Molecularly-imprinted hydrogel beads via self-sacrificing micro-reactors as safe and selective bilirubin adsorbents†
Shiqi
Yin
a,
Yinghui
Xu
a,
Zhoujun
Wang
a,
Zhiwei
Wei
a,
Tao
Xu
a,
Weifeng
Zhao
 *a and
Changsheng
Zhao
*a and
Changsheng
Zhao
 *ab
*ab
aCollege of Polymer Science and Engineering, State Key Laboratory of Polymer Materials Engineering, Sichuan University, Chengdu 610065, China. E-mail: zhaochsh70@scu.edu.cn
bCollege of Chemical Engineering, Sichuan University, Chengdu, 610065, China
First published on 2nd November 2021
Abstract
For patients who are suffering from liver dysfunction or metabolic obstruction, excessive bilirubin (BIL) in their bodies may cause jaundice with irreversible cerebral injury. Traditional exchange transfusion and photodynamic therapy pose a risk of serious adverse reactions or limited curative effects. Therefore, as a generally used treatment, hemoperfusion (HP) purifies patients’ blood with solid adsorbents. However, the development of clinical BIL absorbents is greatly impeded by low selectivity and unsatisfactory blood compatibility. Herein, inspired by oviparity, we propose BIL-imprinted poly(acrylic acid-co-sodium p-styrenesulfonate)–reduced graphene oxide (PAA–SS–rGO@BIL) hydrogel beads as BIL adsorbents via self-sacrificing micro-reactors. In the micro-reactors, cross-linked polymerization is achieved and a solidified gel is formed. The received hydrogel beads show outstanding selective adsorption capabilities toward BIL due to the recognition sites, and π–π and hydrophobic interactions. Such hydrogel beads possess superior blood compatibility owing to their bioinspired heparin-mimicking gel structure. Simulated BIL selective adsorption experiments in vitro demonstrate that the BIL concentrations in the plasma of a patient with severe jaundice can be restored to a moderate level within 3 hours. Therefore, hydrogel beads offer new options for clinical BIL adsorption.
Introduction
In patients with liver dysfunction or metabolic obstruction, the metabolic system cannot excrete excessive bilirubin (BIL) regularly. The accumulated BIL in their bodies will lead to jaundice with cerebral injury which is not reversible, and even death may occur in the most severe cases.1–7 As a consequence, it is indispensable to achieve an efficient, quick and safe removal of BIL concentrations in the human body.8,9 As a typical clinical therapy, blood purification comprises hemodialysis, hemofiltration and hemoperfusion (HP), and HP receives increased attention.10–14 HP can remove toxins when patients’ blood passes through the hemoperfusion apparatus, and HP is effective in the removal of micromolecular toxins, drugs and wastes. Therefore, as a commmonly used therapy, many adsorbents for BIL removal in HP have been developed based on a variety of adsorption mechanisms. Activated carbon, mesoporous silica and amino functionalized polymeric particles are the most frequently used BIL adsorbents.15–17 However, the development of these adsorbents is impeded by some shortcomings, such as the low selective adsorption capacity and undesirable blood compatibility.18–20 Moreover, to prevent coagulation in vitro, patients need low-molecular-weight heparin or heparin sodium injection in the conventional HP process. An overdose of heparin as an anticoagulant increases the cost of treatment and the risk of thrombocytopenia or hemorrhage.21–23 Therefore, it is of great significance to increase the selective adsorption ability and hemocompatibility of the adsorbents.Molecularly imprinted materials have been commonly used in the separation and purification of proteins, since they can offer advantages such as high selectivity and long-term storage stability, which also benefit the adsorption process of adsorbents in blood purification.24–26 In classical imprinting, the templates are added into the polymerization system and are extracted from the bulk after polymerization, followed by several post-treatment processes to obtain the desired particle size and expose the recognition sites. The molecular imprinting technique shows apparent advantages due to the quick and simple preparation process, and high purity of the obtained materials. However, the traditional imprinted matrix suffers from obvious disadvantages of inefficient combination with target molecules and difficulty in extracting templates.27–29
Hydrogel spheres with enormous surface areas are appropriate for column applications in the clinical HP process, which are suitable options to solve the above-mentioned problems.30–32 However, the typically applied methods for the fabrication of hydrogel spheres need to overcome the problems of harsh solvents and complicated processes; moreover, the diameters of the acquired materials are inadaptable for column operations. To solve these problems, Song et al. fabricated hydrogel beads using self-sacrificing micro-reactors, which realize the inner cross-linked polymerization to obtain the gel structure and succeeding automatic redissolution of shell.33 Therefore, inspired by oviparity, we utilize this bioinspired strategy to fabricate imprinted hydrogel spheres to promote the selective adsorption process with target molecules.
Heparin, a natural sulfated polysaccharide, has aroused extensive attention because of its outstanding blood compatibility.34–38 Extensive research on heparin-like hydrogels has been done to enhance the blood compatibility of materials. Due to the abundant carboxyl and sulfonated groups with similar bioactivities to heparin, they can inhibit coagulation activity and promote angiogenesis effectively.39–41 In our previous studies, Deng et al. modified polymeric membranes with p-styrenesulfonate (SS) to improve the hemocompatibility.42 Huang et al. utilized several heparin-mimicking chitosan with varieties of carboxymethyl and sulfate groups for blood purification.43 Song et al. incorporated sulfonic groups, hydroxyl groups and carboxyl groups within the hydrogel 3D networks to synthesize reinforced anticoagulant hydrogel microspheres which had no effect on the structures of hemocyte and plasma proteins.44 Sodium p-styrenesulfonate (SSS) possesses abundant π–π conjugation structures and sulfonated groups, which provide strong interactions for bilirubin and great blood compatibility. Acrylic acid (AA) possesses abundant carboxyl groups, and PAA possesses good mechanical strength. We anticipate that by utilizing self-sacrificing micro-reactors, BIL imprinted heparin-like hydrogel beads with outstanding blood compatibility can be produced.
Herein, a hydrogel precursor aqueous solution (monomers (AA, SSS)), an initiating agent (ammonium persulfate (APS)), a cross-linking agent (N,N′-methylene bisacrylamide (MBA)), templates (BIL) and graphene oxide (GO) suspended in deionized (DI) water and a micro-reactor solution (polyethersulfone (PES) dissolved in N,N′-dimethylacetamide (DMAc)) were prepared first. Then, we dripped the hydrogel precursor aqueous solution into the micro-reactor solution at 70 °C. Based on the liquid–liquid phase separation, the temporary PES protective shells wrapped the hydrogel precursor solution droplets to ensure the completion of the inner polymerization. After that, the PES shell was self-disintegrated and the hydrogel beads were obtained. We introduced GO to ensure that the droplets have suitable viscosity, and utilized the hydrophobicity of reduced GO (rGO) to increase the adsorption capability of hydrogel beads. Therefore, the imprinted hydrogel beads with bioinspired heparin-like structures can remove the BIL through the recognition sites, and π–π and hydrophobic triple-mechanism interactions. To testify the successful synthesis of PAA–SS–rGO@BIL beads, thermogravimetric analysis (TGA), X-ray photoelectron spectroscopy (XPS) and Fourier transform infrared spectroscopy (FTIR) are performed, and their morphologies are observed by scanning electron microscopy (SEM). As blood-contacting materials, the hydrogel beads are also investigated through blood compatibility experiments. Bilirubin adsorption experiments and simulated hemoperfusion experiments in vitro are performed to test the BIL adsorption ability of the hydrogel beads.
Experimental
Materials
DMAc was purchased from Chengdu Kelong Chemical Reagent Co. Ltd. PES was purchased from BASF chemical company. The monomers (AA and SSS), cross-linking agent (MBA), initiating agent (APS), templates (BIL), GO (≥96%) and others were all purchased from Aladdin Reagent Co. Ltd. DI water was used freshly throughout the research.Preparation of hydrogel beads
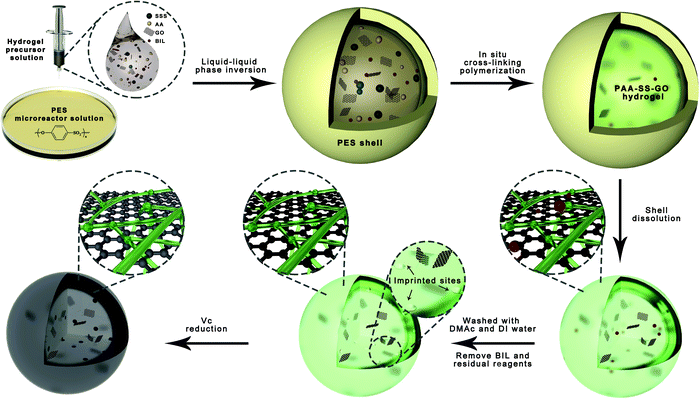
We used a bioinspired strategy via self-sacrificing micro-reactors to produce hydrogel beads. In the first step, we placed 14 g of PES and 86 g of DMAc into a 200 mL glass Petri dish and stirred the mixture vigorously for 2 hours at 40 oC, and then the homogeneous PES micro-reactor solution was received. Second, we prepared the hydrogel precursor solutions of PAA–SS–GO@BIL and PAA–SS–rGO@BIL beads by dissolving the monomers (AA and SSS with a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio and a total concentration of 60 wt%), cross-linking agent (MBA, 6 wt%), initiating agent (APS, 2.4 wt%), templates (BIL, 3.6 wt%) and GO (6 wt%) in DI water homogeneously. Similarly, the hydrogel precursor solutions of PAA–SS–GO and PAA–SS–rGO beads were prepared using the same method, but without the templates. Third, the PES solution was preheated to 70 °C for 1 hour by placing the glass Petri dish on a self-acting thermostat, then the hydrogel precursor solution was dripped into it using a 0.7 mm-diameter syringe needle, and then the PES protective shells wrapped the droplets immediately by the liquid–liquid phase inversion process, as shown in Fig. 1. After incubating the temporary core@shell droplets in the micro-reactor solution at 70 °C for 20 minutes, the hydrogel beads were formed and the PES shells were re-dissolved. Later on, the hydrogel beads were separated and washed with DMAc and DI water to elute the residual solvent and templates completely. Half of the PAA–SS–GO and PAA–SS–GO@BIL beads were immersed into ascorbic acid solution (100 mg L−1) at 60 °C for 4 hours to obtain the PAA–SS–rGO and PAA–SS–rGO@BIL beads, respectively. The dried hydrogel beads can be kept for over three months.
1 mass ratio and a total concentration of 60 wt%), cross-linking agent (MBA, 6 wt%), initiating agent (APS, 2.4 wt%), templates (BIL, 3.6 wt%) and GO (6 wt%) in DI water homogeneously. Similarly, the hydrogel precursor solutions of PAA–SS–GO and PAA–SS–rGO beads were prepared using the same method, but without the templates. Third, the PES solution was preheated to 70 °C for 1 hour by placing the glass Petri dish on a self-acting thermostat, then the hydrogel precursor solution was dripped into it using a 0.7 mm-diameter syringe needle, and then the PES protective shells wrapped the droplets immediately by the liquid–liquid phase inversion process, as shown in Fig. 1. After incubating the temporary core@shell droplets in the micro-reactor solution at 70 °C for 20 minutes, the hydrogel beads were formed and the PES shells were re-dissolved. Later on, the hydrogel beads were separated and washed with DMAc and DI water to elute the residual solvent and templates completely. Half of the PAA–SS–GO and PAA–SS–GO@BIL beads were immersed into ascorbic acid solution (100 mg L−1) at 60 °C for 4 hours to obtain the PAA–SS–rGO and PAA–SS–rGO@BIL beads, respectively. The dried hydrogel beads can be kept for over three months.
 | ||
| Fig. 1 The schematic illustration of the fabrication of hydrogel beads via self-sacrificing micro-reactors. | ||
Characterization of hydrogel beads
A Q500 thermogravimetric analyzer (TG209F1, Netzsch, Germany) was used to obtain the TGA curves from 25 to 900 oC under a nitrogen atmosphere. The XPS spectra were investigated using an X-ray photoelectron spectrometer (Nicolet 560, USA). The chemical structures were obtained from attenuated total reflection FTIR (ATR-FTIR, Nicolet 560) spectra between 600 and 4000 cm−1. A scanning electron microscope (JSM-7610F, JEOL, Japan) was employed to characterize the morphologies on both the surface and cross-section of the hydrogel beads.The average diameters of the hydrogel beads were calculated using the diameters of at least 6 hydrogel beads which were measured with a rectilinear scale. A universal testing machine (SANS CMT4000, MTS) was employed to investigate the mechanical capacities of the hydrogel beads. To further investigate their mechanical properties, we used a blood pump to make water flow through a syringe needle packed with the PAA–SS–rGO@BIL beads circularly for 60 min, to simulate the flowing pressure in hemoperfusion.
Hemocompatibility experiments
The hemocompatibility of the hydrogel beads was investigated by hemolysis analysis, platelet adhesion, contact activation level, blood routine analysis and clotting time. The detailed procedures are shown in the ESI.†Bilirubin adsorption experiments
The hydrogel beads (0.01 g) were added into the BIL solutions (dissolved 3 mg of BIL and 40 mg of NaOH into 10 mL of PBS) under oscillation for 2 h. The concentrations of BIL in the solutions before and after adsorption were measured using a UV-vis spectrophotometer (UV-1750, Shimadzu) at 438 nm. All the adsorption experiments were kept in the dark environment, since BILs are easily oxidized into biliverdin by strong light.Simulated bilirubin selective removal process in HP
To further investigate the practical application of the PAA–SS–rGO@BIL beads as HP absorbents, 0.01 g of the beads were packed into the syringe to simulate the HP process. Restricted to the experimental conditions, the removal ability was tested in simulated plasma instead of whole blood. BILs (340 μg mL−1) (concentration of the patient with severe jaundice) were first dissolved in the plasma (10 mL), and then the plasma was packed into the syringe to investigate the dynamic adsorption process. Uric acid (UA) and creatine (CREA) have a similar structure to BIL. For another group, 340 μg mL−1 BIL, 2.05 mg mL−1 UA and 3.85 mg mL−1 CREA were dissolved in the plasma to discuss the selective adsorption ability of the PAA–SS–rGO@BIL beads.Results and discussion
Preparation and characterization of PAA–SS–rGO@BIL beads
In this study, the structures of PAA–SS–rGO@BIL beads were well designed, and the bioinspired fabrication process of the beads via self-sacrificing micro-reactors is shown in Fig. 1. The actual compositions of the different beads are shown in Table 1.| Samples | AA (g) | SSS (g) | GO/rGO (g) | MBA (g) | APS (g) | BIL (g) |
|---|---|---|---|---|---|---|
| PAA–SS–GO/PAA–SS–rGO | 4.8 | 1.2 | 0.6 | 0.6 | 0.24 | — |
| PAA–SS–GO@BIL/PAA–SS–rGO@BIL | 4.8 | 1.2 | 0.6 | 0.6 | 0.24 | 0.2 |
As shown in Fig. 1, when the hydrogel precursor solution was dropwise added into the PES solution, a liquid–liquid phase inversion process occurred and the PES protective shells wrapped the droplets immediately because PES was insoluble in water. Then, the polymerization reaction began in the micro-reactors and the polymerization time was shorter than the normal cross-linking polymerization time because of the small volume of the droplets and the high-temperature environment outside. In the polymerization process, the micro-reactors served as the containers of the cross-linking polymerization system and the templates of the droplets in shape. Along with polymerization, the PES shells were re-dissolved in DMAc gradually after the formation of hydrogel beads.
GO was introduced into the hydrogel precursor solution because of its thickening properties, so the droplets could maintain a spherical shape. Furthermore, rGO can enhance the adsorption ability by hydrophobic and π–π interactions. Due to the unsatisfactory blood compatibilities of free GO and rGO, it is feasible to assemble them in the hydrogel structures, so that we can utilize them for BIL adsorption. The introduction of carboxyl contained monomer (AA) and sulfonated group contained monomer (SSS) is a promising way to enhance the blood compatibility; the introduced π–π structure of the functional monomers (SSS) can also enhance the BIL adsorption ability of the hydrogel beads.
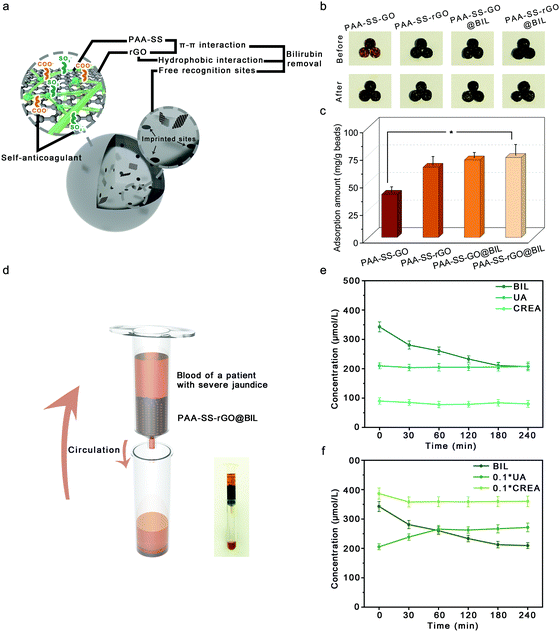
The average diameters of the PAA–SS–GO, PAA–SS–rGO, PAA–SS–GO@BIL and PAA–SS–rGO@BIL beads were 2.32, 1.98, 2.38 and 2.36 mm, respectively (Fig. S1, ESI†). The unsmooth surface of the hydrogel beads resulted from the self-sacrificing micro-reactors, which was inevitable and had no effect on the hemocompatibility. As the color change is shown in the figure, the PAA–SS–GO beads showed amber color, and after the reduction or introduction of the templates, the color turned to homogeneous black, preliminarily illustrating the successful fabrication of the PAA–SS–rGO@BIL beads.
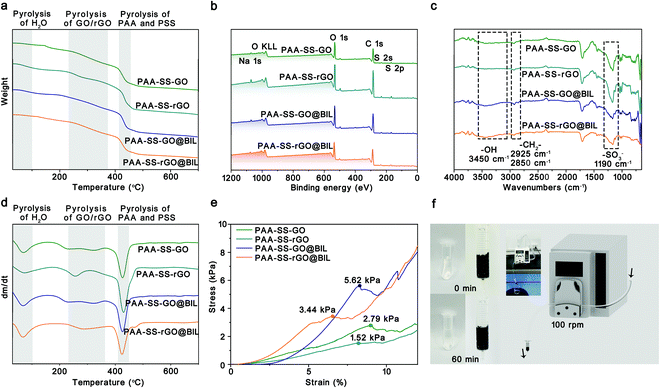
As shown in Fig. 2a–d, the TGA, XPS, and FTIR spectra of the PAA–SS–GO, PAA–SS–rGO, PAA–SS–GO@BIL and PAA–SS–rGO@BIL beads were detected to investigate their chemical structures. In the TGA curves, three degradation platforms corresponding to the pyrolysis of residual bound water (0–100 °C), GO/rGO (230–380 °C) and PAA/PSS (405–430 °C) can be observed, respectively. The mass loss of PAA and PSS (≈24 wt%) appeared between 405 and 430 °C; besides, the mass loss of bound water (≈3 wt%) illustrated that the hydrogel beads possess superior hydrophilic properties. In the XPS survey scan spectra, the Na 1s (1072 eV), O 1s (532 eV), S 2s (229 eV) and S 2p (164 eV) signals of the hydrogel beads can be clearly noticed, and the C, N, O, S and Na element contents are summarized in Table 2. The C, N, O, S and Na atom ratios in the PAA–SS–rGO@BIL beads were 70.64, 0.79, 25.53, 2.60, and 0.43 At%, respectively. By estimation, the general contents of the –SO3− group (corresponding to the S and Na elements) and –COO− group in the PAA–SS–rGO@BIL beads were about 0.813 and 6.759 mmol g−1, respectively. In the FTIR spectra, the characteristic peaks of PAA–SS–rGO@BIL emerged at 3450 (corresponding to the –OH group in bound water and –COOH), 2925, 2850 (corresponding to the C–H in –CH2–) and 1190 cm−1 (corresponding to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in –SO3−), respectively, indicating that the sulfo acid group and carboxy group were abundant on the hydrogel bead interfaces. All the above results demonstrated that the PAA–SS–rGO@BIL hydrogel beads had been synthesized successfully.
O in –SO3−), respectively, indicating that the sulfo acid group and carboxy group were abundant on the hydrogel bead interfaces. All the above results demonstrated that the PAA–SS–rGO@BIL hydrogel beads had been synthesized successfully.
| Samples | Element content (At%) | Estimated –SO3− contenta (mmol g−1) | Estimated –COO− contenta (mmol g−1) | ||||
|---|---|---|---|---|---|---|---|
| C | N | O | S | Na | |||
| a The content of –SO3− is calculated using the S peak area, and the content of –COO− is calculated using the S peak area. | |||||||
| PAA–SS–GO | 69.90 | 1.16 | 26.43 | 1.98 | 0.53 | 0.619 | 7.330 |
| PAA–SS–rGO | 70.45 | 0.97 | 25.34 | 2.32 | 0.92 | 0.725 | 6.831 |
| PAA–SS–GO@BIL | 71.25 | 0.78 | 25.52 | 2.00 | 0.46 | 0.625 | 7.038 |
| PAA–SS–rGO@BIL | 70.64 | 0.79 | 25.53 | 2.60 | 0.43 | 0.813 | 6.759 |
As HP absorbents, good mechanical properties are required to prevent the absorbents from breaking and entering the blood. To further characterize the mechanical properties of the PAA–SS–rGO@BIL beads, as shown in Fig. 2e and f, compression tests were carried out systematically. The stress–strain curves of the PAA–SS–GO, PAA–SS–rGO, PAA–SS–GO@BIL and PAA–SS–rGO@BIL beads were monitored with an electronic universal testing machine. The maximum compressive strengths of PAA–SS–GO, PAA–SS–rGO, PAA–SS–GO@BIL and PAA–SS–rGO@BIL were measured to be 2.79, 1.52, 5.62 and 3.44 kPa corresponding to the first peaks of the curves, respectively. To judge the clinical application of the hydrogel beads objectively, a flowing pressure simulation experiment was employed. DI water in the HP simulating device was clear after 60 min of circulation and no broken beads were observed, which indicated that the PAA–SS–rGO@BIL beads kept the morphology intact during HP for over 60 min.
Morphologies of K-GO/PSS beads
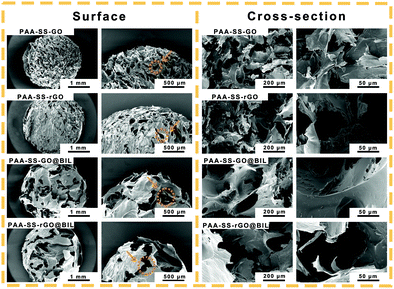
To characterize the morphologies of the prepared hydrogel beads, the hydrogel beads were observed by SEM and the images are shown in Fig. 3. The porous channel structures of the hydrogels were displayed on the surface and inside of the PAA–SS–GO, PAA–SS–rGO, PAA–SS–GO@BIL and PAA–SS–rGO@BIL beads. Furthermore, after adding templates into the hydrogel precursor solution, the diameter of the channel structure showed significant growth, which would improve the removal efficiency of BIL and increase the probability of serving as HP absorbents for the hydrogel beads.Hemocompatibility of hydrogel beads
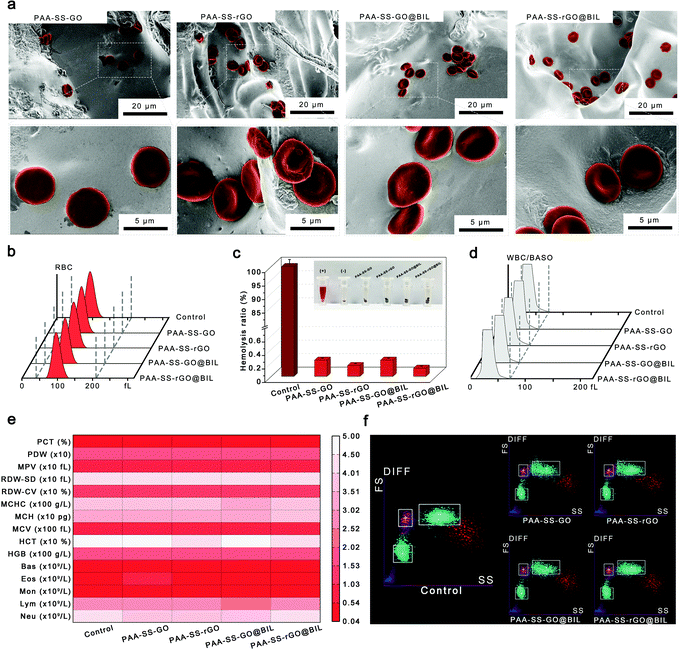
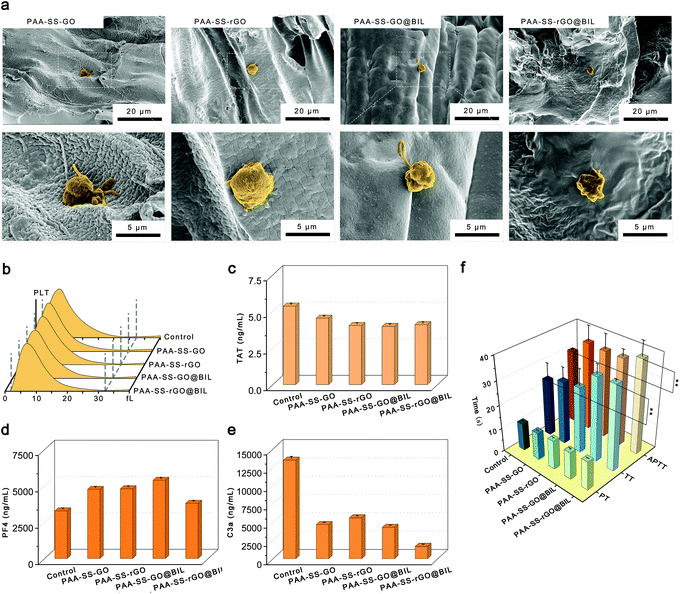
To testify the security as HP absorbents, the hemocompatibility of the PAA–SS–rGO@BIL beads was investigated by hemolysis experiment, platelet adhesion experiment, complement activation assay, blood routine examination and clotting time.Selective bilirubin removal ability of hydrogel beads
As HP absorbents, a single adsorption mechanism always results in low removal efficiency; therefore, the PAA–SS–rGO@BIL beads with triple-model selective adsorption mechanisms were developed to efficiently absorb BIL. As shown in Fig. 6a, on the surfaces of the PAA–SS–rGO@BIL beads, the abundant π–π conjugate structures enhanced the adsorption ability of the hydrogel beads by π–π interactions. Furthermore, the recognition sites and the hydrophobic interaction between rGO and BIL enhanced the combination. The color changes of different beads before and after adsorption are shown in Fig. 6b, and the BIL adsorption results were obtained (Fig. 6c). We calculated the BIL adsorbed amounts M(mg g−1) by the following formula:| M = (C0 − C) * V/M0 |
As shown in Fig. 6d, to further investigate the possibilities for the PAA–SS–rGO@BIL beads as HP BIL absorbents, 0.01 g of the beads were packed into the syringe to simulate the HP process. First, we dissolved 340 μg mL−1 BIL (concentration of the patient with severe jaundice) in the plasma; then, we added the plasma (10 mL) into the device to investigate the dynamic adsorption process; for another group of 340 μg mL−1 BIL, we added 20.5 mg of uric acid and 38.5 mg of creatine in the plasma additionally to discuss the selective adsorption ability of the PAA–SS–rGO@BIL beads. During the process, the BIL, UA and CREA concentration change curves were detected as shown in Fig. 6e and f. The concentration of BIL can be restored to a moderate level within 3 hours by such a simulated HP process, no matter whether the competitive adsorptions are existing or not.
We introduced a selective coefficient (λ), which is the ratio of the adsorption amounts of BIL to UA or CREA, as follows
| λUA = PBIL/PUA, λCREA = PBIL/PCREA |
All these results demonstrated that the PAA–SS–rGO@BIL beads with triple adsorption mechanisms and heparin-like structures had outstanding hemocompatibility and robust BIL selective adsorption capacity; thus, the beads have the potential to serve as HP absorbents.
Conclusions
In summary, we have successfully fabricated BIL-imprinted heparin-like hydrogel beads with triple-model adsorption mechanisms which can be applied as bilirubin absorbents in HP. The PAA–SS–rGO@BIL beads possess homogeneous diameters (2.36 ± 0.44 mm). The bioinspired heparin-like functional groups ensure the low hemolysis ratio (0.2%), low PLT adhesion and inhibition effect of contact activation of the beads, demonstrating the outstanding hemocompatibility of the PAA–SS–rGO@BIL beads. Systematic coagulation tests demonstrate that the clotting time can be prolonged by the hydrogel beads, and the injected dose of heparin can be decreased. Furthermore, the PAA–SS–rGO@BIL beads exhibit superior selective adsorption capabilities of BIL due to their recognition sites, and π–π and hydrophobic triple-mechanism interactions. The simulated BIL removal experiment indicates that the BIL concentrations in the plasma can be restored to a moderate level within 3 hours by such a simulated HP process. Conclusively, the PAA–SS–rGO@BIL beads possess acceptable mechanical strength, outstanding hemocompatibility, and superior selective adsorption capability, which could be used in clinical jaundice treatments.Author contributions
Shiqi Yin: investigation, formal analysis, visualization, and writing – original draft. Yinghui Xu: resources. Zhoujun Wang: visualization. Zhiwei Wei: methodology. Tao Xu: validation. Weifeng Zhao: conceptualization, project administration, and supervision. Changsheng Zhao: supervision.Conflicts of interest
The authors declare no conflict of interest regarding the publication of this article.Acknowledgements
This work was financially sponsored by the National Natural Science Foundation of China (51873115 and 52073190) and the 1.3.5 project for disciplines of excellence, West China Hospital Sichuan University (No. ZYJC21014). Chao He from the College of Polymer Science and Engineering and Yanping Huang from the College of Chemistry and Engineering, Sichuan University, are thanked for their kind support for the SEM analysis.References
- J. F. Watchko and C. Tiribelli, N. Engl. J. Med., 2013, 369, 2021–2030 CrossRef CAS PubMed.
- J. F. Watchko, Clin. Perinatol., 2016, 43, 297 CrossRef PubMed.
- Y. Wang, G. Sheng, T. Shi and X. Cheng, Biosci. Rep., 2020, 40, BSR20192152 CrossRef CAS.
- S. M. Shapiro, Semin. Fetal Neonat. Med., 2010, 15, 157–163 CrossRef PubMed.
- J. D. Ostrow, L. Pascolo, D. Brites and C. Tiribelli, Trends Mol. Med., 2004, 10, 65–70 CrossRef CAS PubMed.
- T. D. Jr. Hinds and D. E. Stec, Curr. Hypertens. Rep., 2019, 21, 1–7 CrossRef.
- L. Guo, L. Zhang, J. Zhang, J. Zhou, Q. He, S. Zeng, X. Cui and J. Shi, Chem. Commun., 2009, 6071–6073 RSC.
- X. Song, T. Xu, L. Yang, Y. Li, Y. Yang, L. Jin, J. Zhang, R. Zhong, S. Sun, W. Zhao and C. Zhao, Biomacromolecules, 2020, 21, 1762–1775 CrossRef CAS.
- B. R. Mueller, Carbon, 2010, 48, 3607–3615 CrossRef CAS.
- J. D. King, M. H. Kern and B. G. Jaar, Clin. J. Am. Soc. Nephrol., 2019, 14, 1408–1415 CrossRef CAS PubMed.
- P. M. Honore, D. De Bels, R. Attou, S. Redant and K. Kashani, Crit. Care, 2019, 23, 1–2 CrossRef PubMed.
- S. Bourcier, P. Hindlet, B. Guidet and A. Dechartres, Crit. Care Med., 2019, 47, 984–992 CrossRef.
- E. G. Clark, S. Hiremath, L. McIntyre, R. Wald, G. L. Hundemer and M. Joannidis, Nat. Rev. Nephrol., 2020, 16, 697–699 CrossRef CAS.
- P. M. Honore, E. Hoste, Z. Molnar, R. Jacobs, O. Joannes-Boyau, M. L. N. G. Mabrain and L. G. Forni, Ann. Intensive Care, 2019, 9, 1–13 CAS.
- J. Chen, Y. Ma, L. Wang, W. Han, Y. Chai, T. Wang, J. Li and L. Ou, Carbon, 2019, 143, 352–361 CrossRef CAS.
- Z. Li, X. Huang, K. Wu, Y. Jiao and C. Zhou, Mater. Sci. Eng., C, 2020, 106, 110282 CrossRef CAS.
- Y. Yang, S. Yin, C. He, X. Wu, J. Yin, J. Zhang, L. Ma, W. Zhao, C. Cheng and C. Zhao, J. Mater. Chem. B, 2020, 8, 1960–1970 RSC.
- R. Zhao, T. Ma, F. Cui, Y. Tian and G. Zhu, Adv. Sci., 2020, 7, 2001899 CrossRef CAS PubMed.
- J. Chen, Y. D. Ma, L. C. Wang, W. Y. Han, Y. M. Chai, T. T. Wang, J. Li and L. L. Ou, Carbon, 2019, 143, 352–361 CrossRef CAS.
- R. Zhao, Y. M. Li, X. Li, Y. Z. Li, B. L. Sun, S. Chao and C. Wang, J. Colloid Interface Sci., 2018, 514, 675–685 CrossRef CAS.
- M. Marchetti, M. G. Zermatten, D. Bertaggia Calderara, A. Aliotta and L. Alberio, J. Clin. Med., 2021, 10, 683 CrossRef CAS PubMed.
- K. Krauel, P. Preusse, T. E. Warkentin, C. Trabhardt, S. Brandt, I. Jensch, M. Mandelkow, E. Hammer, S. Hammerschmidt and A. Greinacher, Blood, 2019, 133, 978–989 CrossRef CAS.
- W. N. Whiteley, H. P. Jr. Adams, P. M. W. Bath, E. Berge, P. M. Sandset, M. Dennis, G. D. Murray, K. S. L. Wong and P. A. G. Sandercock, Lancet Neurol., 2013, 12, 539–545 CrossRef CAS.
- K. Yang, S. Li, L. Liu, Y. Chen, W. Zhou, J. Pei, Z. Liang, L. Zhang and Y. Zhang, Adv. Mater., 2019, 31, 1902048 CrossRef CAS.
- M. J. Whitcombe, I. Chianella, L. Larcombe, S. A. Piletsky, J. Noble, R. Porter and A. Horgan, Chem. Soc. Rev., 2011, 40, 1547–1571 RSC.
- S. Li, K. Yang, B. Zhao, X. Li, L. Liu, Y. Chen, L. Zhang and Y. Zhang, J. Mater. Chem. B, 2016, 4, 2739–2739 RSC.
- L. X. Chen, S. F. Xu and J. H. Li, Chem. Soc. Rev., 2011, 40, 2922–2942 RSC.
- Q. Yang, J. H. Li, X. Y. Wang, H. L. Peng, H. Xiong and L. X. Chen, Biosens. Bioelectron., 2018, 112, 54–71 CrossRef CAS.
- C. S. Mahon and D. A. Fulton, Nat. Chem., 2014, 6, 665–672 CrossRef CAS PubMed.
- N. L. Cuccia, S. Pothineni, B. Wu, J. M. Harper and J. C. Burton, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 11247–11256 CrossRef CAS.
- X. Liu, C. Steiger, S. Lin, G. A. Parada, J. Liu, H. F. Chan, H. Yuk, V. P. Nhi, J. Collins, S. Tamang, G. Traverso and X. Zhao, Nat. Commun., 2019, 10, 493 CrossRef CAS.
- C. C. Piras, P. Slavik and D. K. Smith, Angew. Chem., Int. Ed., 2020, 59, 853–859 CrossRef CAS.
- X. Song, J. An, C. He, J. Zhou, Y. Xu, H. Ji, L. Yang, J. Yin, W. Zhao and C. Zhao, J. Mater. Chem. A, 2019, 7, 21386–21403 RSC.
- I. Capila and R. J. Linhardt, Angew. Chem., Int. Ed., 2002, 41, 391–412 CrossRef PubMed.
- T. E. Warkentin, M. N. Levine, J. Hirsh, P. Horsewood, R. S. Roberts, M. Gent and J. G. Kelton, N. Engl. J. Med., 1995, 332, 1330–1335 CrossRef CAS.
- J. I. Weitz, N. Engl. J. Med., 1997, 337, 688–698 CrossRef CAS PubMed.
- S. Valimaki, Q. Liu, L. Schoonen, D. F. M. Vervoort, Nonappa, V. Linko, R. J. M. Nolte, J. C. M. van Hest and M. A. Kostiainen, J. Mater. Chem. B, 2021, 9, 1272–1276 RSC.
- Z. Xiao, B. R. Tappen, M. Ly, W. Zhao, L. P. Canova, H. Guan and R. J. Linhardt, J. Mater. Chem., 2011, 54, 603–610 CAS.
- L. Ma, C. Cheng, C. He, C. Nie, J. Deng, S. Sun and C. Zhao, Acs Appl. Mater. Inter., 2015, 7, 26050–26062 CrossRef CAS.
- Z. Oezyuerek, K. Franke, M. Nitschke, R. Schulze, F. Simon, K. J. Eichhorn, T. Pompe, C. Werner and B. Voit, Biomaterials, 2009, 30, 1026–1035 CrossRef CAS PubMed.
- S. N. Pawar and K. J. Edgar, Biomaterials, 2012, 33, 3279–3305 CrossRef CAS.
- J. Deng, X. Liu, L. Ma, C. Cheng, W. Shi, C. Nie and C. Zhao, ACS Appl. Mater. Interfaces, 2014, 6, 21603–21614 CrossRef CAS PubMed.
- X. Huang, R. Wang, T. Lu, D. Zhou, W. Zhao, S. Sun and C. Zhao, Biomacromolecules, 2016, 17, 4011–4020 CrossRef CAS.
- X. Song, H. Ji, Y. Li, Y. Xiong, L. Qiu, R. Zhong, M. Tian, J. N. Kizhakkedathu, B. Su, Q. Wei, W. Zhao and C. Zhao, Nat. Biomed. Eng., 2021, 5, 1143–1156 CrossRef CAS PubMed.
- L. Yang, L. Han and L. Jia, ACS Appl. Mater. Interfaces, 2016, 8, 26570–26577 CrossRef CAS.
- P. Wang, J. Liu, X. Luo, P. Xiong, S. Gao, J. Yan, Y. Li, Y. Cheng and T. Xi, J. Mater. Chem. B, 2019, 7, 7314–7325 RSC.
- J. R. Dunkelberger and W. C. Song, Cell Res., 2010, 20, 34–50 CrossRef CAS.
- N. P. Gerard and C. Gerard, Nature, 1991, 349, 614–617 CrossRef CAS PubMed.
- M. Levi, C. H. Toh, J. Thachil and H. G. Watson, Br. J. Haematol., 2009, 145, 24–33 CrossRef CAS PubMed.
- W. C. Lin, T. Y. Liu and M. C. Yang, Biomaterials, 2004, 25, 1947–1957 CrossRef CAS PubMed.
- R. K. Kainthan, J. Janzen, E. Levin, D. V. Devine and D. E. Brooks, Biomacromolecules, 2006, 7, 703–709 CrossRef CAS PubMed.
- M. Guo, J. Wang, C. Wang, P. J. Strong, P. Jiang, Y. S. Ok and H. Wang, Sci. Total Environ., 2019, 682, 340–347 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tb01895g |
| This journal is © The Royal Society of Chemistry 2022 |