar n–i–p structure still shows severe J–V hysteresis, despite the fact that it yields a higher Jsc and Voc.39 The inverted p–i–n structure is the opposite of the n–i–p structure in the order of light, i.e., ITO/FTO, HTL, perovskite, ETL, metal electrode (Al or Au), as shown in Fig. 3c. With the further development of the p–i–n structure, its ETL and HTL have been extended from specific organic substances to inorganic substances.40,41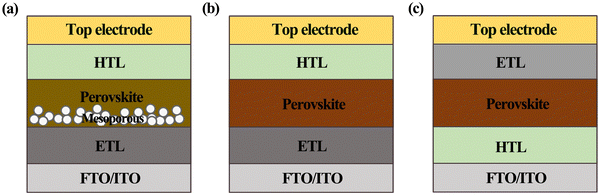 | ||
| Fig. 3 PSCs with the (a) n–i–p mesoporous structure, (b) n–i–p planar structure, and (c) p–i–n planar structure. | ||
The interface of PSCs has a profound effect on their photovoltaic performance and lifetime. Firstly, the photovoltaic performance-related parameters such as Voc, Jsc, fill factor (FF) and PCE are influenced by the charge extraction and transport between the CTL and the active layer of the perovskite.
The photocurrent density of PSCs is defined from the monochromatic incident photon-to-electron conversion efficiency (IPCE), as illustrated by the following equation:42
| IPCE(λ) = α(λ)φinjφc | (1) |
Alternatively, the charge injection efficiency is expressed using the following equation:29
| φinj = kinj/(kinj + kr + knr) | (2) |
Moreover, the non-radiative recombination also has an important effect on the steady-state carrier density, which determines the quasi-Fermi energy level splitting in the device concerning the Voc,45 as is shown by the following equation:30
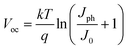 | (3) |
Furthermore, organic materials (0.1 eV and 10 nm, respectively) have a lower exciton binding energy (30–76 meV) and a longer exciton diffusion length (100–1000 nm), making them easier for excitons and free carriers to diffuse to the interface. Non-radiative energy transfer or exciton dissociation between the layers in PSCs can lead to quenching, which affects the device performance.48
In the interfacial engineering of PSCs, the electrode is also an indispensable component, which not only conducts the charge generated by the photovoltaic device to the external circuit to complete the whole photoelectric conversion process, but also affects the quality of the interface layer, the transparency of the photovoltaic device, and the cost of the photovoltaic device preparation. In the preparation of photovoltaic devices, electrodes are an essential and important part. The electrons and holes generated in the device need to reach the top and bottom electrodes through the ETL and HTL, respectively, and finally be collected through the closed external circuit that forms the photocurrents.
For the bottom electrode in the structure of the cells, its morphology and properties have an important influence on the wettability and film quality of the upper interfaces, so the modification of its surface is very important for improving the performance of PSCs. Currently, the most commonly used metal oxide transparent conductive films for the bottom electrode of PSCs are indium-doped tin oxide (ITO) and fluorine-doped tin oxide (FTO), etc., whose photovoltaic properties are closely related to the film thickness and film quality.49,50 Among them, ITO is commonly used as the bottom electrode for planar structured PSCs. Compared with ITO, FTO has a lower light transmission rate due to its larger thickness, but it is more suitable as a substrate for interfacial layers that require high-temperature heating, such as TiO2, due to its corrosion resistance and high-temperature tolerance, and is therefore widely used as the bottom electrode for mesoporous structured PSCs. The top electrode plays a crucial role in charge collection in PSCs, and common top electrodes in PSCs are Ag, Al and Au. However, due to their high cost and the degradation of PSCs caused by Ag electrodes in contact with perovskite for a long time, carbon electrodes, transparent conductive metal oxide electrodes, and polymer electrodes are being developed and utilized in addition to metal electrodes.31,51 In particular, carbon has many advantages such as being an abundant resource, and having a low cost, good conductivity, and good chemical stability. Moreover, its work function (5.0 eV) is similar to that of Au (5.1 eV), which makes carbon an ideal choice for the top electrode of PSCs. In addition, since carbon electrode-based PSCs may not require an HTL, the preparation of HTL-free carbon-based PSCs (C-PSCs) with a lower energy consumption and simpler operation has become a hot research topic in the field of PSCs.52 In addition, a tight ohmic contact between the electrode and the interface should be achieved to reduce the contact potential barrier at the device interface and obtain a higher PCE.53
In particular, semitransparent PSCs (ST-PSCs), with their unique transparent electrode design, can absorb light from both sides and produce a greater energy output compared with single-sided PSCs. Furthermore, ST-PSCs can be stacked with other cells to make full use of the sunlight, and thus have received a lot of attention. At present, the process of silicon-based cells in high-efficiency four-terminal tandem devices has matured, and the cell performance of tandem modules is mainly determined by the upper layer of ST-PSCs.54,55 Modification of the interface between the electrode and the CTL in ST-PSCs, to make the energy bands more compatible and the charge transfer smoother, will make the preparation of stacked cells more favorable. According to the above analysis, interfacial engineering has been developed as an important tool to accomplish these purposes.
3. Interfacial engineering in the normal structure
3.1 Bottom electrode/perovskite interface
Unlike the top electrode, which needs to be deposited on a fragile and complex perovskite layer or an organic CTL, the bottom electrode can generally be sufficiently stable when combined with a suitable charge transport intermediate layer. In ETL-free PSCs, the majority of work focused on the interfacial modification is devoted to correcting the mismatch in energy levels at the electrode/perovskite interface. A polar non-conjugated small molecule, 4,4-(((methyl(4-sulphonatobutyl)ammonio)bis(propane-3,1-diyl))bis(dimethyl ammoniumdiyl))bis-(butane-1-sulfonate) (MSAPBS) was designed as a modifier to reduce the work function of ITO and improve the energy level alignment at the interface through an intrinsic dipole. The electron extraction efficiency was thus greatly improved, followed by significant enhancement of the Voc, Jsc and FF of the device. Ultimately, the champion device achieved a PCE of 20.55% (Fig. 4a and b). Simultaneously, its stability was improved.56 In a similar work, rubidium fluoride (RbF) was inserted on the FTO surface as a modification layer. The RbF layer created a dipole layer, which adjusted the work function of the FTO, eliminated the electron transfer potential barrier, and optimized the energy level arrangement of the FTO/perovskite interface. Consequently, charges were transferred more efficiently and charge-carrier recombination was suppressed. As a result, the efficiency of ETL-free PSCs with the RbF layer reached as high as 18.79% (Fig. 4c and d).57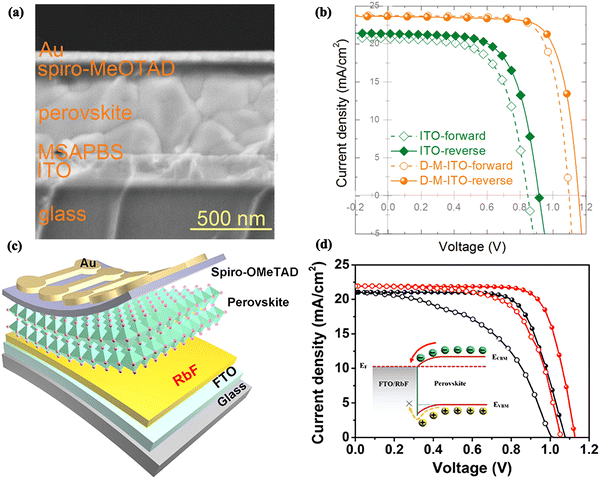 | ||
| Fig. 4 (a) Cross-sectional SEM image and (b) J–V curves of PSCs for the bottom electrode/perovskite interface of normal structural PSCs based on MSAPBS. Reproduced with permission from ref. 56. Copyright 2020 Wiley. (c) Device structure diagram and (d) J–V curves of PSCs for the bottom electrode/perovskite interface of normal structural PSCs based on RbF. Reproduced with permission from ref. 57. Copyright 2020 Elsevier. | ||
3.2 ETL/perovskite interface
As one of the key interfaces in PSCs, energy-level matching between the ETL and the perovskite layer is crucial for improving the carrier extraction and collection efficiency, as well as the Voc of the device. Too large a difference between the ETL and perovskite layer energy bands will lead to inefficient electron injection into the ETL and Voc reduction. However, a small energy band difference between the ETL and the perovskite layer will also cause ineffective separation of the photogenerated electrons and holes, resulting in significant recombination of the electron–hole pairs.58 Commonly used ETL materials (e.g., TiO2, ZnO, SnO2, etc.) and the conduction band minimum (CBM) of general perovskite materials have energy-level-matching issues, which are not conducive to efficient electron extraction and transport, and thus impact the performance of the cells.59 By altering the interface between the ETL and the perovskite layer, the energy-level structure can be matched to promote the separation and collection of photogenerated charge carriers, preventing the electrons from recombining with the holes.High-performance PSCs usually require a flat, uniform, dense, and well-crystallized film of the light-absorbing layer as well as the transmission layer. For example, TiO2 is a commonly used ETL material, but there are many oxygen vacancies and defect states on its surface, and the photogenerated electrons will not be able to form photocurrents due to severe recombination with holes, leading to significant performance degradation.60 It is widely known that when perovskite thin films are prepared using a fast crystallization technique, defects on the material surface or at the grain boundaries frequently arise, which will enhance non-radiative recombination thus reducing the charge carrier lifetime, causing energy loss and affecting the efficiency of the cells. Furthermore, defects not only promote water–oxygen penetration and accelerate perovskite decomposition but also provide a major pathway for ion migration. Therefore, it is imperative to reduce defects to form a high-quality thin film.28,31,61 Therefore, interfacial engineering is important to reduce the photodegradation of perovskite layers caused by external factors (e.g., water, oxygen, and light) and internal factors (e.g., ion migration, electron migration, and interfacial reactions) to obtain efficient and stable PSCs. In the following subsections, we will elaborate on the classification according to the type of material chosen, which are mainly two-dimensional materials, fullerenes and derivatives, quantum dots, self-assembled molecules, inorganic salts and organic materials.
 | ||
| Fig. 5 Two-dimensional materials for the ETL/perovskite interface of normal structural PSCs. (a)–(d) Statistics of the photovoltaic parameters based on pristine and different post-processing of MXene. Reproduced with permission from ref. 66. Copyright 2019 Springer Nature. (e) J–V curves of PSCs based on SnO2 and SnO2/GDY. Reproduced with permission from ref. 68. Copyright 2020 Wiley. (f) J–V curves of PSCs based on SnO2 and SnO2/GDYO. Reproduced with permission from ref. 69. Copyright 2021 The Royal Society of Chemistry. | ||
As a new all-carbon nanostructured material after fullerenes, carbon nanotubes, and graphene, graphdiyne (GDY) is gradually being applied in the field of optoelectronic semiconductors because of its favorable charge transport capabilities and excellent semiconductor properties.67 Zhang et al. utilized GDY to effectively increase the power-level matching between the SnO2 ETL and the perovskite layer, resulting in a 4-fold increase in electron mobility.68 High-quality perovskite films with low defect density were also formed through interfacial modification using GDY. Systematic DFT studies further explained that the underlying mechanism is the facilitation of charge extraction and transport through the coupling of GDY and SnO2. By modifying the SnO2/perovskite interfaces with GDY, the final device obtained a 21.11% efficiency with negligible hysteresis (Fig. 5e). Similarly, graphdiyne oxide (GDYO) was also applied to modify the SnO2 ETL.69 GDYO can passivate the surface defects of SnO2 to enhance electron transport while inhibiting non-radiative recombination due to the content of hydrophilic carboxyl and hydroxyl functional groups that can establish chemical interactions with uncoordinated Sn. The WF of GDYO-modified SnO2 matched well with perovskite, leading to a higher PCE of 21.23% in the final device (Fig. 5f). After 24 days of holding at 80 °C, the unencapsulated device efficiency declined to 84%, and after 160 hours of continuous light illumination, it reduced to 71%.
 | ||
| Fig. 6 Fullerene (C60) and derivatives for ETL/perovskite interfaces of normal structured PSCs. (a) and (b) Photovoltaic parameters of C60-based PSCs. Reproduced with permission from ref. 70. Copyright 2020 American Chemical Society. (c) and (d) J–V curves of PSCs based on TiO2 and SnO2/NMBF-X monomers and dimers. Reproduced with permission from ref. 71. Copyright 2020 Wiley. (e) and (f) J–V curves of PSCs based on SnO2 and SnO2/DPC60 and measured curves of environmental stability. Reproduced with permission from ref. 72. Copyright 2019 Wiley. | ||
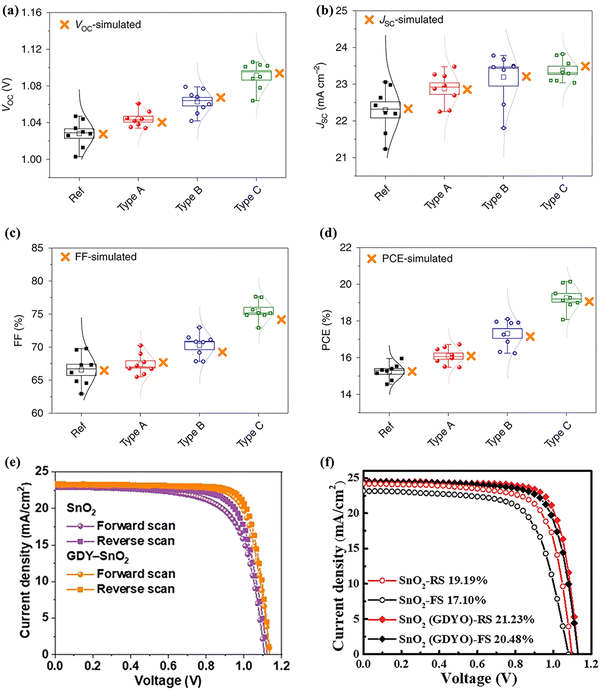
Novel fulleropyrrolidine (NMBF-X, where X = H or Cl) monomers and dimers were inserted between the metal oxide ETL and the perovskite layer to form an interfacial modification layer.71 The ETLs were TiO2 and SnO2, and the perovskite materials were chosen as MAPbI3 and (FAPbI3)x(MAPbBr3)1−x. After modifying the ETL/perovskite interfaces with fulleropyrrolidine, the device performance increased significantly (Fig. 6c and d). The chlorinated fullerene dimer was found to coordinate more effectively with metal oxides and perovskites through chloride terminations, resulting in excellent stability and an efficiency enhancement. This unencapsulated planar device achieved an efficiency of 22.3% without hysteresis. More importantly, the initial efficiency of over 98% was maintained after 1000 hours of environmental storage.
Similarly, a fullerene derivative with amine functional groups, i.e., 2,5-diphenyl C60 fulleropyrrolidine (DPC60), was introduced as an interfacial layer between perovskite and SnO2.72 Owing to its unique structure and functional groups, DPC60 can interact with the perovskite, passivating the interfacial defects of the perovskite layer. Meanwhile, better energy-level matching led to a substantial increase in electron extraction. Therefore, a PSC with the addition of DPC60 achieved a PCE of 20.4%, which is higher than the control device (18.8%) (Fig. 6e and f). In addition, the hydrophobic DPC60 layer significantly increased the stability, maintaining an initial efficiency of 82% after continuous exposure under one sun illumination and thermal aging (55 ± 5 °C) for 200 h.
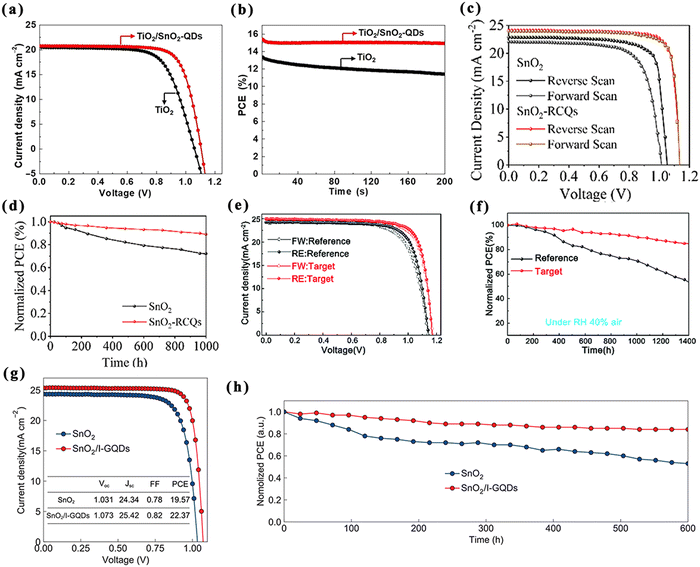 | ||
| Fig. 7 QDs for the ETL/perovskite interfaces of normal structural PSCs. J–V curves (a) and stability curves (b) of PSCs based on pristine and SnO2-QDs. Reproduced with permission from ref. 73. Copyright 2019 Elsevier. J–V curves (c) and stability curves (d) of PSCs based on SnO2 and SnO2-RCQs. Reproduced with permission from ref. 74. Copyright 2020 Wiley. J–V curves (e) and stability curves (f) of PSCs based on SnO2 and SnO2/MQDs. Reproduced with permission from ref. 75. Copyright 2021 The Royal Society of Chemistry. J–V curves (g) and stability curves (h) of PSCs based on SnO2 and SnO2/I-GQDs. Reproduced with permission from ref. 76. Copyright 2021 Wiley. | ||
A recent study showed that MXene quantum dot-modified SnO2 can modulate the crystallization process of the perovskite for preparing effective and reliable PSCs, and the crystallization kinetics were explored for the first time using a synchrotron-based 2D grazing incidence X-ray diffraction technique.75 It was found that titanium carbide (Ti3C2)-MXene quantum dot-modified SnO2 (MQDs-SnO2) could rapidly induce perovskite nucleation in the precursor solutions and form intermediate perovskite phases after antisolvent treatment, thus improving the crystalline quality and phase stability of the perovskite films. The optimal performance of the corresponding PSCs reached 23.3% with excellent moisture resistance and photostability (Fig. 7e and f).
Shortly thereafter, Gao et al. proved the role of imidazole bromide functionalized graphene quantum dots (I-GQDs) in regulating the ETL and the perovskite layer (FAPbI3).76 Better energy-band docking can be achieved via modification of the I-GQDs for reducing charge accumulation and promoting electron transport. Furthermore, the surface functional groups of the I-GQDs facilitated the formation of high-quality perovskite films to decrease the non-radiative recombination within the films. Furthermore, the material can enhance the stability of PSCs without modifying the band gap of the FA-based perovskite layer due to strong interactions between the imidazole moiety at the surface of I-GQDs and the perovskite. As a result, an improved PCE of 22.37% was achieved, and better long-term stability was obtained (Fig. 7g and h).
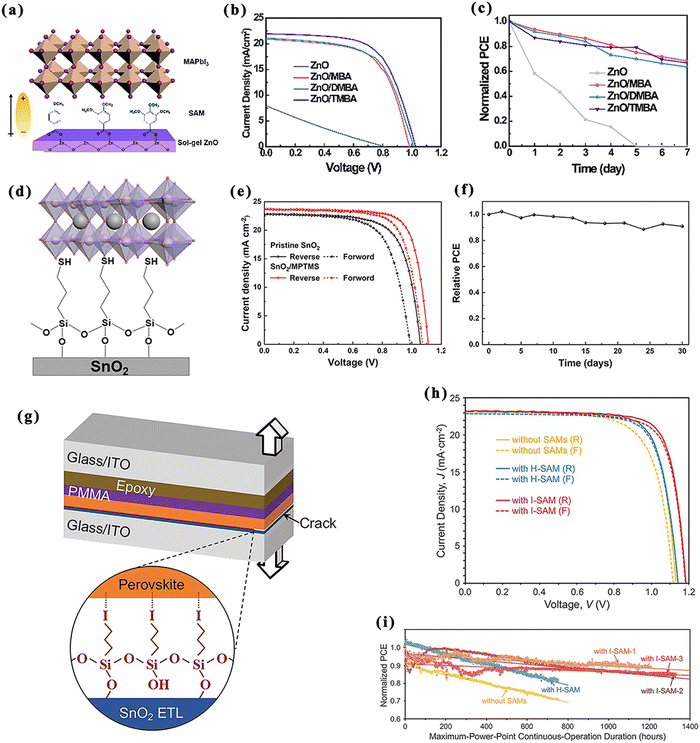 | ||
| Fig. 8 SAMs for ETL/perovskite interfaces of normal structural PSCs. Schematic diagram of the structure of (a) MBA (left), DMBA (middle), and TMBA (right), (d) MPTMS, and (g) I-SAM. J–V curves ((b), (e) and (h)) and stability curves ((c), (f) and (i)) of PSCs based on ZnO, ZnO/MBA, ZnO/DMBA and ZnO/TMBA (b) and (c), pristine ETL SnO2 and SnO2/MPTMS (e) and (f), and pristine ETL SnO2 and with ETL H-SAM or I-SAM (h) and (i). (a)–(c) Reproduced with permission from ref. 80. Copyright 2020 The Royal Society of Chemistry. (d)–(f) Reproduced with permission from ref. 81. Copyright 2021 Wiley. (g)–(i) Reproduced with permission from ref. 82. Copyright 2021 The American Association for the Advancement of Science. | ||
An iodine-terminated self-assembled molecule (I-SAM, i.e., [Si(OCH3)3(CH2)3I]) was adopted as the interfacial modifier, while [Si(OCH3)3(CH2)3H] was applied as the control molecule to investigate the role of iodine as an end-group atom (Fig. 8g).82 The silane structure in this molecule can be modified well at the interface as a SAM layer structure, while eliminating the –OH groups at the interface by reacting with them. This resulted in a 50% increase in the mechanical bond strength of the ETL/perovskite compared with [Si(OCH3)3(CH2)3H]. The efficiency of the device modified using the I-SAM increased from 20.2% to 21.4%. In addition, the results for the long-term operating stability of the PSCs at the maximum efficiency point proved that its T80 (lifetime to maintain 80% of the initial efficiency) was projected to increase from 700 h to 4000 h (Fig. 8h and i).
Vacancy defects can lead to the occurrence of ion migration. By inducing a local chemical doping effect, the Fermi energy level in the ion aggregation zone moves away from the conduction/valence band as a result of ion migration, causing a bending of the energy level, which affects the separation, transport and extraction of carriers, ultimately degrading the performance of the device. I− has been proved to have the shortest migration path in the MAPbI3 perovskite, while Pb2+ is difficult to migrate.85,86 NdCl3 as an interfacial modification layer was systematically studied.87 It demonstrated that the NdCl3 interfacial layer (NdCl3-IL) fills the I− vacancies resulting in a reduction of the vacancy and an increase in the activation energy, which inhibits the migration of I− from the top surface to the rear interface, thus reducing the trap density (Fig. 9a). The device modified using this interfacial layer obtained a PCE of 22.16% and a higher Voc with a negligible hysteresis effect. The stability of the device was also improved, maintaining 83% of the initial performance after 100 hours of the maximum power point tracking test. Notably, the method in combination with the 2D/3D passivation strategy was applied to a large-area (1cm2) device, obtaining a certified efficiency of 21.68%.
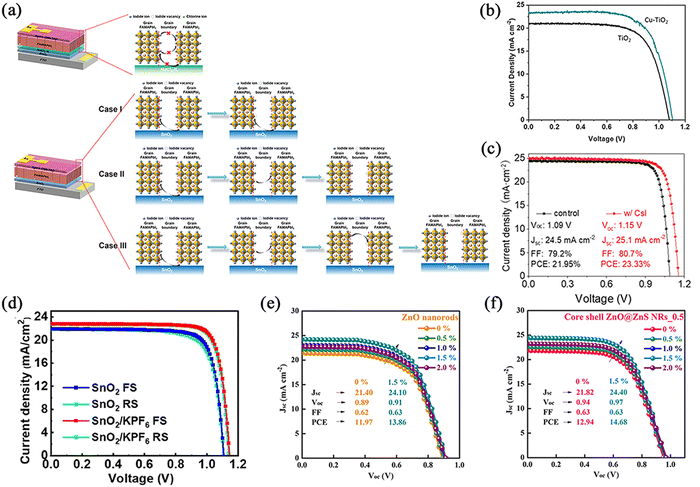 | ||
| Fig. 9 Inorganic salts for ETL/perovskite interfaces of normal structural PSCs. (a) Diagram of I− migration in control and NdCl3-IL-based PSCs. Reproduced with permission from ref. 87. Copyright 2021 Wiley. (b) J–V curves of PSCs based on TiO2 and Cu–TiO2. Reproduced with permission from ref. 88. Copyright 2021 Elsevier. (c) J–V curves of PSCs based on SnO2 and SnO2/CsI. Reproduced with permission from ref. 89. Copyright 2021 Wiley. (d) J–V curves of PSCs based on SnO2 and SnO2-KPF6. Reproduced with permission from ref. 90. Copyright 2021 Elsevier. (e) and (f) J–V curves of PSCs based on ZnO (e) and ZnO–ZnS (f) on flexible substrates. Reproduced with permission from ref. 92. Copyright 2021 Elsevier. | ||
Monovalent Cu cations (Cu+) were also utilized to solve the problems of low electron mobility, poor electrical conductivity, and the possibility of acting as a photocatalyst for chemical reactions in TiO2.88 The uniform distribution of Cu+ not only increased the extraction rate of photogenerated carriers but also reduced the surface trap states, thus improving the device performance. The final obtained planar PSCs achieved a PCE of 18.15%, which was better than 15.78% for the blank device (Fig. 9b). To verify the generalizability, the method was applied to mesoporous structures, and the experimental results proved the same modification capability. Notably, aging tests as well as stability experiments demonstrated that the Cu-modified device exhibited superior long-term stability.
Another effective strategy was to utilize CsI-doped SnO2, thus effectively improving the SnO2/perovskite interfacial quality of the PSCs.89 The presence of CsI during the formation of perovskite films passivated the interfacial imperfections. In addition, the gradient distribution of Cs+ contributed to a more acceptable energy-band alignment of SnO2 with the perovskite. The resistance of FAPbI3-based PSCs to UV irradiation was enhanced after doping with Cs+, achieving an excellent efficiency of up to 23.3% (Fig. 9c).
The importance of KPF6 for modification of the SnO2/perovskite interface was also demonstrated.90 It was found that PF6− remained at the interface while most of the K+ ions diffused into the perovskite. In addition, PF6− formed hydrogen bonds with organic cations in the perovskite component, and coordination bonds with SnO2, which can effectively passivate interfacial defects and release interfacial stresses. The PCE of the device obtained using KPF6 was increased from 19.66% to 21.39%, and the unencapsulated device maintained its initial efficiency of 80.1% after 960 hours of aging at 60 °C (Fig. 9d). Similarly, multifunctional potassium sulfate (K2SO4) was also used as an interface modification material for SnO2/perovskite.91 K2SO4 achieved the goals of both defect passivation and optimized energy-band alignment owing to the synergistic interfacial and internal modification of the perovskite. With the modification of K2SO4, the photovoltaic performance was increased from 19.45% to 21.18%. Moreover, the initial efficiency values of the modified PSCs can be maintained above 85% after storage at 25% relative humidity for 1000 hours.
More recently, interfacial engineering has been continuously devoted to the study of wearable and portable flexible PSCs. Fahim et al. explored the modification process after the ZnO surface was converted to ZnS.92 The bifunctional interfacial layer reduces the hydroxyl (–OH) groups on the ZnO surface, and passivates the ZnO/perovskite interface, thereby improving the stability. It also modulated the energy level for effective electron transport through the strain-induced piezoelectric effect (Zn–S–Pb pathway). The piezoelectric effect regulates the energy band structure through the internal piezoelectric potential generated by the piezoelectric semiconductor under pressure, controlling the separation, transport and recombination of excitons.93,94 As a result, the efficiency of the flexible PSC was dramatically improved (Fig. 9e and f).
In another study, Sun et al. employed the amphiphilic linker molecule p-aminobenzenesulfonic acid (ABSA) at the interface of the SnO2/perovskite.96 The authors explained the mechanism of the interfacial modification in terms of energy-band bending. Both experimental and theoretical studies comprehensively demonstrated that the sulfonic acid group of ABSA can modify the SnO2, thus reducing the energy-band potential barrier on the surface of the ETL and enhancing the charge transport. The alkaline amino groups were used to passivate deep-energy-level defects on the perovskite surface and reduced non-radiative recombination. The PCE of MAPbI3-based PSCs modified with this material was significantly improved from 18.02% to 20.32% with better long-term stability.
Although fullerenes and their derivatives dominate the organic photovoltaic field due to their unique structure and other advantages, the material itself has some insurmountable drawbacks, however, such as a low absorption in the visible range, difficulties with modification, and high cost, all of which restrict the device performance and wide-scale use. In recent years, the emergence of non-fullerene-type small-molecule materials, especially the Y series of non-fullerenes (Y-NFAs; mainly Y1–Y6), has attracted increasing attention from the scientific community.97 For example, Lou et al. designed the Y-series derivative BTAC4 to modify the SnO2 layer via side-chain engineering.98 An efficiency of more than 23% was achieved. By exploring the interaction between the π-conjugated n-type small molecules and the SnO2/perovskite interfaces, the team revealed the outstanding interfacial modification effect of organic passivators with a more compact and ordered molecular filling ability.
In the same year, Kong et al. demonstrated that doping EDTA-2M molecules (M = K, Rb, or Cs) with SnO2 colloids improved the electrical and surface properties of the SnO2 films, resulting in a substantial increase in electron mobility.99 In addition, EDTA-2M molecules can successfully passivate the surface imperfections of SnO2 and improve its crystalline quality by promoting perovskite crystallization. The PCE of the device with perovskite band gaps of 1.69 eV and 1.57 eV, respectively, reached up to 23.30%. Unencapsulated PSCs on the basis of EDTA-modified SnO2 were maintained at 95% of their initial performance after exposure to an ambient atmosphere for 1200 h.
Shortly thereafter, (2-carboxyethyl)(dimethyl)sulfonium chloride (CDSC) and 3-(dimethylamino)propionic acid hydrochloride (DPAH) (reference molecule) were employed to modify the SnO2/perovskite interfaces.100 It was theoretically and experimentally proved that the CDSC and DPAH molecules are capable of strong chemical interactions with both SnO2 and the perovskite layer, thus linking the two through chemical bonding and improving interfacial contacts. Both salt molecules were able to effectively passivate defects from the surface of the perovskite and the ETL, and the CDSC-modified device achieved a higher PCE (22.22%) as well as better stability. The structures of the small organic molecules used for normal structural PSCs are shown in Fig. 10.
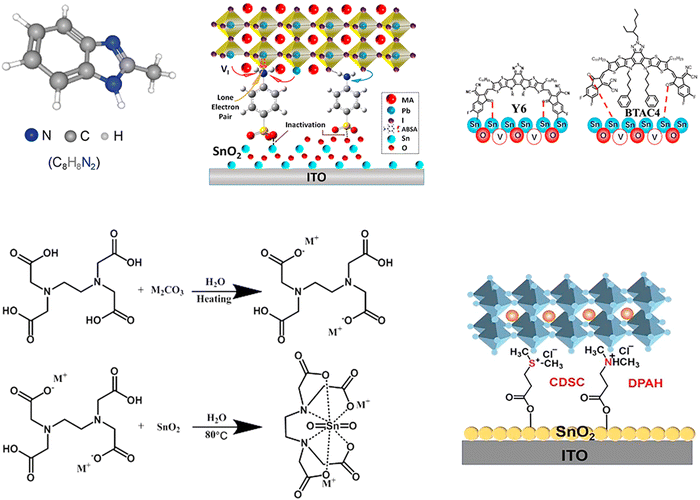 | ||
| Fig. 10 Structure diagram of various organic molecules for the ETL/perovskite interface of normal structural PSCs. Reproduced with permission from ref. 95–98 and 100. Copyrights 2020, 2021, 2022 Elsevier, 2021 American Chemical Society, and 2021 Wiley. | ||
To address the severe charge-trap problem in perovskite films, 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4) and triphenylphosphine oxide (TPPO) were synergistically applied to PSCs.101 [BMIM]BF4 formed an interfacial dipole layer that reduced the surface WF of the ETL and filled the organic/Cs cation vacancies. The P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the TPPO molecule interacts with the uncoordinated Pb2+, which reduced the defect states in the perovskite films and reduced the non-radiative recombination. As a result, the PCE of the PSC increased from 18.7% to 21.1% with long-term stability, even under dry air conditions (about 22% relative humidity at 25 °C).
O bond in the TPPO molecule interacts with the uncoordinated Pb2+, which reduced the defect states in the perovskite films and reduced the non-radiative recombination. As a result, the PCE of the PSC increased from 18.7% to 21.1% with long-term stability, even under dry air conditions (about 22% relative humidity at 25 °C).
ZnO possesses excellent optoelectronic properties, it can be prepared at low temperatures, and it has a higher electron mobility than TiO2, thus attracting much attention.102,103 However, since ZnO is an amphoteric oxide with an extremely unstable surface, it tends to react with MAI for the preparation of perovskites, leading to an increment in interfacial defects in the perovskite layer, which increases the carrier recombination and induces degradation of the optoelectronic performance of the device.104 Methylammonium iodide can be used to solve this problem by modifying the ETL.105 It was shown that methylammonium iodide modification can effectively block the reaction between ZnO and the perovskite layers. The obtained PSCs exhibited excellent thermal stability, and retained 82% of the initial efficiency after aging at 50 mW cm−2 of white light and 65 °C for 2100 hours, which is the highest known performance.
Adhesion of the weakly alkaline precursor of biguanide hydrochloride (BGCl) on the surface of the tin oxide substrate can significantly improve the denseness and uniformity of the tin oxide layer.106 At the same time, the amino group in BGCl can undergo Lewis coordination with Sn4+ to achieve n-type doping of tin oxide, while the Cl− effectively passivates the oxygen vacancy defects in the surface layer through electrostatic coupling. In addition, the amino and ammonium functional groups enriched with BGCl can effectively anchor I− in the solution of the perovskite precursor, ensuring the growth of the perovskite with an ordered array arrangement and a vertical crystallographic orientation. Therefore, carrier extraction at the interface was significantly enhanced, achieving better carrier transport inside the device. The prepared devices achieved up to 24.4% certified PCE and long-term stability.
It is worth noting that organic interfacial layers can also be applied to flexible PSCs. You et al. designed a biological polymer (heparin potassium, HP) as an SnO2–HP composite layer to enhance the interfacial contact between the ETL and the perovskite layer.107 On the one hand, owing to the interaction between the Sn atoms of SnO2 and the O atoms of –COO–, HP is able to adhere to the surface of the SnO2 nanocrystals. On the other hand, the polymerization chain of HP could attenuate the interaction between the adjacent SnO2 nanocrystals and promote their uniform dispersion. Meanwhile, because of the larger domains and higher surface free energy of the SnO2–HP layer, the perovskite may undergo more uniform crystal nucleation, which leads to better growth of the perovskite crystals. The SnO2–HP-based device exhibited a highly reproducible average efficiency of 23.03% and excellent operating stability on the rigid substrate. More importantly, the average efficiency of the HP-modified SnO2 ETL on flexible substrates is 19.47%, showing excellent potential for flexible and large-area device studies.
Xiong et al. reported poly(ethylene glycol) diacrylate (PEGDA) as a polymer backbone for modifying SnO2.108 The ETL treated with this material showed a significant impact on both the device performance and stability. On the one hand, more homogeneous and energy-level-aligned interfaces were achieved, increasing the electrical conductivity. On the other hand, PEGDA can passivate the defects at the ETL/perovskite interfaces. The final device achieved a champion photovoltaic performance of 23.31% with excellent stability. Typical modification materials that can enhance both the device performance and stability in this interface are screened and summarized in Table 1.
| Modification materials | Perovskite | Main roles | PCE [%] | Stability | Ref. | |
|---|---|---|---|---|---|---|
| Two-dimensional materials | GDYO | (FAPbI3)1−x(MAPbBr3)x | Facilitate electron transport, suppresses non-radiative recombination | 21.23 | 84% of initial efficiency after 24 d at 80 °C | 69 |
| Fullerene (C60) and derivatives | NMBF-X | (FAPbI3)x(MAPbBr3)1−x | Better coordination with ETL and perovskite | 22.3 | Over 98% of initial efficiency after 1000 h of storage | 71 |
| DPC60 | FA0.81MA0.10Cs0.04PbI2.55Br0.40 | Passivate interface defects, improve electron extraction | 20.4 | 82% of initial efficiency after 200 h of continuous sunlight exposure and thermal aging (55 ± 5 °C) | 72 | |
| Quantum dots (QDs) | RCQs | Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | Improve electromigration | 22.77 | 95% of initial efficiency at 25 °C and 40–60% air humidity | 74 |
| MQDs | FA0.9MA0.05Cs0.05PbI0.98Br0.02 | Improve crystalline quality and phase stability of perovskite films | 23.3 | Over 90% of initial efficiency after 500 h of continuous light exposure | 75 | |
| I-GQDs | FAPbI3 | Improve energy-level matching and quality of perovskite films | 22.37% | 84% of initial efficiency after 600 h | 76 | |
| Self-assembled molecules (SAMs) | I-SAM | Cs0.05(FA0.85MA0.15)0.95Pb(I0.85Br0.15)3 | Increase the mechanical bonding strength of the interface | 21.4 | Projected T80 from 700 h to 4000 h | 82 |
| Inorganic salts | NdCl3 | FA1−xMAxPbI3 | Inhibit the migration of I− | 22.16 | 83% of initial efficiency after 100 h of maximum power point tracking testing | 87 |
| Cu1+ | MAPbI3 | Improve extraction of carriers, reduce surface trap states | 18.15 | 53% of the initial efficiency under aging test conditions, 91% of initial efficiency under light stabilization experimental conditions | 88 | |
| KPF6 | Rb0.05(FA0.95MA0.05)0.95PbI2.85Br0.15 | Improvement of perovskite film quality, passivate interface defects, release interfacial stresses | 21.39 | 80.1% of initial efficiency after 960 h of aging at 60 °C and 57.2% after 960 h of exposure to 1 Sun illumination | 90 | |
| Organic materials | MBIm | Cs0.05(MA0.15FA0.85)0.95Pb(I0.85Br0.15)3 | Optimize energy-level matching, passivate interface defects, reduce non-radiative recombination | 21.6 | 85% and 90% of initial efficiency under high-humidity and continuous-light conditions | 95 |
| ABSA | MAPbI3 | Enhance charge transport, passivate defects, reduce non-radiative recombination | 20.32 | 56.7% of the initial efficiency after 30 d in the atmosphere | 96 | |
| EDTA-2M | FA0.95MA0.05Pb(I0.95Br0.05)3 | Passivate interface defects and improve the crystalline quality of perovskite | 23.30 | 95% of initial efficiency after 1200 h in the atmosphere | 99 | |
| CDSC | Rb0.02(FA0.95Cs0.05)0.98PbI2.91Br0.03Cl0.06 | Passivate interface defects | 22.22 | 92.5% of initial efficiency after aging at 60 °C for 1272 h | 100 | |
| [BMIM]BF4 and TPPO | Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | Reduce surface WF of ETL and non-radiative recombination | 21.1 | 98.3% of initial efficiency after 214 d under dry air conditions | 101 | |
| BGCl | (FAPbI3)x(MAPbI3)y | Enhance extraction and transport of carriers | 24.4 | 95% of initial efficiency with over 500 h of aging | 106 | |
| HP | Cs0.05FA0.85MA0.10Pb(I0.97Br0.03)3 | Improve the growth of perovskite crystals, promote the uniform dispersion of SnO2 | 23.03 | 97% of the initial efficiency after 1000 h under 1 Sun illumination | 107 | |
| PEGDA | FAPbI3 | Increase electrical conductivity, passivate interface defects | 23.31 | Over 90% of initial efficiency with 850 h of continuous lighting | 108 | |
3.3 Perovskite/HTL interface
Similar to the ETL, it is vital to establish effective hole injection and stable transport in order to generate high-efficiency PSCs. Too large a gap between the HTL and the energy band of the perovskite layer can lead to inefficient hole injection into the HTL and a decrease in Voc.109,110 In addition, there are a large number of defect sites in the perovskite/HTL interface, and these defect sites can capture carriers and cause carrier loss due to recombination.111 Moreover, these defect sites will become adsorption sites for water oxygen, which will accelerate the decomposition and degradation of the perovskite film and cause rapid degradation of the performance of the PSCs.112 Optimization at the perovskite/HTL interface is essential to passivate the defects and obtain high-quality films.59,113 Since the existing hole materials do not fully satisfy the objectives of energy-level alignment, enhanced charge dynamics, trap passivation, and reduced ion migration, interfacial modification has been developed as an effective method for the perovskite/HTL interface.It has recently been reported that the modification effect of the 2D structure is affected by the length of its alkyl chain.115 Furthermore, the surface defects of the 2D perovskite films themselves may also be related to the interaction of their structures with the HTL. In this study, a 3D perovskite was treated with 2D structures of butylammonium iodide (BAI), octylammonium iodide (OAI) and dodecylammonium iodide (DAI) with different alkyl chain lengths, and the PCE and humidity stability of the devices were compared. The results revealed a significant improvement in the electron-blocking ability and humidity resistance as the alkyl chain length was increased from BA to OA to DA. The OAI-treated PSC obtained a champion PCE of 22.9% in comparison with the other two materials.
Similarly, Liu et al. focused on an interfacial passivation strategy of a 2D perovskite.116 Four structured aromatic organic cations without halogen functional groups were designed to form low-dimensional perovskite (LDP) passivation layers, namely benzylammonium (BA), 4-fluorobenzylammonium (FBA), 4-chlorobenzylammonium (CBA) and 4-bromobenzylammonium (BBA), with their respective modification effects being investigated (Fig. 11a and b). The various halogenated LDP passivation layers exhibited different effects due to their unique crystal parameters and chemical properties. The experimental results demonstrate that all four materials can reduce the non-radiative recombination loss and trap states. Remarkably, the device exhibits significantly enhanced long-term stability under different aging conditions due to the good hydrophobicity of the halogenated LDP and its ability to inhibit organic molecules and iodide ions from diffusing.
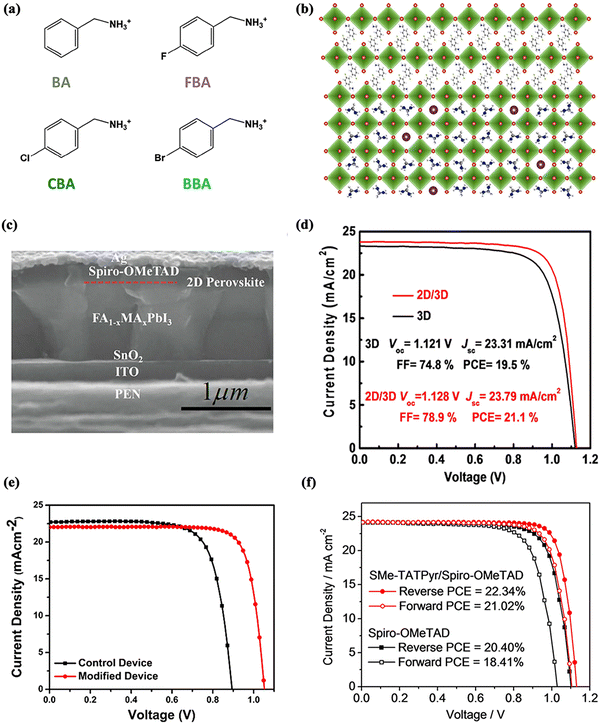 | ||
| Fig. 11 Two-dimensional (2D) materials for perovskite/HTL interfaces of normal structural PSCs. (a) Schematic diagrams of the structure of BA and its halogenated derivatives (FBA, CBA and BBA) and (b) crystal diagram of the BA-based 2D perovskite layer on 3D perovskite. Reproduced with permission from ref. 116. Copyright 2020 Elsevier. (c) Cross-sectional SEM image and (d) J–V curves of PSCs based on the 2D perovskite structure. Reproduced with permission from ref. 118. Copyright 2021 Wiley. (e) J–V curves of PSCs based on pristine and MoS2-modified devices. Reproduced with permission from ref. 119. Copyright 2020 Elsevier. (f) J–V curves of PSCs based on Spiro-OMeTAD and Spiro-OMeTAD/SMe-TATPyr. Reproduced with permission from ref. 120. Copyright 2021 Elsevier. | ||
The 2D PEA2PbI4 layer was found to be used as a passivation layer on 3D perovskite films by Chen et al.117 This further tuned the Fermi energy level, significantly reduced the trap density on the 3D perovskite surface, and suppressed non-radiative recombination. Final PSCs with an efficiency of 18.51% were obtained. More importantly, the device could maintain an initial efficiency of nearly 90% after 1000 h under high humidity ambient conditions of 60 ± 10%.
In contrast to the above research directions, Long et al. worked out the effect of a 2D perovskite on flexible PSCs.118 They introduced a 2D perovskite formed from 4-trifluoromethylphenylethylamine iodide (CF3PEAI) on the surface of a 3D perovskite. The 2D perovskite not only improved the energy-level alignment between the HTL and the perovskite layer but also reduced the trap density in the 3D perovskite, lowering the energy loss and carrier recombination at the interface and promoting hole transfer from the perovskite to the HTL. The flexible PSCs prepared after the modification achieved an outstanding efficiency of 21.1% with a certified PCE of 20.5% (Fig. 11c and d). Notably, the device showed enhanced long-term stability due to the supportive impact of the 2D perovskite layer on the underlying 3D perovskite.
In 2020, 2D-MoS2 was applied to the perovskite/HTL interface.119 Insertion of this material as an interfacial layer can regulate the energy-level alignment of the HTL and the perovskite, which can suppress interfacial charge accumulation and increase the charge extraction rate. Moreover, the defect state was reduced, and the degradation of the active layer was improved. Ultimately, the efficiency of the device structure based on FTO/c-TiO2/SnO2 QD/Cs0.1FAPbI3(0.81) (MAPbBr3(0.09))/MoS2/PTAA/Au reached an efficiency of 18.54% compared with 15.05% for the control device without the MoS2 layer (Fig. 11e).
Recent studies have shown that sulfur atoms can act as Lewis bases and thus effectively passivate uncoordinated Pb2+ deficiencies in perovskites. With this in mind, Li et al. designed and synthesized a class of sulfur-rich 2D conjugated small molecules (SMe-TATPyr).120 After introducing an ultrathin layer of SMe-TATPyr between the perovskite layer and the HTL, they found that SMe-TATPyr matched better with the valence band of the perovskite, so it can effectively reduce the energy loss and improve the Voc of the device. Simultaneously, the S atoms in SMe-TATPyr can passivate the uncoordinated Pb2+ defects in the perovskite through Pb–S interactions, thus reducing the interfacial defect recombination. The PCE of the device increased significantly from 20.4% to 22.3%, and 95% of the initial efficiency was preserved after 1500 h of storage under ambient conditions (Fig. 11f).
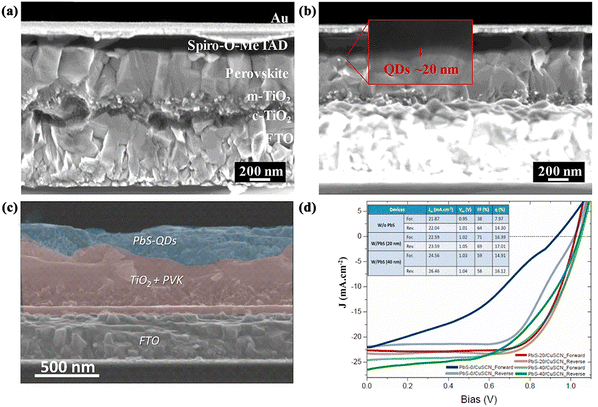 | ||
| Fig. 12 Quantum dots (QDs) for perovskite/HTL interfaces of normal structural PSCs. Cross-sectional SEM images of PSCs based on (a) an unmodified perovskite surface and (b) the perovskite layer modified with PQDs. Reproduced with permission from ref. 121. Copyright 2019 Elsevier. (c) Cross-sectional SEM image of PSC modified with PbS-QDs, and (d) J–V curves of pristine and PbS-QD-modified PSCs. Reproduced with permission from ref. 127. Copyright 2021 Elsevier. | ||
PbS-QDs with a broad light absorption and outstanding hole transport ability have also been employed and investigated between the perovskite layer and the HTL. The valence band of PbS-QDs is in the range of −(5.0 to 5.1) eV, which matches the valence band of MAPbI3. Zhu et al. synthesized lead sulfide QDs and dynamically spin coated them onto perovskite films using the reported method.126 The incorporation of PbS-QDs enhanced the hole extraction rate and reduced carrier recombination. Furthermore, the morphology of the perovskite film is significantly improved as a result. Ultimately, the device obtained a PCE of 19.24%, which was better than that of the unmodified device. Similarly, Ka et al. reported the application of PbS-QDs as an interface modification layer for the HTL and the perovskite/HTL interface, respectively, where CuSCN was used as the HTL material instead of the expensive organic HTL.127 The PbS-QDs were grown directly on the perovskite films using physical deposition, and two devices were subsequently prepared. They found that the PbS-QDs provided a beneficial passivation effect on the perovskite films, enabling better charge separation and collection. The experimental results demonstrated that PbS-QDs exhibited the best performance of 17% when used as an intermediate layer between the perovskite and the CuSCN films (Fig. 12c and d). In particular, the presence of PbS-QDs greatly improved the stability of the device and avoided hysteresis effects.
As mentioned earlier, CuSCN has commonly been considered by researchers as a replacement for expensive organic materials such as spiro-OMeTAD due to its low cost and superior hole mobility and stability, but devices prepared using CuSCN often suffer from Voc loss.128 Based on this, organic functional molecules were added to the surface of the perovskite layer to passivate imperfections and improve interfacial contacts.129 The researchers looked for potential functional groups with a high CuSCN-binding affinity among several pyridine derivatives and found the best functional molecules that bind to both perovskite and CuSCN (Fig. 13a). Ultimately, CuSCN-based devices were prepared using a mixture of 3-pyridyl isothiocyanate (Pr-ITC) and phenylene-1,4-diisothiocyanate (Ph-DITC) as the interfacial modification material and achieved an average PCE of 18.57%, which was very close to that of spiro-OMeTAD-based PSCs.
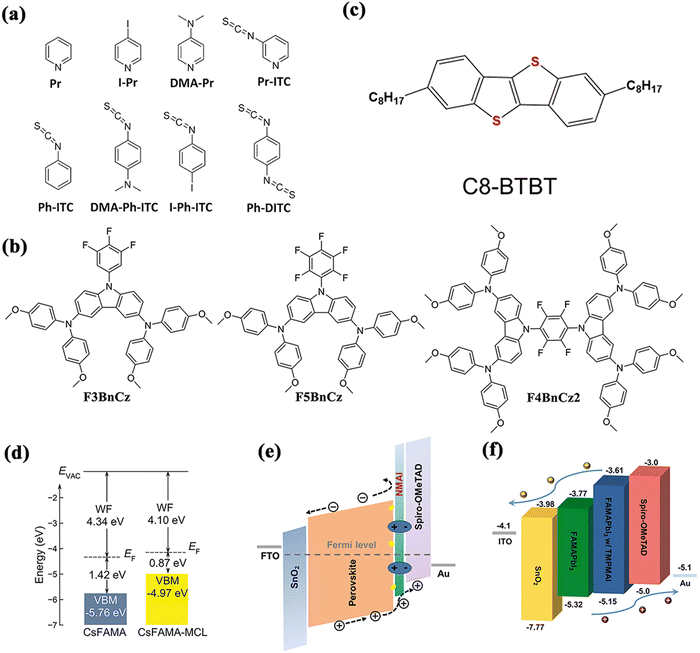 | ||
| Fig. 13 (a)–(c) Structure diagrams of various organic molecules used to modify the perovskite/HTL interface of normal structural PSCs. (a) Reproduced with permission from ref. 129. Copyright 2019 The Royal Society of Chemistry. (b) Reproduced with permission from ref. 130. Copyright 2020 The Royal Society of Chemistry. (c) Reproduced with permission from ref. 132. Copyright 2021 American Chemical Society. (d) Energy level diagram of PSCs based on MCL. Reproduced with permission from ref. 133. Copyright 2021 Wiley. (e) Energy level diagram of PSCs based on NMAI. Reproduced with permission from ref. 136. Copyright 2020 Wiley. (f) Energy level diagram of PSCs based on TMPMAI. Reproduced with permission from ref. 138. Copyright 2022 Elsevier. | ||
To improve the FF of PSCs during operation, Wang et al. synthesized three carbazole-based fluorinated small molecules, that is, F3BnCz, F5BnCz and F4BnCz2, and explored their effects on the perovskite/HTL interfaces (Fig. 13b).130 It was investigated that the use of these organic molecules as interfacial layers not only effectively prevented electron transport through holes in the material but also formed an improved contact between the perovskite and the HTL. As a result, the FF was increased from 75% to 80% without affecting the Jsc and Voc values, leading to an increase in the PCE from 18.99% to a maximum of 19.96%. In particular, the modified PSCs demonstrated high long-term stability due to the addition of fluorine atoms that make the interfacial layer highly hydrophobic.
Bearing in mind that interfacial organic molecular engineering has been unsatisfactory in improving the lifetime of PSCs, an interfacial engineering strategy using multifunctional ligands was reported, demonstrating that 2,5-thiophenanthrene carboxylic acid ligands can effectively passivate interfacial defects and significantly improve the stability of PSCs.131 This modification was ascribed to the interaction between the ligand and the perovskite layer at the nanomolecular/sub-nanomolecular level. This ligand improved various defects, inhibited interfacial reorganization, and reduced ion migration in multiple ways.
Most of the organic molecules used to passivate interfacial defects create an electrically insulating layer at the interface that hinders charge extraction. Zhao et al. introduced an interfacial engineering strategy using the highly crystalline small molecule 2,7-dioctyl[1]benzothieno[3,2-b][1]benzothiophene (C8-BTBT) (Fig. 13c). C8-BTBT interacts with the perovskite surface, thus reducing the interfacial defects.132 In addition, this strategy stabilized the interface by reducing the electrically insulating aggregates. Ultimately, the absolute efficiency of the C8-BTBT-modified device was increased by more than 2% and the heat resistance of the spiro-OMeTAD layer resulted in a significant improvement.
Also to solve this drawback, researchers explored the possibility of 2-(3′′′,4′-dimethyl-[2,2′:5′,′′:5′′,2′′′-quaterthiophen]-5-yl)ethan-1-ammonium iodide (4Tm) to modify the perovskite/HTL interface.133 It was found that the 4Tm molecular layer and the thin 2D perovskite (4Tm)2PbI4 layer combined to form a multifunctional capping layer (MCL) as a conjugated ligand between the perovskite and the HTL. This promoted charge transfer throughout the device by improving the energy-level alignment and stabilized the perovskite interface and lattice by inhibiting ion migration to reduce interfacial non-radiative recombination (Fig. 13d). The PCE of the MCL-based modified PSC was improved to 22.06% with long-term operational stability.
Currently, the passivation mechanism of most organic ammonium salts such as PEAI works by reacting with ions not bound to ligands or via the formation of a low-dimensional perovskite phase. For instance, cetyltrimethylammonium bromide (CTAB) was applied to the surface of the perovskite layer and subjected to a simple post-treatment.134 Two useful effects here were that the ammonium group in CTAB may anchor to the MAPbI3 film surface to passivate defects, and that the moisture stability was improved through the attachment of long carbon chains at the perovskite surface. The experimental results indicated that the champion photovoltaic performance of 18.95% and the steady-state output PCE of 18.11% were obtained by modifying the optimal CTAB concentration. Specifically, the CTAB-treated MAPbI3 device exhibited better operating stability than the control device under the same conditions.
Considering the importance of improving the thermal stability of PSCs, Salado et al. explored the effect of three different substituted thiazolium iodide (TMI) salts on the perovskite/HTL interface.135 Not only can TMI control the possible recombination paths at the perovskite/HTL interface but it can also reduce the density of shallow and deep traps due to the passivation of iodide vacancies in the lattice, thus increasing both Voc and FF. More importantly, unencapsulated devices utilizing TMI exhibited outstanding intrinsic and thermal stability due to the interaction between the thiazole salt and the perovskite surface.
In contrast to previous reports, Liang et al. investigated the interfacial modification of 1-naphthylmethylamine iodide (NMAI) and demonstrated an alternative passivation mechanism, namely chemical passivation.136 The NMAI, with a high formation energy, left NMA+ functional groups on the surface of the perovskite film, thus effectively inhibiting defect recombination. Simultaneously, the charge accumulation was controlled by inducing energy-level bending, which improved the minority carrier recombination due to charge blockage (Fig. 13e). The final prepared device revealed an efficiency of 21.04% and a Voc of up to 1.20 V as well as long-term stability.
Another unique strategy uses (Me-PDA)Pb2I6, which can form 3D ‘perovskitoid’ structures instead of a low-dimensional perovskite.137 This interfacial layer was induced to form a thin 3D perovskite-like structure acting at the perovskite/HTL interface. Compared with the commonly used 2D structure of (PEA)2PbI4, the device derived using (Me-PDA)Pb2I6 was characterized by a superior film quality, a longer carrier lifetime and a higher carrier mobility, together with a lower surface defect density. The device efficiency was thus significantly improved to 22.0% with long-term operational stability.
In 2022, a new organic ammonium salt, namely N,N,N-trimethyl phenylmethyl ammonium iodide (TMPMAI), was successfully designed and applied at the perovskite/HTL interface.138 This strategy not only passivated interfacial defects (mainly targeting Pb2+ as a deep defect in the perovskite) via interaction of the introduced benzene ring with the perovskite but also created a gradient energy distribution at the interfaces, thus effectively suppressing non-radiative recombination (Fig. 13f). Ultimately, the device based on TMPMAI modification obtained an outstanding PCE of 23.1% with long-term stability.
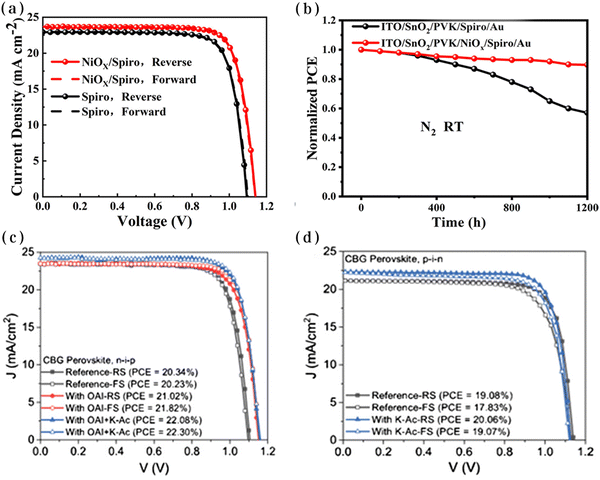 | ||
| Fig. 14 J–V curves (a) and stability curves (b) for the perovskite/HTL interfaces of normal structural PSCs based on pristine and NiOx-modified interfaces. Reproduced with permission from ref. 139. Copyright 2019 American Chemical Society. J–V curves for n–i–p (c) and p–i–n (d) structures of normal structural PSCs based on pristine and K-Ac-modified interfaces. Reproduced with permission from ref. 140. Copyright 2021 Wiley. | ||
A passivation strategy for the perovskite/HTL interface using potassium acetate (K-Ac) was presented.140 The unique K-Ac can be soluble in a wide range of solvents, enabling it to be used as a bottom and top interface layer. In n–i–p devices, this interfacial passivation layer improved the energy-band alignment of the perovskite/HTL interface, ultimately achieving a superior efficiency of 21.57% compared with the control devices. In particular, the potassium passivation layer in the inverted device changed the orientation of the perovskite particles and improved the charge transport, which also resulted in a more outstanding optoelectronic performance (Fig. 14c and d). The generalizability of this passivation strategy deserves continued attention from researchers.
In addition, other interface-modification techniques have been used to deposit the modification materials at the perovskite/HTL interface. All of these approaches showed promise and significant advantages for the commercial application of PSCs, although the process often requires significant time. Typical modification materials that can enhance both the device performance and the stability in the above interfaces are screened and summarized in Table 2.
| Modification materials | Perovskite | Main roles | PCE [%] | Stability | Ref. | |
|---|---|---|---|---|---|---|
| Two-dimensional materials | CF3PEAI | FA1−xMAxPbI3 | Reduce energy loss and carrier recombination at the interface | 20.5 | Over 86% of initial efficiency after 880 h of aging | 118 |
| SMe-TATPyr | FA1−xMAxPbI3 | Reduce energy loss and interface defect recombination | 22.3 | 95% initial efficiency for 1500 h of storage under ambient conditions | 120 | |
| Quantum dots (QDs) | PQDs (CsPbBrCl2) | CsPbBr1.85I1.15 | Fill defective sites, inhibit water penetration | 21.5 | 80% of initial efficiency after 500 h of continuous light exposure | 122 |
| PbS-QDs | MAPbI3−x−yClxBry | Passivate interface defects, improve charge separation and collection | 17 | Over 84% of initial efficiency under continuous sunlight exposure and storage in ambient air for over 30 d | 127 | |
| Small organic molecules and ammonium salts | F4BnCz2 | MAPbI3 | Prevent electrons from transporting material through holes | 19.96 | 80% of initial efficiency for 23 d of storage | 130 |
| 2,5-Thiophenanthrene carboxylic acid ligands | FA0.97MA0.03PbI2.91Br0.09 | Passivate interface defects, improve the stability of PSCs | 23.4 | 87% of initial efficiency after 1440 h | 131 | |
| 4Tm | Cs0.05(FA0.87MA0.13)0.95Pb(I0.87Br0.13)3 | Improve energy-level alignment, reduce interfacial non-radiative recombination | 22.06 | 85% of maximum efficiency after 700 h | 133 | |
| CTAB | MAPbI3 | Passivate defects, improve moisture stability | 18.11 | Over 60% of initial efficiency after 1800 m | 134 | |
| TMI | MAPbI3 | Control the possible reorganization paths on the interface, reduce the trap density | 18.93 | 95% of initial efficiency after 800 h | 135 | |
| NMAI | Cs0.05(MA0.15FA0.85)0.95Pb(I0.85Br0.15)3 | Suppress defects and minority carrier recombination | 21.04 | 98.9% of the initial efficiency after 3240 h | 136 | |
| (Me-PDA)Pb2I6 | (FAPbI3)0.85(MAPbI2Br)0.10(CsPbI3)0.05 | Improve film quality, carrier lifetime and carrier mobility, reduce surface defect density | 22.0 | 90% of the initial efficiency after 1008 h | 137 | |
| TMPMAI | FAMAPbI3 | Passivate interfacial defects, inhibit non-radiative recombination | 23.1 | Maintain initial efficiency after 96 d | 138 | |
| Other interface modification methods | NiOx | (FAPbI3)x(MAPbBr3)1−x | Reduce interfacial defect density, improve carrier extraction capability and energy-level alignment | 21.66 | 90% initial efficiency for 1200 h | 139 |
3.4 Dual interfacial modification engineering
In recent years, there has been an increasing number of researchers exploring the novel strategy of simultaneously modifying the ETL/perovskite and perovskite/HTL dual interfaces in order to improve the device performance more comprehensively. Either the same material is applied or different materials are modified separately, with similar approaches as described in the previous section. For example, organic cationic-based n-BAI compounds were induced to form 2D perovskite layers as double-side passivation layers.141 It was found that the double-side-modified layer effectively suppressed the non-radiative recombination on both sides of the active layer. The discontinuity of the passivation layer remained unaffected by the conductive channel between the perovskite and the CTL to the extent that effective charge extraction was maintained (Fig. 15a and b). The modified device revealed one of the highest Voc values of 1.20 V and a PCE of 22.60% with negligible photocurrent hysteresis.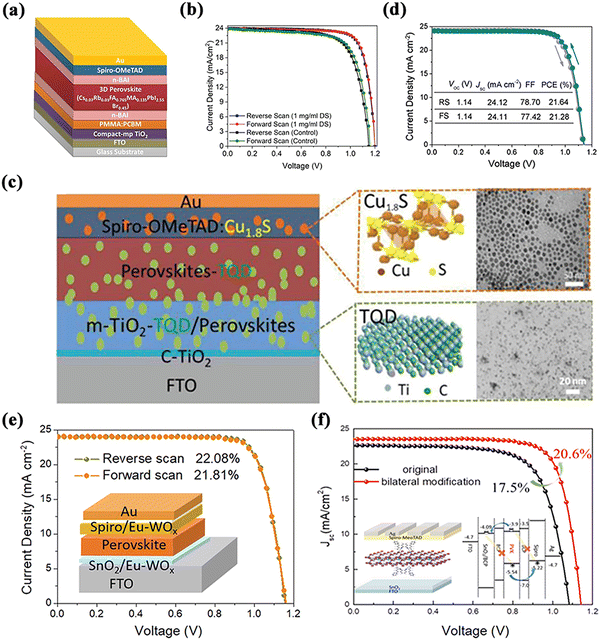 | ||
| Fig. 15 Schematic diagram of the structure of dual-interface-modified PSCs (a) and J–V curves (b) based on n-BAI. Reproduced with permission from ref. 141. Copyright 2019 Wiley. Modification with TQD and Cu1.8S (c) and J–V curves (d) based on this device architecture. Reproduced with permission from ref. 142. Copyright 2020 Wiley. (e) Structure and J–V curves of dual-interface-modified PSC with Eu-WOx. Reproduced with permission from ref. 143. Copyright 2021 Elsevier. (f) Structure and J–V curves of dual-interface-modified PSC with BCP. Reproduced with permission from ref. 144. Copyright 2021 American Chemical Society. | ||
Such modification has also been made for QDs. Chen et al. applied Ti3C2Tx quantum dots (TQDs) to both the ETL and perovskite layers, and, in particular, they also explored the doping effect of Cu1.8S nanoparticles on the HTL – thus it can be called a triple interfacial modification.142 On the one hand, TQDs in mesoporous titania reduced the interfacial defects, improving the electron transport in the ETL/perovskite. On the other hand, the TQDs located in the perovskite/HTL increased the crystallite size and conductivity of the perovskite by passivating the recombination centers of charge, thus charge separation and transport were significantly promoted (Fig. 15c and d). Furthermore, the HTL with the incorporation of Cu1.8S showed a higher hole mobility and conductivity as well as a better film quality, and also slowed down the decomposition of the perovskite. This resulted in PSCs with multiple interfacial modifications with TQDs and Cu1.8S nanoparticles, reaching a PCE of 21.64%.
Similarly, WOx is an n-type transition metal semiconductor oxide with a wide bandgap and a high electron mobility, which has been commonly employed in photocatalysts, electrochromic materials, optical devices, etc.77 WOx nanorods doped with europium ions (Eu-WOx) were also introduced into both the ETL and HTL to improve the performance.143 The modified PSCs obtained a 22.08% hysteresis-free photovoltaic performance and better operational stability (Fig. 15e). This result was attributed to the significant improvement in electrical conductivity, carrier mobility, and crystal quality of the perovskite films by the Eu-WOx nanorods, as well as the significant improvement in the electron/hole extraction efficiency at the perovskite/ETL and perovskite/HTL interfaces through proper energy-band alignment. Likewise, n-type semiconductor materials (such as BCP) have been used for bi-directional modification studies of PSCs.144 The films modified with BCP achieved better crystallinity and superior interfacial contact, resulting in high-performance devices with a PCE of 20.6% (Fig. 15f).
AgBiS2 has also been investigated as a modification layer for the ETL/perovskite interface, improving the energy-level alignment and enhancing the charge transport capability.145 In addition, the researchers avoided the problem of inferior conductivity by using polyethylene glycol (PEG) in various solvents to modify the perovskite/HTL interfaces instead of adding it directly to the perovskite precursor solution. The grain size and crystallinity of the perovskite films were significantly improved. Consequently, the device modified with the dual interface achieved an efficiency of 21.19% and showed strong UV stability and stability against moisture absorption.
Interestingly, Chen et al. developed a guided transfer strategy to achieve the simultaneous modification of the perovskite and ETL films.146 Firstly, Eu3+ was doped into SnO2 to passivate its surface defects, and then Eu3+ diffused into the perovskite/ETL interface via directional motion. Under the synergistic effect of both, the trap density in the different films was significantly reduced and the electron mobility of the ETL was increased markedly. The experimental results indicated that the obtained PSCs showed a PCE of up to 20.14%. In particular, the unencapsulated PSCs were efficient and slowly degraded by only 13% after 84 days of storage in an ambient atmosphere.
3.5 Perovskite/top electrode interface
Recent work on the interfacial modification of the top electrode of normal PSCs has largely focused on the interface between the perovskite layer and the carbon electrode. In 2013, Han et al. used a carbon electrode as the top electrode of the PSCs and prepared the first fully printed HTL-free PSCs, obtaining a PCE of 6.64%.49,52 Since then, research into replacing the traditional noble metal with carbon as the top electrode has been gradually developed. However, this device structure has so far suffered from severe performance losses at the back electrode interface, including energy-level mismatch and ineffective hole extraction. Based on this, a 2D perovskite passivation layer was utilized as an electron-blocking layer (EBL) to achieve the purpose of suppressing non-radiative recombination and reducing interfacial recombination losses (Fig. 16a). The final HTL-free PSCs treated with 2D perovskite interfaces achieved a maximum efficiency of 18.5%, accompanied by enhanced device stability (Fig. 16b).147 Wu et al. utilized a thin layer of poly(ethylene oxide) (PEO) to improve the interfacial energy-level alignment, which was verified through UV emission spectroscopy measurements. Accordingly, a high efficiency value of 14.9% was exhibited. In particular, the modified device retains 77% of its initial PCE after 192 h of storage under unencapsulated dual 85 °C aging conditions (Fig. 16c–e).148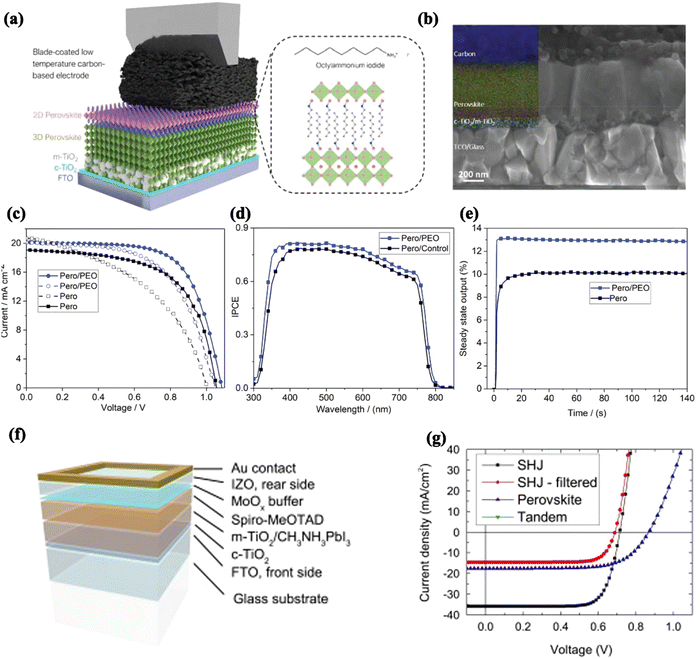 | ||
| Fig. 16 (a) Schematic diagram and (b) SEM image of the perovskite/top electrode interface of HTM-free C-PSCs based on a 2D perovskite. Reproduced with permission from ref. 147. Copyright 2022 Wiley. (c) I–V curves, (d) IPCE curves and (e) steady-state output curves for the perovskite (Pero)/top electrode interface based on PEO. Reproduced with permission from ref. 148. Copyright 2019 Wiley. (f) Schematic diagram of the structure of a typical ST-PSC and (g) J–V curves for different device structures based on MoOx. Reproduced with permission from ref. 149. Copyright 2015 Elsevier. | ||
In addition to carbon electrodes, PSCs with an efficiency greater than 9% were obtained by applying amorphous indium zinc oxide (IZO) layers via sputtering as broadband transparent back electrodes (Fig. 16f and g). When MoOx was inserted as a buffer layer, the efficiency could be increased to 10.3%.149 In particular, an efficiency of more than 18% was obtained after applying the modified cells to a four-terminal tandem device.
4. Interfacial engineering in the inverted structure
4.1 Bottom electrode/HTL interface
In PSCs with inverted structures, the acidic nature and electrical inhomogeneity of PEDOT:PSS can negatively affect the chemical stability and electron-blocking ability of the ITO/PEDOT:PSS interface. Some SAMs can be used to solve this problem by modulating the WF of ITO. For example, SAMs containing fluorinated functionalized boronic acid derivatives were investigated for their effectiveness in modifying the ITO surface of planar PSCs.150 The results after SAM treatment showed that the short-circuit current was enhanced and the trap states of the interface were improved via passivation when the WF of ITO was increased. In addition, SAM modification can also be used to protect electrodes that are made of ITO and PEDOT:PSS. Planar PSCs with a 16% efficiency were successfully designed with low hysteresis, high reproducibility, high stability, and high efficiency after SAM modification.4.2. HTL/perovskite interface
Among inverted and normal PSCs, the main difference lies in the swapping of the ETL and HTL positions and the matching of the functional valence bands after the swapping. Polymer-based hole-transport materials have a relatively high hole mobility and can be easily modified from a molecular perspective, making them ideal for inverted PSCs. The extensively employed poly(3, 4-ethylenedioxythiophene):polystyrene sulfonate (PEDOT:PSS) affects the Voc of the cells due to its energy-level problem, and its acidic aqueous solution is corrosive to ITO glass, which is detrimental to the long-term durability of the PSC.151,152 Therefore, numerous modification strategies have been explored to address these described defects.Zhou et al. used the multifunctional ionic liquid 1-ethyl-3-methylimidazolium chloride (EMIC) to modify the PEDOT:PSS HTL/perovskite interface, which regulated the interfacial contact between the HTL and the perovskite layer.153 Furthermore, the crystalline quality of the perovskite film was improved and the charge complex loss was reduced. Notably, they pioneered the replacement of PCBM with the S-acetylthiocholine chloride molecule. The efficiency of the finally prepared inverted PSCs reached 20.06% with outstanding stability (Fig. 17a).
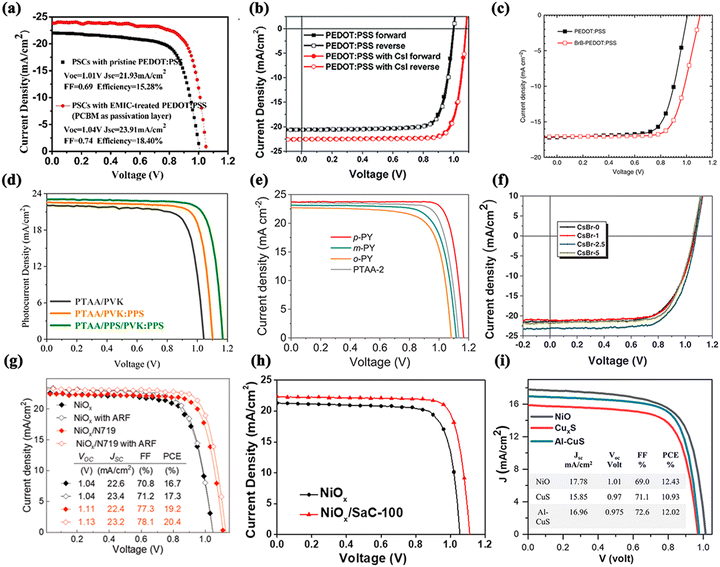 | ||
| Fig. 17 J–V curves and stability curves for the HTL/perovskite interface of an inverted structure PSC based on (a) pristine PEDOT:PSS and EMIC-treated PEDOT:PSS. Reproduced with permission from ref. 153. Copyright 2019 Elsevier. (b) J–V curves for the HTL/perovskite interface of an inverted structure PSC based on PEDOT:PSS and PEDOT:PSS/CsI. Reproduced with permission from ref. 154. Copyright 2019 The Royal Society of Chemistry. (c) J–V curves for the HTL/perovskite interface of an inverted structure PSC based on PEDOT:PSS and BrB/PEDOT:PSS. Reproduced with permission from ref. 155. Copyright 2019 Springer Nature. (d) J–V curves for the HTL/perovskite interface of an inverted structure PSC based on PTAA/PVK, PTAA/PVK:PPS, and PTAA/PPS/PVK:PPS. Reproduced with permission from ref. 161. Copyright 2021 American Chemical Society. (e) J–V curves for the HTL/perovskite interface of an inverted structure PSC based on p-PY, m-PY, o-PY and PTAA-2. Reproduced with permission from ref. 162. Copyright 2022 Wiley. (f) J–V curves for the HTL/perovskite interface of an inverted structure PSC based on pristine and different concentrations of CsBr. Reproduced with permission from ref. 168. Copyright 2020 Wiley. (g) J–V curves for the HTL/perovskite interface of an inverted structure PSC based on NiOx and NiOx/N719. Reproduced with permission from ref. 169. Copyright 2021 Wiley. (h) J–V curves for the HTL/perovskite interface of an inverted structure PSC based on NiOx and NiOx/SaC-100. Reproduced with permission from ref. 170. Copyright 2021 Elsevier. (i) J–V curves for the HTL/perovskite interface of an inverted structure PSC based on NiO and CuxS and Al–CuS. Reproduced with permission from ref. 171. Copyright 2021 Wiley. | ||
Cesium iodide (CsI) was also applied using the interfacial modification strategy by doping into PEDOT:PSS.154 The material reacts with PbI2 to form CsPbI3, improving the interfacial contact and charge transport of the PEDOT:PSS/perovskite interface. In addition, the reduction of the WF of the HTL enhanced its hole-transport properties and suppressed non-radiative recombination. The inverted PSCs modified with CsI exhibited an excellent PCE of 20.22% with negligible hysteresis (Fig. 17b). More importantly, a higher Voc of 1.084 V was achieved.
Quasi-2D (Q-2D) perovskite films have been shown to have multiphase structures with different n values (the number of laminated inorganic substances) and to facilitate electron–hole separation.155 However, Q-2D perovskite materials are still far from being comparable to 3D perovskite materials. Liu et al. found that in inverted PSCs, the deposition of Q-2D perovskite led to the disappearance of this vertical phase distribution. This was mainly attributed to the acidic PEDOT:PSS caused the conversion of perovskite precursors from colloids to solutions. Based on this, they introduced a self-assembly layer of 4-bromobenzenediazonium tetrafluoroborate (BrB), which enabled the Q-2D perovskite films to form the required vertical phase distribution (Fig. 17c). The champion device exhibited a high Voc of 1.11 V and a PCE of 13.74%, as well as superior stability.
Recently, to address the problem of recombination loss due to doping of PEDOT:PSS, aqueous NaOH was used to de-dope the PEDOT:PSS layer and to increase the conductivity by adjusting the thickness of the layer to achieve effective charge transport.156 The de-doped PEDOT:PSS/perovskite interface demonstrated a significantly lower recombination loss, leading to an increase in the device Voc, FF and PCE. This modification strategy provided a potential direction for the application of PEDOT:PSS in inverted PSCs.
Poly(triarylamine) (PTAA) was introduced into PSCs by Nam Joong Jeon et al. to show excellent performance as a hole transport material.157 Interestingly, the material can also be utilized as an interfacial modification layer to play a role such as defect passivation. Its mechanism of action to reduce interfacial recombination was evidenced through a simulation strategy based on a drift–diffusion model.158,159
Although PTAA is currently the preferred material because of its better matching energy levels, higher transport properties, and its ability to significantly improve the long-term stability of the cells, the poor conductivity and surface wettability of PTAA lead to an impaired device performance.160 Thus, it is crucial to carry out suitable modifications to the PTAA layer. The 3-(1-pyridinio)-1-propanesulfonate (PPS) molecule was designed as a chemical bridge at the PTAA/perovskite interface.161 The sulfonate in the PPS molecule can form coordination bonds with Pb2+ in the perovskite, and pyridine can be chemically coupled with the phenyl group of PTAA through π–π stacking, which synergistically improves the interfacial properties of the PTAA/perovskite interface. Furthermore, PPS molecules can significantly reduce the non-radiative recombination by passivating the surface defects of the perovskite. As a result, the efficiency of the device was increased to 21.7% with negligible hysteresis (Fig. 17d). Similarly, Xu et al. tuned the energy levels and surface wettability by removing the alkyl group of PTAA and adding a multifunctional pyridine unit, together with passivation of the interfacial trap.162 The difficulties of PTAA as the HTL were thus comprehensively solved. The resulting device achieved a PCE of more than 22% with the benefit of the dense and homogeneous perovskite film (Fig. 17e).
Except for the commonly used organic polymer cavity transport materials, inorganic cavity layer materials such as NiOx, V2O5, and CuSCN have also been extensively explored and researched.163–165 Among them, NiOx has received more attention due to its stable chemistry and valence band that matches with the halide perovskite absorber layer. With the application of interface modifiers suitable for NiOx, a number of significant advances have been made that are accompanied by certified high PCE values.
Alkali chloride was reported to markedly inhibit the interfacial recombination of NiOx hole transport layers in inverted PSCs as an interfacial modification layer.166 This was mainly attributed to the ordering of the perovskite films being tuned by alkali chloride ions, which reduced the defect density and led to a reduction in interfacial recombination. The Voc of the treated devices reached a high of 1.15 eV with the resulting PCE being significantly improved. Potassium salts, such as KI, KSCN and KNO3, and other inorganic salts worked in a similar way to this material.167 However, distinct from the inorganic salts mentioned above, Zhang et al. highlighted the focus of CsBr for inverted PSCs to alleviate interfacial stresses caused by lattice mismatch.168 The resulting treated device achieved a certified efficiency of 19.7% and favorable stability. The optimization of the perovskite/NiOx HTL interface led to a substantial reduction in the interfacial defect density and obtained the fast extraction/transfer of holes (Fig. 17f). In this work, a new direction is proposed for modification of the NiOx/perovskite interface. The organometallic dye molecule (N719) with a mesoporous configuration can not only passivate the defects at the HTL/perovskite interface but can also accelerate the carrier transport.169 The Lewis basic and acidic groups in the N719 molecule applied to both the NiOx and perovskite surfaces. At the same time, the hole extraction ability of the perovskite was enhanced. The optimized device achieves a PCE of 20.4%, as well as a higher Voc (Fig. 17g). This strategy provides an opportunity for the selection of dye molecules in terms of interfacial modification materials.
The interfacial reaction between Ni3+ on the NiOx surface and the A-site cation salt in the perovskite precursor solution has been systematically investigated.170 It was concluded that this reaction affects the effective transport of holes and increases the possibility of non-radiative recombination, thus compromising the device performance. In addition, the stability of the device was degraded due to deprotonation of the precursor amine and the oxidation of iodine to interstitial iodine. Based on this, trimethylolpropane tris(2-methyl-1-aziridinepropionate) (SaC-100) was applied as an interfacial modification layer to target this undesirable reaction. SaC-100 can effectively hinder the reaction of Ni3+ with MAI and reduce the Voc loss (Fig. 17h). Through surface treatment, they obtained a 20.21% efficiency and long-term stability.
Meanwhile, Al-doped CuS has been reported to be incorporated into inverted PSCs as an efficient inorganic HTL as well as an interfacial modification material, where the role of aluminum was mainly to modulate the energy-band matching of CuS and the perovskite.171 This method not only optimizes the crystallization process of the perovskite by passivating the interfacial defects but can also be applied as a new HTL for inverted PSCs, rendering sulfide-based materials remarkably promising as HTL materials (Fig. 17i). Typical modification materials were similarly screened and summarized in Table 3.
| Modification materials | Perovskite | Main roles | PCE [%] | Stability | Ref. |
|---|---|---|---|---|---|
| EMIC | MAPbI3 | Improve crystalline quality of perovskite films, reduce charge compound loss | 20.06 | 85% of initial efficiency after 35 d of storage in 60% humidity air | 153 |
| BrB | (BA)2(MA0.95Cs0.05)3Pb4I13 | Form vertical phase distribution of Q-2D perovskite films | 13.74 | 88% of initial efficiency after 1400 h of storage in air. | 155 |
| KCl | CsFAMA | Reduce defect density and interface recombination | 20.96 | Over 95% of initial performance after 150 d storage in a nitrogen-filled dry box | 166 |
| CsBr | MA1−xFAxPbI3−yCly | Reduce defect density, improve extraction/transfer of cavities | 19.70 | 82% of the initial efficiency after 900 h | 168 |
| SaC-100 | MAPbI3 | Reduce Voc loss and non-radiative recombination by hindering the reaction of Ni3+ with MAI | 20.21 | Over 80% and over 85% of initial efficiency in thermal stability tests and light-stability tests | 170 |
| Al-doped CuS | FAPbI3 | Improve energy-band matching, passivate interfacial defects | 12.02 | 60% of its initial efficiency after 650 h | 171 |
4.3 Perovskite/ETL interface
In inverted PSCs, the charge separation and transport at the perovskite/ETL interface determine the overall optoelectronic performance of the device. Compared with the large progress made with hole transport materials, the development of electron transport materials has been relatively slow. Electron transport materials commonly used in inverted PSCs include C60 and its derivatives and other non-fullerene electron transport materials. So far, PCBMs are the most extensively utilized electron transport materials due to their suitable energy-level matching, low-temperature solution processability, and favorable electron mobility.172,173The chlorinated n-type fused-ring molecule Cl6SubPc was introduced into the interface between the PEAI-treated 3D/2D perovskite layer and the C60 ETL, resulting in a significant increase in device efficiency from 20.5% to 22%.174 More importantly, the stability of the device was also significantly improved to the extent that it rivals those of crystalline silicon cells (Fig. 18a). Although PCBM on its own has a high electron transport capacity, it still suffers from a low coverage on the perovskite layers and interfacial recombination. [6,6]-Phenyl-C61-butyric acid-N,N-dimethyl-3-(2-thienyl)propanam ester (PCBB-S-N) was demonstrated to act as an intermediate layer to aid the growth of subsequent PCBM layers, forming a smooth and continuous film.175,176 Interestingly, PCBB-S-N can also form a bilayer ETL with PCBM. On the one hand, it regulates the energy-band shift between the perovskite layer and the PCBM ETL. On the other hand, PCBB-S-N contains Lewis bases thiophene and amino groups to play a synergistic role by binding to Pb2+ as well as forming hydrogen bonds, respectively (Fig. 18b). Thus, the prepared inverted PSCs have a champion photovoltaic performance of 21.08% as well as a more prominent water resistance and thermal stability.
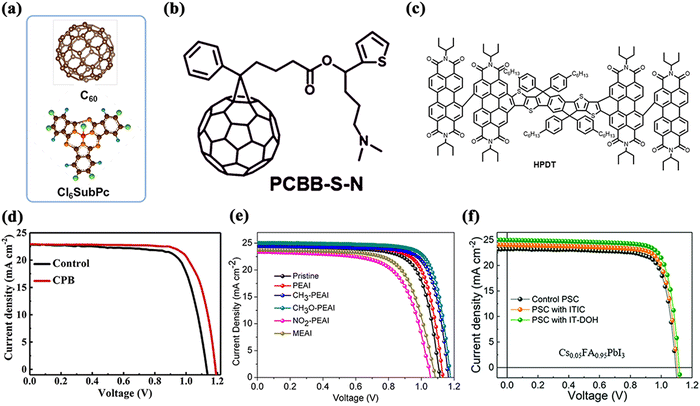 | ||
| Fig. 18 Structural diagram for the HTL/perovskite interface of inverted structural PSCs based on (a) C60 and Cl6SubPc. Reproduced with permission from ref. 174. Copyright 2021 Elsevier. (b) Structure of PCBB-S-N. Reproduced with permission from ref. 176. Copyright 2019 Wiley. (c) Structure of HPDT. Reproduced with permission from ref. 177. Copyright 2020 Elsevier. (d) J–V curves based on a pristine device and modification with CPB. Reproduced with permission from ref. 178. Copyright 2020 Elsevier. (e) J–V curves based on a pristine device and a device with different post-processing. Reproduced with permission from ref. 180. Copyright 2019 American Chemical Society. (f) J–V curves based on a pristine device and modification with ITIC and IT-DOH. Reproduced with permission from ref. 181 Copyright 2021 The Royal Society of Chemistry. | ||
Similarly, a report demonstrated that the n-type organic molecule homologous perylene diimide tetramer (HPDT) can improve the quality of PCBM films and enhance the performance of inverted PSCs.177 Its suitable energy level and high electron mobility resulted in the suppression of non-radiative recombination (Fig. 18c). The PCE was thus increased to 19.75% with negligible hysteresis.
The doping of PQDs in triple-cation perovskite was studied to improve the device performance and demonstrated its interfacial passivation properties.178 The presence of PQDs in the anti-solvent avoided lattice distortion of the different components due to their similar crystal structure to the perovskite films, thus optimizing the interfacial defects. Moreover, the high quality of the obtained perovskite films ensured the reduction of non-radiative recombination and the improvement of the carrier transport capacity during the device operation (Fig. 18d). Finally, more than 21% of the PCE was obtained for the devices modified with CPB-QDs. Another material for modifying the perovskite/ETL interface is PbS-QDs.179 By depositing PbS-QDs on the perovskite, significant passivation of interfacial defects and suppression of non-radiative recombination of carriers were achieved. This was mainly due to the suppression of ion-migration phenomena and the acquisition of high-quality PbS-QDs films. The treated inverted PSCs achieved an efficiency of 20.64% as well as a significant improvement in stability.
In addition to Lewis acids/bases and QDs, some ammonium halides have been used to improve the device performance. Zhuang et al. systematically investigated the passivation effects of four phenethylammonium iodide (PEAI) compounds that have different substituents.180 They concluded that the interfacial passivation results were influenced by the electron density of the benzene ring. Among them, phenethylammonium iodides with electron-donating groups, i.e., methoxy and methyl, can yield excellent passivation. By contrast, phenethylammonium iodides with electron-accepting groups, i.e., nitro, showed poor effects (Fig. 18e). The enhancement of the PCE and stability was attributed to the interaction between the uncoordinated Pb2+ and the benzene ring. IT-DOH, a hydroxylated non-fullerene acceptor (NFA) was introduced to modify the perovskite/ETL interface to suppress non-radiative recombination, defects and optimize carrier transport.181 The unique structure of IT-DOH enabled a more ordered orientation and effective passivation of defects (Fig. 18f). The final experimental results showed that the NFA-doped devices achieved a champion photovoltaic performance of 22.09% (Table 4).
| Modification materials | Perovskite | Main roles | PCE [%] | Stability | Ref. |
|---|---|---|---|---|---|
| Cl6SubPc | CsFAMA | Passivate interface defects, inhibit halide diffusion | 22 | 98% of initial performance after 2000 h at 80 °C in an inert environment | 174 |
| PCBB-S-N | MAPbI3 | Regulate energy-band shift, inhibit invading water molecules | 21.08 | 92–95% of initial efficiency after annealing at 85 °C for 500 h at 40–50% ambient atmosphere for nearly 1000 h | 175 |
| HPDT | MAPbI3 | Improve the quality of PCBM films, inhibit non-radiative recombination | 19.75 | Maintain a stable efficiency of 19.45% | 177 |
| PbS-QDs | MAPbI3 | Passivate interfacial defects, inhibit non-radiative recombination | 20.64 | Maintain a stable efficiency of 20.23% | 179 |
| Phenethylammonium iodides with electron-donating groups, i.e., methoxy and methyl | (FAPbI3)1−x(MAPbBr3−yCly)x | Passivate interface defects | 22.98 | Maintain almost constant efficiency over 1000 h of storage | 180 |
4.4 Dual-interfacial-modification engineering
The dual-interfacial modification strategy has also attracted extensive attention in inverted PSCs, for example, by depositing PFN-Br and PEAI on the HTL and perovskite layers, respectively, to simultaneously improve the HTL/perovskite interface and perovskite/ETL interface quality, which synergistically brought about the inhibition of non-radiative recombination.182 The optimized PCE reached a champion value of 21.33% and a stable PCE of 21.01% (Fig. 19a and b).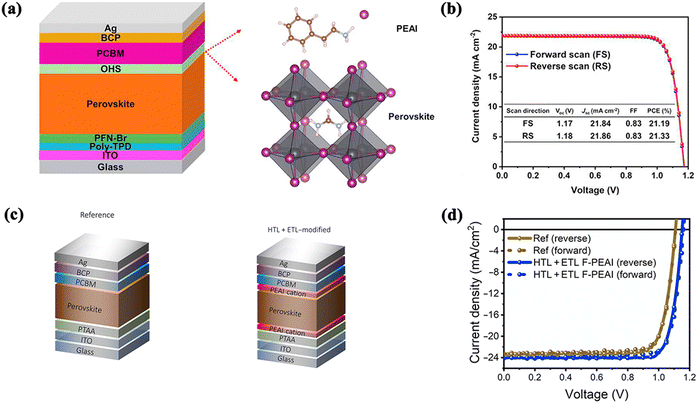 | ||
| Fig. 19 Structural diagram of dual-interface-modified PSCs (a) and J–V curves (b) based on PFN-Br and PEAI. Reproduced with permission from ref. 182. Copyright 2020 Elsevier. Structural diagram of dual-interface-modified PSCs (c) and J–V curves (d) based on PEAI cations. Reproduced with permission from ref. 184. Copyright 2021 The American Association for the Advancement of Science. | ||
The application of KSCN for dual interfacial layer treatment has also been reported.183 Firstly, the passivation effect of K+ is the same as that mentioned above. Then, KSCN can effectively reduce the defect density of the dual surface and inhibit the ion migration through the electrostatic interaction of the perovskite and the strong covalent bonding of NiOx. In addition, the energy-level alignment of the HTL and the perovskite can be optimized. This strategy eventually led to a Voc of 1.14 V and an FF of 0.8 with a champion PCE of 21.23%.
In a recent study, various PEAI-based compounds were used as modified layers at different interfaces.184 It was proposed that the wettability of the modified HTL/perovskite interface was improved, resulting in more homogeneous films as well as avoiding the generation of nanovoids at the HTL surface. Modification of the perovskite/ETL interface achieved an active passivation effect with the suppression of non-radiative recombination. It is worth mentioning that the joint implementation of both modifications resulted in a simultaneous increase in all the photovoltaic parameters and enabled the easy combination of the ionic liquid with the perovskite active layer. The final device with a maximum conversion efficiency of 23.7% was obtained (Fig. 19c and d).
4.5 ETL/top electrode interface
The same problem of poor interfacial contact and severe energy loss between the electron transport layer and the metal electrode in inverted PSCs hinders their development. The electrode modification material can adjust the function of the metal electrode, so that the modified low function metal forms an ohmic contact with the ETL to promote electron transport and improve the device performance. A perylene diimide-based zwitterion, QAPDI, has been successfully developed as an interface-modification layer for the ETL/electrode. The material, with its excellent solubility, suitable energy level and high electron mobility, enabled enhanced interfacial contact, a lower energy loss and consequently enhanced electron injection, transport and collection. In particular, the environmental stability was further enhanced by the good hydrophobicity of the modified layer (Fig. 20a).185 Wang et al. inserted a phosphine-inlaid polymer between the top Ag metal electrode and the PCBM.186 By bonding tightly to the top metal electrode, the modified layer prevented the direct reaction between the electrode and the perovskite, enhancing the rate of electron transfer between the two layers. This study demonstrates that an efficiency of 20.2% can be obtained even in air. It is worth mentioning that the 85 °C aging test further demonstrates that the device can maintain 80% of the initial efficiency for at least 1100 hours (Fig. 20b). The carbolong-derived complexes were also used as the cathode interface layer material for the inverse PSCs, and the results showed that the optimal device FF after modification was as high as 83.52%.187 The final solar cells were prepared with a 21% (Ag electrode) and a 20% (Au electrode) photoelectric conversion efficiency (Fig. 20c). The final stability test results proved that the use of carbolong-derived complexes combined with high functional Au electrodes greatly improved the stability of the devices in air and inert gas.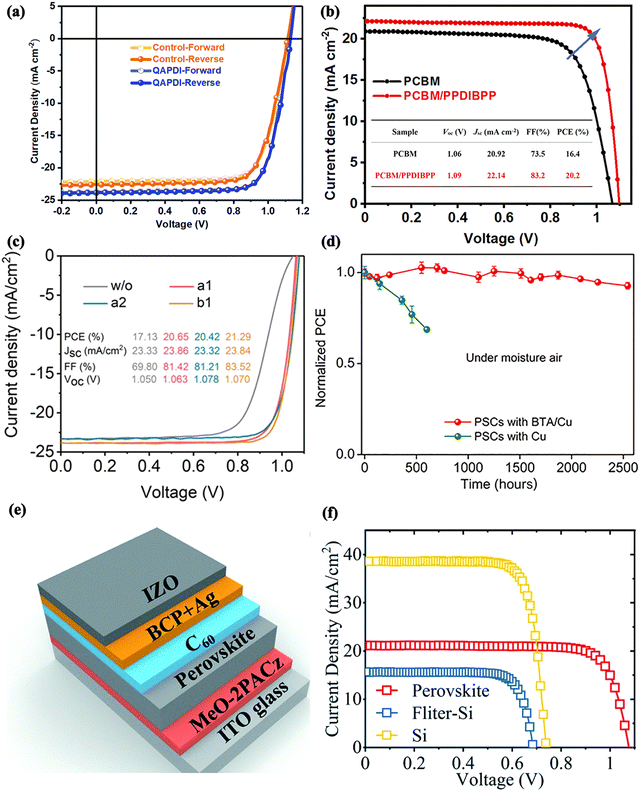 | ||
| Fig. 20 (a) J–V curves for the ETL/top electrode interface of inverted PSCs based on QAPDI. Reproduced with permission from ref. 185 Copyright 2020 The Royal Society of Chemistry. (b) J–V curves for the ETL/top electrode interface of inverted PSCs based on PPDIBPP. Reproduced with permission from ref. 186. Copyright 2021 Elsevier. (c) J–V curves for the ETL/top electrode interface of inverted PSCs based on carbolong-derived complexes. Reproduced with permission from ref. 187. Copyright 2021 American Chemical Society. (d) Stability curves for the ETL/top electrode interface of inverted PSCs based on BTA. Reproduced with permission from ref. 188. Copyright 2020 The American Association for the Advancement of Science. (e) Schematic diagram of the structure of a typical ST-PSC and (f) J–V curves for different device structures based on BCP:Ag. Reproduced with permission from ref. 189 Copyright 2021 The Royal Society of Chemistry. | ||
Not to be overlooked, another major challenge for inverted PSCs is the reaction of commonly used metal electrodes with the perovskite layer, leading to electrode corrosion and degradation of the device performance. Based on this, Fang et al. were inspired by metal corrosion protection to improve the stability of devices via the introduction of the organic preservative benzotriazole (BTA) before deposition of Cu electrodes.188 The BTA molecule chemically coordinates with Cu electrodes and forms an insoluble polymer film of [BTA-Cu], which inhibited the electrochemical corrosion and reaction between the perovskite and the Cu electrodes. The devices with the chemical preservative BTA have an initial PCE of approximately 92.8% after 2500 hours of aging in air (Fig. 20d).
Ying et al. applied an interfacial modification strategy to tandem solar cells using a 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP):Ag complex to regulate the electrical contact between C60 (ETL) and IZO (Fig. 20e).189 The material not only improves electron transport but also acts as a hole hindrance layer to inhibit charge recombination and has the effect of protecting the C60 layer. A final perovskite/silicon four-terminal tandem solar cell with an overall efficiency of 27.59% was achieved (Fig. 20f).
5. Summary and outlook
The multilayer structure of PSCs facilitates the existence of four main interfaces, each of which is directly related to the interfacial carrier dynamics and plays a decisive role in the photovoltaic performance and stability of the devices. Among them, the interfaces of ETL/perovskite, perovskite/HTL, and CTL/electrode are the most critical. Based on these, this Review discusses and summarizes the main research progress for these types of interface engineering. We believe that the main functions of these different interfacial modifications can be summarized as follows: (1) passivation of interfacial defects by filling vacancies or fixing interstitial atoms through chemical interactions of specific functional groups; (2) more rapid charge extraction by modulating the energy-band alignment of neighboring interfacial layers; (3) optimization of the perovskite growth process to assist in the formation of homogeneous, densified, high-performance perovskite films; and (4) improvement of the device stability by blocking external moisture penetration or inhibiting ion migration as a blocking layer.For the photovoltaic industry, the efficiency, lifetime and cost are considered to be the important indicators of a device's industrial viability. The PSC efficiency has increased dramatically in recent years to the standard point for industrial production, which is no longer the focus of attention. Currently, the maximum lifetime reported for PSCs is at most a few thousand hours, which is far below the lifetime of commercially available photovoltaic devices. Interfacial modification, as a simple and accessible way of optimizing PSCs, not only offers an extremely enhanced effect on the efficiency but also holds significant research potential for making improvements in the PSC lifetime. Interfacial modification is expected to improve the long-term stability of PSCs by isolating water and oxygen and passivating defects, thus attracting increasing attention in recent years, which will be a hot spot for PSC research in the coming years.
In addition, the PCE decreases significantly as the effective area of the PSC is increased, which is limited by the solution-processing method of perovskite films. The efficiency is attributed to the defects in film coverage, the homogeneity and the smoothness of each functional layer that increasingly affect the extraction and transmission of photogenerated carriers as the effective area of the device is enlarged. Therefore, the industrialization of large-area devices for PSCs is a problem that needs to be solved as soon as possible. Future research should focus on the interface-modification methods and materials applicable to large-area devices.
Last but not least, interfacial modification materials need to meet not only the requirements of improving the device performance but also the impact on the operator and the environment. In the future development of PSCs, materials with environmental and recyclable properties, e.g., being biodegradable or naturally degradable, should be developed further. Overall, the interface engineering of PSCs will proceed toward finding effective material systems and processing methods that will completely eradicate defects as well as providing stabilization of the perovskite surface stress. It is predicted that interface engineering will contribute significantly to the future commercialization of PSCs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from the Joint Research Funds of Department of Science & Technology of Shaanxi Province and Northwestern Polytechnical University (No. 2020GXLH-Z-018, and 2020GXLH-Z-007), the Natural Science Basic Research Plan in Shaanxi Province of China (2021JLM-43), the Fundamental Research Funds for the Central Universities, and the Natural Science Foundation of Jiangsu Province for Distinguished Young Scholars, China (Grant BK20200034).References
- S. Wang, X. Wang, B. Liu, Z. Guo, K. K. Ostrikov, L. Wang and W. Huang, Nanoscale, 2021, 13, 17989–18009 RSC.
- J. Cao, X. Lv, P. Zhang, T. T. Chuong, B. Wu, X. Feng, C. Shan, J. Liu and Y. Tang, Adv. Mater., 2018, 30, 1800568 CrossRef PubMed.
- M. K. Rao, D. N. Sangeetha, M. Selvakumar, Y. N. Sudhakar and M. G. Mahesha, Sol. Energy, 2021, 218, 469–491 CrossRef.
- S. Wang, Y. Li, X. Wang, G. Zi, C. Zhou, B. Liu, G. Liu, L. Wang and W. Huang, J. Mater. Sci. Technol., 2022, 104, 155–162 CrossRef.
- S. Wang, L. Wang and W. Huang, J. Mater. Chem. A, 2020, 8, 24307–24352 RSC.
- S. Wang, B. Liu, X. Wang, Y. Zhang and W. Huang, Nano Res., 2022, 15, 7026–7033 CrossRef CAS.
- L. Chao, T. Niu, W. Gao, C. Ran, L. Song, Y. Chen and W. Huang, Adv. Mater., 2021, 33, 2005410 CrossRef CAS PubMed.
- Z. Sun, X. Chen, Y. He, J. Li, J. Wang, H. Yan and Y. Zhang, Adv. Energy Mater., 2022, 12, 2200015 CrossRef CAS.
- K. Yoshikawa, H. Kawasaki, W. Yoshida, T. Irie, K. Konishi, K. Nakano, T. Uto, D. Adachi, M. Kanematsu, H. Uzu and K. Yamamoto, Nat. Energy, 2017, 2, 17032–17040 CrossRef CAS.
- T. Takamoto1, T. Agui1, A. Yoshida1, K. Nakaido1, H. Juso1, K. Sasaki1, K. Nakamora1, H. Yamaguchi1, T. Kodama1, H. Washio1, M. Imaizumi and M. Takahashi, Sharp Tech. J., 2010, 100, 1–10 Search PubMed.
- Z. Yang, J. Z. Fan, A. H. Proppe, F. P. G. Arquer, D. Rossouw, O. Voznyy, X. Lan, M. Liu, G. Walters, R. Quintero-Bermudez, B. Sun, S. Hoogland, G. A. Botton, S. O. Kelley and E. H. Sargent, Nat. Commun., 2017, 8, 1325 CrossRef PubMed.
- H. Zhou, Y. Zhang, J. Seifter, S. D. Collins, C. Luo, G. C. Bazan, T. Q. Nguyen and A. J. Heeger, Adv. Mater., 2013, 25, 1646–1652 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- P. Chen, Y. Bai, M. Lyu, J.-H. Yun, M. Hao and L. Wang, Sol. RRL, 2018, 2, 1700186 CrossRef.
- J. Gebhardt and A. M. Rappe, ACS Energy Lett., 2017, 2, 2681–2685 CrossRef CAS.
- T. M. Brenner, D. A. Egger, L. Kronik, G. Hodes and D. Cahen, Nat. Rev. Mater., 2016, 1, 15007 CrossRef CAS.
- T. Miyasaka, A. Kulkarni, G. M. Kim, S. Öz and A. K. Jena, Adv. Energy Mater., 2019, 10, 1902500 CrossRef.
- M. Saba, F. Quochi, A. Mura and G. Bongiovanni, Acc. Chem. Res., 2016, 49, 166–173 CrossRef CAS PubMed.
- A. R. Srimath Kandada and A. Petrozza, Acc. Chem. Res., 2016, 49, 536–544 CrossRef CAS PubMed.
- H. S. Kim, C. R. Lee, J. H. Im, K. B. Lee, T. Moehl, A. Marchioro, S. J. Moon, R. Humphry-Baker, J. H. Yum, J. E. Moser, M. Gratzel and N. G. Park, Sci. Rep., 2012, 2, 591 CrossRef PubMed.
- https://www.nrel.gov/pv/cell-efficiency.html .
- M. R. Filip, S. Hillman, A. A. Haghighirad, H. J. Snaith and F. Giustino, J. Phys. Chem. Lett., 2016, 7, 2579–2585 CrossRef CAS PubMed.
- N. K. Noel, S. D. Stranks, A. Abate, C. Wehrenfennig, S. Guarnera, A.-A. Haghighirad, A. Sadhanala, G. E. Eperon, S. K. Pathak, M. B. Johnston, A. Petrozza, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 3061–3068 RSC.
- G. Volonakis, M. R. Filip, A. A. Haghighirad, N. Sakai, B. Wenger, H. J. Snaith and F. Giustino, J. Phys. Chem. Lett., 2016, 7, 1254–1259 CrossRef CAS PubMed.
- W. Hui, L. Chao, H. Lu, F. Xia, Q. Wei, Z. Su, T. Niu, L. Tao, B. Du, D. Li, Y. Wang, H. Dong, S. Zuo, B. Li, W. Shi, X. Ran, P. Li, H. Zhang, Z. Wu, C. Ran, L. Song, G. Xing, X. Gao, J. Zhang, Y. Xia, Y. Chen and W. Huang, Science, 2021, 371, 1359–1364 CrossRef CAS PubMed.
- P. Y. Lin, A. Loganathan, I. Raifuku, M. H. Li, Y. Y. Chiu, S. T. Chang, A. Fakharuddin, C. F. Lin, T. F. Guo, L. Schmidt-Mende and P. Chen, Adv. Energy Mater., 2021, 11, 2100818 CrossRef CAS.
- K. Wang, W. S. Subhani, Y. Wang, X. Zuo, H. Wang, L. Duan and S. F. Liu, Adv. Mater., 2019, 31, 1902037 CrossRef CAS PubMed.
- S. Shao and M. A. Loi, Adv. Mater. Interfaces, 2019, 7, 1901469 CrossRef.
- A. Fakharuddin, L. Schmidt-Mende, G. Garcia-Belmonte, R. Jose and I. Mora-Sero, Adv. Energy Mater., 2017, 7, 1700623 CrossRef.
- T. H. Han, S. Tan, J. Xue, L. Meng, J. W. Lee and Y. Yang, Adv. Mater., 2019, 31, 1803515 CrossRef CAS PubMed.
- Y. Li, H. Xie, E. L. Lim, A. Hagfeldt and D. Bi, Adv. Energy Mater., 2022, 12, 2102730 CrossRef CAS.
- W. Yu, X. Sun, M. Xiao, T. Hou, X. Liu, B. Zheng, H. Yu, M. Zhang, Y. Huang and X. Hao, Nano Res., 2021, 15, 85–103 CrossRef.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- M. A. Loi and J. C. Hummelen, Nat. Mater., 2013, 12, 1087–1089 CAS.
- H. Zhou, Q. Chen, G. Li, S. Luo, T. Song, H. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542–548 CrossRef CAS.
- J. J. Choi, X. Yang, Z. M. Norman, S. J. Billinge and J. S. Owen, Nano Lett., 2014, 14, 127–133 CrossRef CAS.
- Z. Song, S. C. Watthage, A. B. Phillips and M. J. Heben, J. Photonics Energy, 2016, 6, 1947–7988 Search PubMed.
- D. Li, L. Chao, C. Chen, X. Ran, Y. Wang, T. Niu, S. Lv, H. Wu, Y. Xia, C. Ran, L. Song, S. Chen, Y. Chen and W. Huang, Nano Lett., 2020, 20, 5799–5806 CrossRef CAS PubMed.
- Z. C. Cheng, Y. Y. Fang, A. F. Wang, T. T. Ma, F. Liu, S. Gao, S. H. Yan, Y. Di and T. S. Qin, J. Cent. South Univ., 2021, 28, 3714–3727 CrossRef CAS.
- Q. Dong, Y. Yuan, Y. Shao, Y. Fang, Q. Wang and J. Huang, Energy Environ. Sci., 2015, 8, 2464–2470 RSC.
- P. Schulz, ACS Energy Lett., 2018, 3, 1287–1293 CrossRef CAS.
- T. Handa, D. M. Tex, A. Shimazaki, A. Wakamiya and Y. Kanemitsu, J. Phys. Chem. Lett., 2017, 8, 954–960 CrossRef CAS.
- V. D'Innocenzo, A. R. Srimath Kandada, M. De Bastiani, M. Gandini and A. Petrozza, J. Am. Chem. Soc., 2014, 136, 17730–17733 CrossRef PubMed.
- M. Stolterfoht, C. M. Wolff, Y. Amir, A. Paulke, L. Perdigón-Toro, P. Caprioglio and D. Neher, Energy Environ. Sci., 2017, 10, 1530–1539 RSC.
- Q.-D. Ou, C. Li, Q.-K. Wang, Y.-Q. Li and J.-X. Tang, Adv. Mater. Interfaces, 2017, 4, 1600694 CrossRef.
- J. W. Lee, S. G. Kim, S. H. Bae, D. K. Lee, O. Lin, Y. Yang and N. G. Park, Nano Lett., 2017, 17, 4270–4276 CrossRef CAS PubMed.
- I. G. Scheblykin, Adv. Energy Mater., 2020, 10, 2001724 CrossRef CAS.
- R. Chen, W. Zhang, X. Guan, H. Raza, S. Zhang, Y. Zhang, P. A. Troshin, S. A. Kuklin, Z. Liu and W. Chen, Adv. Funct. Mater., 2022, 32, 2200651 CrossRef CAS.
- C. Ding, L. Yin, L. Zhang, R. Huang, S. Fan, Q. Luo, J. Lin, F. Li, C. Zhao, R. Österbacka and C. Q. Ma, Adv. Funct. Mater., 2021, 31, 2103820 CrossRef CAS.
- D. Luo, X. Li, A. Dumont, H. Yu and Z. H. Lu, Adv. Mater., 2021, 33, 2006004 CrossRef CAS PubMed.
- J. Liu, Q. Zhou, N. K. Thein, L. Tian, D. Jia, E. M. J. Johansson and X. Zhang, J. Mater. Chem. A, 2019, 7, 13777–13786 RSC.
- C. H. Lin, L. Hu, X. Guan, J. Kim, C. Y. Huang, J. K. Huang, S. Singh and T. Wu, Adv. Mater., 2022, 34, 2108616 CrossRef CAS PubMed.
- B. Chen, S. W. Baek, Y. Hou, E. Aydin, M. De Bastiani, B. Scheffel, A. Proppe, Z. Huang, M. Wei, Y. K. Wang, E. H. Jung, T. G. Allen, E. Van Kerschaver, F. P. Garcia de Arquer, M. I. Saidaminov, S. Hoogland, S. De Wolf and E. H. Sargent, Nat. Commun., 2020, 11, 1257 CrossRef CAS PubMed.
- C. Yan, J. Huang, D. Li and G. Li, Mater. Chem. Front., 2021, 5, 4538–4564 RSC.
- L. Huang, D. Zhang, S. Bu, R. Peng, Q. Wei and Z. Ge, Adv. Sci., 2020, 7, 1902656 CrossRef CAS PubMed.
- S. Huang, Q. Dong, Y. Shi, L. Duan and L. Wang, Chem. Eng. J., 2020, 394, 125024 CrossRef CAS.
- X. Gong, Q. Sun, S. Liu, P. Liao, Y. Shen, C. Gratzel, S. M. Zakeeruddin, M. Gratzel and M. Wang, Nano Lett., 2018, 18, 3969–3977 CrossRef CAS PubMed.
- S. Wang, T. Sakurai, W. Wen and Y. Qi, Adv. Mater. Interfaces, 2018, 5, 1800260 CrossRef.
- P. Chen, Z. Wang, S. Wang, M. Lyu, M. Hao, M. Ghasemi, M. Xiao, J.-H. Yun, Y. Bai and L. Wang, Nano Energy, 2020, 69, 104392 CrossRef CAS.
- L. Tao, J. Qiu, B. Sun, X. Wang, X. Ran, L. Song, W. Shi, Q. Zhong, P. Li, H. Zhang, Y. Xia, P. Müller-Buschbaum and Y. Chen, J. Energy Chem., 2021, 61, 395–415 CrossRef.
- L. Yin, Y. Li, X. Yao, Y. Wang, L. Jia, Q. Liu, J. Li, Y. Li and D. He, Nano Lett., 2021, 13, 96–112 CrossRef PubMed.
- H. Zheng, Y. Wang, B. Niu, R. Ge, Y. Lei, L. Yan, J. Si, P. Zhong and X. Ma, J. Phys. Chem. C, 2021, 125, 15210–15222 CrossRef CAS.
- K. F. Chen, P. Cai, H. L. Peng, X. G. Xue, Z. M. Wang and L. X. Sun, J. Cent. South Univ., 2021, 28, 3935–3958 CrossRef CAS.
- M. Dadashi Firouzjaei, M. Karimiziarani, H. Moradkhani, M. Elliott and B. Anasori, Mater. Today Adv, 2022, 13, 100202 CrossRef CAS.
- A. Agresti, A. Pazniak, S. Pescetelli, A. Di Vito, D. Rossi, A. Pecchia, M. Auf der Maur, A. Liedl, R. Larciprete, D. V. Kuznetsov, D. Saranin and A. Di Carlo, Nat. Mater., 2019, 18, 1228–1234 CrossRef CAS PubMed.
- J. Li, T. Jiu, S. Chen, L. Liu, Q. Yao, F. Bi, C. Zhao, Z. Wang, M. Zhao, G. Zhang, Y. Xue, F. Lu and Y. Li, Nano Lett., 2018, 18, 6941–6947 CrossRef CAS PubMed.
- S. Zhang, H. Si, W. Fan, M. Shi, M. Li, C. Xu, Z. Zhang, Q. Liao, A. Sattar, Z. Kang and Y. Zhang, Angew. Chem., Int. Ed., 2020, 59, 11573–11582 CrossRef CAS PubMed.
- L. Yao, M. Zhao, L. Liu, S. Chen, J. Wang, C. Zhao, Z. Jia, S. Pang, X. Guo and T. Jiu, Mater. Chem. Front., 2021, 5, 6913–6922 RSC.
- J. Jimenez-Lopez, B. M. D. Puscher, D. M. Guldi and E. Palomares, J. Am. Chem. Soc., 2020, 142, 1236–1246 CrossRef CAS PubMed.
- H. Wang, F. Li, P. Wang, R. Sun, W. Ma, M. Chen, W. Miao, D. Liu and T. Wang, Adv. Energy Mater., 2020, 10, 2000615 CrossRef CAS.
- C. Tian, K. Lin, J. Lu, W. Feng, P. Song, L. Xie and Z. Wei, Small Methods, 2019, 4, 1900476 CrossRef.
- T. Wu, C. Zhen, J. Wu, C. Jia, M. Haider, L. Wang, G. Liu and H.-M. Cheng, Sci. Bull., 2019, 64, 547–552 CrossRef CAS.
- W. Hui, Y. Yang, Q. Xu, H. Gu, S. Feng, Z. Su, M. Zhang, J. Wang, X. Li, J. Fang, F. Xia, Y. Xia, Y. Chen, X. Gao and W. Huang, Adv. Mater., 2020, 32, 1906374 CrossRef CAS PubMed.
- Y. Yang, H. Lu, S. Feng, L. Yang, H. Dong, J. Wang, C. Tian, L. Li, H. Lu, J. Jeong, S. M. Zakeeruddin, Y. Liu, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2021, 14, 3447–3454 RSC.
- Z. W. Gao, Y. Wang, H. Liu, J. Sun, J. Kim, Y. Li, B. Xu and W. C. H. Choy, Adv. Funct. Mater., 2021, 31, 2101438 CrossRef CAS.
- Y. Hou, C. O. R. Quiroz, S. Scheiner, W. Chen, T. Stubhan, A. Hirsch, M. Halik and C. J. Brabec, Adv. Energy Mater., 2015, 5, 1501056 CrossRef.
- F. Ali, C. Roldán-Carmona, M. Sohail and M. K. Nazeeruddin, Adv. Energy Mater., 2020, 10, 2002989 CrossRef CAS.
- C. M. Wolff, L. Canil, C. Rehermann, N. Ngoc Linh, F. Zu, M. Ralaiarisoa, P. Caprioglio, L. Fiedler, M. Stolterfoht, S. Kogikoski, Jr., I. Bald, N. Koch, E. L. Unger, T. Dittrich, A. Abate and D. Neher, ACS Nano, 2020, 14, 1445–1456 CrossRef CAS PubMed.
- J. Han, H. Kwon, E. Kim, D.-W. Kim, H. J. Son and D. H. Kim, J. Mater. Chem. A, 2020, 8, 2105–2113 RSC.
- Y. Shi, H. Zhang, X. Tong, X. Hou, F. Li, Y. Du, S. Wang, Q. Zhang, P. Liu and X. Zhao, Sol. RRL, 2021, 5, 2100128 CrossRef CAS.
- Z. Dai, S. K. Yadavalli, M. Chen, A. Abbaspourtamijani, Y. Qi and N. P. Padture, Science, 2021, 372, 618–622 CrossRef CAS PubMed.
- P. Zhu, S. Gu, X. Luo, Y. Gao, S. Li, J. Zhu and H. Tan, Adv. Energy Mater., 2019, 10, 1903083 CrossRef.
- R. Azmi, N. Nurrosyid, S.-H. Lee, M. Al Mubarok, W. Lee, S. Hwang, W. Yin, T. K. Ahn, T.-W. Kim, D. Y. Ryu, Y. R. Do and S.-Y. Jang, ACS Energy Lett., 2020, 5, 1396–1403 CrossRef CAS.
- L. Wang, X. Wang, L. Zhu, S.-B. Leng, J. Liang, Y. Zheng, Z. Zhang, Z. Zhang, X. Liu, F. Liu and C.-C. Chen, Chem. Eng. J., 2022, 430, 132730 CrossRef CAS.
- Z. Huang, A. H. Proppe, H. Tan, M. I. Saidaminov, F. Tan, A. Mei, C.-S. Tan, M. Wei, Y. Hou, H. Han, S. O. Kelley and E. H. Sargent, ACS Energy Lett., 2019, 4, 1521–1527 CrossRef CAS.
- Q. Xiong, C. Wang, Q. Zhou, L. Wang, X. Wang, L. Yang, J. Ding, C. C. Chen, J. Wu, X. Li and P. Gao, Adv. Funct. Mater., 2021, 32, 2107823 CrossRef.
- A. A. Zaky, E. Christopoulos, K. Gkini, M. K. Arfanis, L. Sygellou, A. Kaltzoglou, A. Stergiou, N. Tagmatarchis, N. Balis and P. Falaras, Appl. Catal., B, 2021, 284, 119714 CrossRef CAS.
- H. Xu, Y. Miao, N. Wei, H. Chen, Z. Qin, X. Liu, X. Wang, Y. Qi, T. Zhang and Y. Zhao, Adv. Energy Mater., 2021, 12, 2103151 CrossRef.
- H. Bi, B. Liu, D. He, L. Bai, W. Wang, Z. Zang and J. Chen, Chem. Eng. J., 2021, 418, 129375 CrossRef CAS.
- C. Zhang, H. Wang, H. Li, Q. Zhuang, C. Gong, X. Hu, W. Cai, S. Zhao, J. Chen and Z. Zang, J. Energy Chem., 2021, 63, 452–460 CrossRef.
- M. Fahim, I. Firdous, W. Zhang and W. A. Daoud, Nano Energy, 2021, 86, 106127 CrossRef CAS.
- P. Finkel, M. G. Cain, T. Mion, M. Staruch, J. Kolacz, S. Mantri, C. Newkirk, K. Kavetsky, J. Thornton, J. Xia, M. Currie, T. Hase, A. Moser, P. Thompson, C. A. Lucas, A. Fitch, J. M. Cairney, S. D. Moss, A. G. A. Nisbet, J. E. Daniels and S. E. Lofland, Adv. Mater., 2022, 34, 2106827 CrossRef CAS PubMed.
- Y. M. You, W. Q. Liao, D. Zhao, H. Y. Ye, Y. Zhang, Q. Zhou, X. Niu, J. Wang, P. F. Li, D. W. Fu, Z. Wang, S. Gao, K. Yang, J. M. Liu, J. Li, Y. Yan and R. G. Xiong, Science, 2017, 357, 306–309 CrossRef CAS PubMed.
- S. Sonmezoglu and S. Akin, Nano Energy, 2020, 76, 105127 CrossRef CAS.
- Y. Sun, J. Zhang, H. Yu, J. Wang, C. Huang and J. Huang, Chem. Eng. J., 2021, 420, 129579 CrossRef CAS.
- Y. Yang, ACS Nano, 2021, 15, 18679–18682 CrossRef CAS PubMed.
- Q. Lou, Y. Han, C. Liu, K. Zheng, J. Zhang, X. Chen, Q. Du, C. Chen and Z. Ge, Adv. Energy Mater., 2021, 4, 2101416 CrossRef.
- J. Tao, X. Liu, J. Shen, H. Wang, J. Xue, C. Su, H. Guo, G. Fu, W. Kong and S. Yang, Chem. Eng. J., 2022, 430, 132683 CrossRef CAS.
- X. Zuo, B. Kim, B. Liu, D. He, L. Bai, W. Wang, C. Xu, Q. Song, C. Jia, Z. Zang, D. Lee, X. Li and J. Chen, Chem. Eng. J., 2022, 431, 133209 CrossRef CAS.
- D. Liu, H. Zheng, Y. Wang, L. Ji, H. Chen, W. Yang, L. Chen, Z. Chen and S. Li, Chem. Eng. J., 2020, 396, 125010 CrossRef CAS.
- H. Liang, Y. C. Hu, Y. Tao, B. Wu, Y. Wu and J. Cao, ACS Appl. Mater. Interfaces, 2019, 11, 43116–43121 CrossRef CAS PubMed.
- M. Tai, X. Zhao, H. Shen, Y. Guo, M. Zhang, Y. Zhou, X. Li, Z. Yao, X. Yin, J. Han, X. Li and H. Lin, Chem. Eng. J., 2019, 361, 60–66 CrossRef CAS.
- D. Zhang, X. Zhang, S. Bai, C. Liu, Z. Li, W. Guo and F. Gao, Sol. RRL, 2019, 3, 1900154 CrossRef.
- S. Tsarev, S. Olthof, A. G. Boldyreva, S. M. Aldoshin, K. J. Stevenson and P. A. Troshin, Nano Energy, 2021, 83, 105774 CrossRef CAS.
- Z. Xiong, X. Chen, B. Zhang, G. O. Odunmbaku, Z. Ou, B. Guo, K. Yang, Z. Kan, S. Lu, S. Chen, N. A. N. Ouedraogo, Y. Cho, C. Yang, J. Chen and K. Sun, Adv. Mater., 2021, 34, 2106118 CrossRef PubMed.
- S. You, H. Zeng, Z. Ku, X. Wang, Z. Wang, Y. Rong, Y. Zhao, X. Zheng, L. Luo, L. Li, S. Zhang, M. Li, X. Gao and X. Li, Adv. Mater., 2020, 32, 2003990 CrossRef CAS PubMed.
- Z. Xiong, L. Lan, Y. Wang, C. Lu, S. Qin, S. Chen, L. Zhou, C. Zhu, S. Li, L. Meng, K. Sun and Y. Li, ACS Energy Lett., 2021, 6, 3824–3830 CrossRef CAS.
- J. Luo, F. Lin, J. Xia, H. Yang, R. Zhang, H. A. Malik, H. Shu, Z. Wan, K. Han, R. Wang, X. Yao and C. Jia, Nano Energy, 2021, 82, 105751 CrossRef CAS.
- W. Ke, P. Priyanka, S. Vegiraju, C. C. Stoumpos, I. Spanopoulos, C. M. M. Soe, T. J. Marks, M. C. Chen and M. G. Kanatzidis, J. Am. Chem. Soc., 2018, 140, 388–393 CrossRef CAS PubMed.
- C. Xiao, F. Zhang, Z. Li, S. P. Harvey, X. Chen, K. Wang, C.-S. Jiang, K. Zhu and M. Al-Jassim, Matter, 2020, 2, 261–272 CrossRef.
- C. Liu, X. Zhou, S. Chen, X. Zhao, S. Dai and B. Xu, Adv. Sci., 2019, 6, 1801169 CrossRef PubMed.
- L. Calió, D. S. Kazim, P. M. Grätzel and D. S. Ahmad, Angew. Chem., 2016, 55, 14522–14545 CrossRef PubMed.
- M. E. F. Bouduban, V. I. E. Queloz, V. M. Caselli, K. T. Cho, A. R. Kirmani, S. Paek, C. Roldan-Carmona, L. J. Richter, J. E. Moser, T. J. Savenije, M. K. Nazeeruddin and G. Grancini, J. Phys. Chem. Lett., 2019, 10, 5713–5720 CrossRef CAS PubMed.
- H. Kim, S. U. Lee, D. Y. Lee, M. J. Paik, H. Na, J. Lee and S. I. Seok, Adv. Energy Mater., 2019, 9, 1902740 CrossRef CAS.
- G. Liu, H. Zheng, H. Xu, L. Zhang, X. Xu, S. Xu and X. Pan, Nano Energy, 2020, 73, 104753 CrossRef CAS.
- P. Chen, Y. Bai, S. Wang, M. Lyu, J. H. Yun and L. Wang, Adv. Funct. Mater., 2018, 28, 1706923 CrossRef.
- C. Long, K. Huang, J. Chang, C. Zuo, Y. Gao, X. Luo, B. Liu, H. Xie, Z. Chen, J. He, H. Huang, Y. Gao, L. Ding and J. Yang, Small, 2021, 17, 2102368 CrossRef CAS PubMed.
- N. H. Hemasiri, S. Kazim and S. Ahmad, Nano Energy, 2020, 77, 105292 CrossRef CAS.
- M.-H. Li, T.-G. Sun, J.-Y. Shao, Y.-D. Wang, J.-S. Hu and Y.-W. Zhong, Nano Energy, 2021, 79, 105462 CrossRef CAS.
- S. Akin, Y. Altintas, E. Mutlugun and S. Sonmezoglu, Nano Energy, 2019, 60, 557–566 CrossRef CAS.
- X. Zheng, J. Troughton, N. Gasparini, Y. Lin, M. Wei, Y. Hou, J. Liu, K. Song, Z. Chen, C. Yang, B. Turedi, A. Y. Alsalloum, J. Pan, J. Chen, A. A. Zhumekenov, T. D. Anthopoulos, Y. Han, D. Baran, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Joule, 2019, 3, 1963–1976 CrossRef CAS.
- X. D. Zhu, X. J. Ma, Y. K. Wang, Y. Li, C. H. Gao, Z. K. Wang, Z. Q. Jiang and L. S. Liao, Adv. Funct. Mater., 2019, 29, 1807094 Search PubMed.
- Z. Hawash, L. K. Ono and Y. Qi, Adv. Mater. Interfaces, 2018, 5, 1700623 CrossRef.
- F. Cheng, R. He, S. Nie, C. Zhang, J. Yin, J. Li, N. Zheng and B. Wu, J. Am. Chem. Soc., 2021, 143, 5855–5866 CrossRef CAS PubMed.
- X. Zhu, B. Cheng, X. Li, J. Zhang and L. Zhang, Appl. Surf. Sci., 2019, 487, 32–40 CrossRef CAS.
- I. Ka, I. M. Asuo, R. Nechache and F. Rosei, Chem. Eng. J., 2021, 423, 130334 CrossRef CAS.
- G. A. Sepalage, S. Meyer, A. R. Pascoe, A. D. Scully, U. Bach, Y.-B. Cheng and L. Spiccia, Nano Energy, 2017, 32, 310–319 CrossRef CAS.
- I. S. Yang, S. Lee, J. Choi, M. T. Jung, J. Kim and W. I. Lee, J. Mater. Chem. A, 2019, 7, 6028–6037 RSC.
- R. Wang, R. Nakar, Y. Jiang, N. Berton, S. Wu, Q. Wang, J.-M. Liu, G. Zhou, K. Kempa, B. Schmaltz and J. Gao, J. Mater. Chem. A, 2020, 8, 16527–16533 RSC.
- A. Krishna, H. Zhang, Z. Zhou, T. Gallet, M. Dankl, O. Ouellette, F. T. Eickemeyer, F. Fu, S. Sanchez, M. Mensi, S. M. Zakeeruddin, U. Rothlisberger, G. N. Manjunatha Reddy, A. Redinger, M. Gratzel and A. Hagfeldt, Energy Environ. Sci., 2021, 14, 5552–5562 RSC.
- R. Zhao, L. Xie, R. Zhuang, T. Wu, R. Zhao, L. Wang, L. Sun and Y. Hua, ACS Energy Lett., 2021, 6, 4209–4219 CrossRef CAS.
- K. Ma, H. R. Atapattu, Q. Zhao, Y. Gao, B. P. Finkenauer, K. Wang, K. Chen, S. M. Park, A. H. Coffey, C. Zhu, L. Huang, K. R. Graham, J. Mei and L. Dou, Adv. Mater., 2021, 33, 2100791 CrossRef CAS PubMed.
- S. Wang, Y. Zhu, C. Wang and R. Ma, J. Mater. Chem. A, 2019, 7, 11867–11876 RSC.
- M. Salado, M. Andresini, P. Huang, M. T. Khan, F. Ciriaco, S. Kazim and S. Ahmad, Adv. Funct. Mater., 2020, 30, 1910561 CrossRef CAS.
- L. Liang, H. Luo, J. Hu, H. Li and P. Gao, Adv. Energy Mater., 2020, 10, 2000197 CrossRef CAS.
- F. Zhang, H. Lu, B. W. Larson, C. Xiao, S. P. Dunfield, O. G. Reid, X. Chen, M. Yang, J. J. Berry, M. C. Beard and K. Zhu, Chem, 2021, 7, 774–785 CAS.
- M. Hou, Y. Wang, X. Yang, M. Han, H. Ren, Y. Li, Q. Huang, Y. Ding, Y. Zhao, X. Zhang and G. Hou, Nano Energy, 2022, 94, 2270009 CrossRef.
- R. Li, P. Wang, B. Chen, X. Cui, Y. Ding, Y. Li, D. Zhang, Y. Zhao and X. Zhang, ACS Energy Lett., 2019, 5, 79–86 CrossRef.
- P. Boonmongkolras, S. D. H. Naqvi, D. Kim, S. R. Pae, M. K. Kim, S. Ahn and B. Shin, Sol. RRL, 2021, 2000793 CrossRef CAS.
- M. A. Mahmud, T. Duong, Y. Yin, H. T. Pham, D. Walter, J. Peng, Y. Wu, L. Li, H. Shen, N. Wu, N. Mozaffari, G. Andersson, K. R. Catchpole, K. J. Weber and T. P. White, Adv. Funct. Mater., 2019, 30, 1907962 CrossRef.
- X. Chen, W. Xu, N. Ding, Y. Ji, G. Pan, J. Zhu, D. Zhou, Y. Wu, C. Chen and H. Song, Adv. Funct. Mater., 2020, 30, 2003295 CrossRef CAS.
- X. Chen, W. Xu, Z. Shi, G. Pan, J. Zhu, J. Hu, X. Li, C. Shan and H. Song, Nano Energy, 2021, 80, 105564 CrossRef CAS.
- R. Chen, B. Long, S. Wang, Y. Liu, J. Bai, S. Huang, H. Li and X. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 24747–24755 CrossRef CAS PubMed.
- L. Liu, D. Liu, R. Sun, D. Zhou, Y. Wu, X. Zhuang, S. Liu, W. Bi, N. Wang, L. Zi, B. Zhang, Z. Shi and H. Song, Sol. RRL, 2021, 5, 2000652 CrossRef CAS.
- Y. Chen, X. Zuo, Y. He, F. Qian, S. Zuo, Y. Zhang, L. Liang, Z. Chen, K. Zhao, Z. Liu, J. Gou and S. F. Liu, Adv. Sci., 2021, 8, 2001466 CrossRef CAS PubMed.
- S. Zouhair, S. M. Yoo, D. Bogachuk, J. P. Herterich, J. Lim, H. Kanda, B. Son, H. J. Yun, U. Würfel, A. Chahboun, M. K. Nazeeruddin, A. Hinsch, L. Wagner and H. Kim, Adv. Energy Mater., 2022, 12, 2200837 CrossRef CAS.
- Z. Wu, Z. Liu, Z. Hu, Z. Hawash, L. Qiu, Y. Jiang, L. K. Ono and Y. Qi, Adv. Mater., 2019, 31, 1804284 CrossRef PubMed.
- J. Werner, G. Dubuis, A. Walter, P. Löper, S. Moon, S. Nicolay, M. Masis, S. Wolf, B. Niesen and C. Ballif, Sol. Energy Mater. Sol. Cells, 2015, 141, 407–413 CrossRef CAS.
- D. Akin Kara, K. Kara, G. Oylumluoglu, M. Z. Yigit, M. Can, J. J. Kim, E. K. Burnett, D. L. Gonzalez Arellano, S. Buyukcelebi, F. Ozel, O. Usluer, A. L. Briseno and M. Kus, ACS Appl. Mater. Interfaces, 2018, 10, 30000–30007 CrossRef CAS PubMed.
- J. Huang, K.-X. Wang, J.-J. Chang, Y.-Y. Jiang, Q.-S. Xiao and Y. Li, J. Mater. Chem. A, 2017, 5, 13817–13822 RSC.
- F. Wu, K. Yan, H. Wu, B. Niu, Z. Liu, Y. Li, L. Zuo and H. Chen, J. Mater. Chem. A, 2021, 9, 14920–14927 RSC.
- X. Zhou, M. Hu, C. Liu, L. Zhang, X. Zhong, X. Li, Y. Tian, C. Cheng and B. Xu, Nano Energy, 2019, 63, 103866 CrossRef CAS.
- K. Jiang, F. Wu, G. Zhang, P. C. Y. Chow, C. Ma, S. Li, K. S. Wong, L. Zhu and H. Yan, J. Mater. Chem. A, 2019, 7, 21662–21667 RSC.
- T. Liu, Y. Jiang, M. Qin, J. Liu, L. Sun, F. Qin, L. Hu, S. Xiong, X. Jiang, F. Jiang, P. Peng, S. Jin, X. Lu and Y. Zhou, Nat. Commun., 2019, 10, 878 CrossRef PubMed.
- Y.-C. Chin, M. Daboczi, C. Henderson, J. Luke and J.-S. Kim, ACS Energy Lett., 2022, 7, 560–568 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- D. Głowienka, D. Zhang, F. Di Giacomo, M. Najafi, S. Veenstra, J. Szmytkowski and Y. Galagan, Nano Energy, 2020, 67, 104186 CrossRef.
- M. Wang, H. Wang, W. Li, X. Hu, K. Sun and Z. Zang, J. Mater. Chem. A, 2019, 7, 26421–26428 RSC.
- H. Liu, H.-R. Liu, F. Yang, J.-E. Yang, J. Song, M. Li, Z. Li, W. C. Tsoi, M. Chinweokwu Eze, Z.-Y. Liu, H. Ma, M. Gao and Z.-K. Wang, J. Power Sources, 2020, 448, 227420 CrossRef CAS.
- Q. Zhou, J. Qiu, Y. Wang, M. Yu, J. Liu and X. Zhang, ACS Energy Lett., 2021, 1596–1606 CrossRef CAS.
- X. Xu, X. Ji, R. Chen, F. Ye, S. Liu, S. Zhang, W. Chen, Y. Wu and W. H. Zhu, Adv. Funct. Mater., 2022, 32, 2109968 CrossRef CAS.
- J. Y. Jeng, K. C. Chen, T. Y. Chiang, P. Y. Lin, T. D. Tsai, Y. C. Chang, T. F. Guo, P. Chen, T. C. Wen and Y. J. Hsu, Adv. Mater., 2014, 26, 4107–4113 CrossRef CAS PubMed.
- A. S. Subbiah, A. Halder, S. Ghosh, N. Mahuli, G. Hodes and S. K. Sarkar, J. Phys. Chem. Lett., 2014, 5, 1748–1753 CrossRef CAS PubMed.
- C. X. Guo, K. Sun, J. Ouyang and X. Lu, Chem. Mater., 2015, 27, 5813–5819 CrossRef CAS.
- W. Chen, Y. Zhou, G. Chen, Y. Wu, B. Tu, F. Z. Liu, L. Huang, A. M. C. Ng, A. B. Djurišić and Z. He, Adv. Energy Mater., 2019, 9, 1803872 CrossRef.
- X. Liu, H. W. Qiao, M. Chen, B. Ge, S. Yang, Y. Hou and H. G. Yang, Mater. Chem. Front., 2021, 5, 3614–3620 RSC.
- B. Zhang, J. Su, X. Guo, L. Zhou, Z. Lin, L. Feng, J. Zhang, J. Chang and Y. Hao, Adv. Sci., 2020, 7, 1903044 CrossRef CAS PubMed.
- S. Zhumagali, F. H. Isikgor, P. Maity, J. Yin, E. Ugur, M. De Bastiani, A. S. Subbiah, A. J. Mirabelli, R. Azmi, G. T. Harrison, J. Troughton, E. Aydin, J. Liu, T. Allen, A. U. Rehman, D. Baran, O. F. Mohammed and S. De Wolf, Adv. Energy Mater., 2021, 11, 2101662 CrossRef CAS.
- J. Zhang, J. Long, Z. Huang, J. Yang, X. Li, R. Dai, W. Sheng, L. Tan and Y. Chen, Chem. Eng. J., 2021, 426, 131357 CrossRef CAS.
- A. Sadhu, M. Rai, T. Salim, X. Jin, J. M. R. Tan, S. W. Leow, M. G. Ahmed, S. Magdassi, S. G. Mhaisalkar and L. H. Wong, Adv. Funct. Mater., 2021, 31, 2103807 CrossRef CAS.
- J. Y. Jeng, Y. F. Chiang, M. H. Lee, S. R. Peng, T. F. Guo, P. Chen and T. C. Wen, Adv. Mater., 2013, 25, 3727–3732 CrossRef CAS PubMed.
- J. You, Z. Hong, Y. M. Yang, Q. Chen, M. Cai, T. B. Song, C. C. Chen, S. Lu, Y. Liu, H. Zhou and Y. Yang, ACS Nano, 2014, 8, 1674–1680 CrossRef CAS PubMed.
- W. Chen, B. Han, Q. Hu, M. Gu, Y. Zhu, W. Yang, Y. Zhou, D. Luo, F.-Z. Liu, R. Cheng, R. Zhu, S.-P. Feng, A. B. Djurišić, T. P. Russell and Z. He, Sci. Bull., 2021, 66, 991–1002 CrossRef CAS.
- P. Patil, D. S. Mann, U. T. Nakate, Y.-B. Hahn, S.-N. Kwon and S.-I. Na, Chem. Eng. J., 2020, 397, 125504 CrossRef CAS.
- S. Wang, H. Chen, J. Zhang, G. Xu, W. Chen, R. Xue, M. Zhang, Y. Li and Y. Li, Adv. Mater., 2019, 31, 1903691 CrossRef CAS PubMed.
- H. Wang, F. Yang, N. Li, J. Song, J. Qu, S. Hayase and W.-Y. Wong, Chem. Eng. J., 2020, 392, 123677 CrossRef CAS.
- W. Yang, R. Su, D. Luo, Q. Hu, F. Zhang, Z. Xu, Z. Wang, J. Tang, Z. Lv, X. Yang, Y. Tu, W. Zhang, H. Zhong, Q. Gong, T. P. Russell and R. Zhu, Nano Energy, 2020, 67, 104189 CrossRef CAS.
- R. Ma, Z. Ren, C. Li, Y. Wang, Z. Huang, Y. Zhao, T. Yang, Y. Liang, X. W. Sun and W. C. H. Choy, Small, 2020, 16, 2002628 CrossRef CAS PubMed.
- J. Zhuang, P. Mao, Y. Luan, X. Yi, Z. Tu, Y. Zhang, Y. Yi, Y. Wei, N. Chen, T. Lin, F. Wang, C. Li and J. Wang, ACS Energy Lett., 2019, 4, 2913–2921 CrossRef CAS.
- Q. Yang, X. Liu, S. Yu, Z. Feng, L. Liang, W. Qin, Y. Wang, X. Hu, S. Chen, Z. Feng, G. Hou, K. Wu, X. Guo and C. Li, Energy Environ. Sci., 2021, 14, 6536–6545 RSC.
- B. Li, Y. Xiang, K. D. G. I. Jayawardena, D. Luo, Z. Wang, X. Yang, J. F. Watts, S. Hinder, M. T. Sajjad, T. Webb, H. Luo, I. Marko, H. Li, S. A. J. Thomson, R. Zhu, G. Shao, S. J. Sweeney, S. R. P. Silva and W. Zhang, Nano Energy, 2020, 78, 105249 CrossRef CAS.
- Z. W. Gao, Y. Wang, D. Ouyang, H. Liu, Z. Huang, J. Kim and W. C. H. Choy, Small Methods, 2020, 4, 2000478 CrossRef CAS.
- M. Degani, Q. An, M. A. Siguan, Y. J. Hofstetter, C. Cho, F. Paulus, G. Grancini and Y. Vaynzof, Sci. Adv., 2021, 7, eabj7930 CrossRef CAS PubMed.
- H. Wang, J. Song, J. Qu, J. Lian, P. C. Qian and W. Y. Wong, J. Mater. Chem. A, 2020, 8, 18117–18124 RSC.
- G. Wang, K. Zhang, Z. Wang, J. Wang, R. Xu, L. Li, X. Xu, Y. Li, S. Xiao, S. Zheng, X. Li and S. Yang, Nano Energy, 2021, 89, 106374 CrossRef CAS.
- J. Wang, J. Li, Y. Zhou, C. Yu, Y. Hua, Y. Yu, R. Li, X. Lin, R. Chen, H. Wu, H. Xia and H. L. Wang, J. Am. Chem. Soc., 2021, 143, 7759–7768 CrossRef CAS PubMed.
- X. Li, S. Fu, W. Zhang, S. Ke, W. Song and J. Fang, Sci. Adv., 2020, 6, eabd1580 CrossRef CAS PubMed.
- Z. Ying, X. Yang, J. Zheng, Y. Zhu, J. Xiu, W. Chen, C. Shou, J. Sheng, Y. Zeng, B. Yan, H. Pan, J. Ye and Z. He, J. Mater. Chem. A, 2021, 9, 12009–12018 RSC.
| This journal is © The Royal Society of Chemistry 2022 |