 Open Access Article
Open Access ArticleFlexible polymeric nanosized micelles for ophthalmic drug delivery: research progress in the last three years
Zhiguo
Li
a,
Minting
Liu
a,
Lingjie
Ke
a,
Li-Juan
Wang
a,
Caisheng
Wu
*a,
Cheng
Li
*b,
Zibiao
Li
 *c and
Yun-Long
Wu
*c and
Yun-Long
Wu
 *a
*a
aFujian Provincial Key Laboratory of Innovative Drug Target Research, State Key Laboratory of Cellular Stress Biology, School of Pharmaceutical Sciences, Xiamen University, Xiamen 361102, China. E-mail: wucsh@xmu.edu.cn; wuyl@xmu.edu.cn
bFujian Provincial Key Laboratory of Ophthalmology and Visual Science & Ocular Surface and Corneal Diseases, Eye Institute & Affiliated Xiamen Eye Center, School of Medicine, Xiamen University, Xiamen 361102, China. E-mail: cheng-li@xmu.edu.cn
cDepartment of Materials Science and Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore 117576, Singapore. E-mail: lizb@imre.a-star.edu.sg
First published on 10th August 2021
Abstract
The eye is a complex structure with a variety of anatomical barriers and clearance mechanisms, so the provision of safe and effective ophthalmic drug delivery technology is a major challenge. In the past few decades, a number of reports have shown that nano-delivery platforms based on polymeric micelles are of great interest, because of their hydrophobic core that encapsulates lipid-soluble drugs and small size with high penetration, allowing long-term drug retention and posterior penetration in the eye. Furthermore, as an ocular delivery platform, polymeric micelles not only cover the single micellar drug delivery system formed by poloxamer, chitosan or other polymers, but also include composite drug delivery systems like micelle-encapsulated hydrogels and micelle-embedded contact lenses. In this review, a number of ophthalmic micelles that have emerged in the last three years will be systematically reviewed, with a summary of and discussion on their unique advantages or unique drug delivery performance. Last but not least, the current challenges of polymeric micelle formulations in potential clinical ophthalmic therapeutic applications will also be proposed, which might be helpful for future design of ocular drug delivery formulations.
1. Introduction
The eye is a small and complex organ with unique anatomical barriers that make effective intraocular drug delivery a great challenge.1,2 Although the hydrophilic corneal stroma mainly allows the diffusion of hydrophilic molecules,3 the delivery of many effective hydrophobic drugs has been significantly hampered. In order to solve this problem, a number of nanomaterial based formulations have been designed and have emerged as a possible solution to solve this issue in the past few decades,4,5 in terms of nanosized micelles, liposomes, or microemulsions.6–9 As a typical example, nanomicelles have attracted much attention due to their high hydrophobic drug loading and controllable drug release abilities.10 Although there are many approaches for nanosized micelle construction, the polymeric formulation remains one of the most popular designs as polymeric micelles can be easily functionally optimized due to their flexible polymer chains.11,12Considering the design towards effective ophthalmic delivery, polymeric nanoscale carriers have great potential owing to their small size, which results in good corneal penetration, intraocular absorption, and eye irritation or tearing reduction.10,13 Furthermore, polymeric micelles can be self-assembled from hydrophobic cores and hydrophilic shells.14 This unique feature endows them with the ability to trap lipophilic drugs and prevent undesired drug exposure to a variety of biological barriers in the eye, such as corneal or conjunctival barriers.1,15,16 More importantly, made of amphiphilic molecules or block copolymers, polymeric nanosized micelles can be formed at low concentration above the critical micelle concentration (CMC).17 In comparison with low molecular weight non-ionic surfactants, polymeric micelles have a lower CMC and thus maintain a more stable shape, resulting in longer drug retention and enhanced therapeutic effects.18,19 Although there are a number of advantages of polymeric nanomicelle based formulations for ophthalmic drug delivery, drug retention time will still be shortened due to eye clearance mechanisms such as tears and blinking, leading to low efficiency of administration in the process of topical ocular administration, which leads to poor drug penetration against eye barriers.20
In recent years, polymeric nanosized micellar eye preparations have emerged in order to solve the above problems hampering ophthalmic drug delivery,21 as illustrated in Scheme 1. For example, the physical properties of polymeric micelles could be tuned by using different hydrophilic or hydrophobic fragments, such as polyethylene glycol (PEG)-based micelles for good dispersity, chitosan (CS) micelles for enhanced uptake, etc.22,23 On the one hand, the stability, adhesion, or penetration of polymeric micelle formulations could also be tailored, which is conducive to the long-term ophthalmic delivery of therapeutic drugs.24,25 Last but not least, polymer micelles have been developed as a promising technique for preventing premature disintegration and release of therapeutic drugs. To better understand the recent development in this field, this report aims to review the novel polymeric micelle preparations for ocular drug delivery published in the last three years, which are mainly divided into two categories (in terms of direct delivery carriers or indirect delivery carriers). The unique advantages, excellent properties, and drug delivery performance of these polymeric micelle formulations will be carefully described in detail and summarized. Furthermore, the characteristics of these polymer micelles and the current challenges will be discussed in a comprehensive manner. Lastly, a summary of the clinical value of these polymeric micelle formulations as well as future prospects will also be presented.
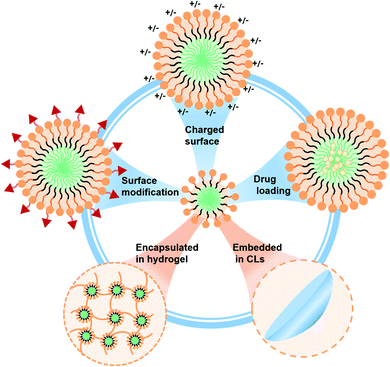 | ||
| Scheme 1 Illustration of polymeric nanosized micelle formulations with tailorable behavior for potential ophthalmic drug delivery applications. | ||
2. Characteristics of polymeric micelles
Micelles are a kind of ordered molecular aggregate, including nonionic surfactants26 and polymeric micelles.11 They both have a common feature of containing hydrophilic fragments and hydrophobic fragments. Similar to surfactants, polymeric micelles are self-assembly structures formed spontaneously by amphiphilic segments in water.10 When the concentration of block or graft copolymers in water reaches a certain level, microphase separation in the hydrophobic and hydrophilic segments of polymers will occur. As a result, the typical core–shell micelles with hydrophobic segment inward and hydrophilic segment outward will be formed automatically.Among the blocks of polymeric micelles, the hydrophilic blocks are mostly biocompatible copolymers such as polyethylene glycol (PEG), polyoxyethylene (PEO), etc.27,28 The hydrophobic blocks are mainly biodegradable copolymers such as polylactic acid (PLA),6 poly-ε-caprolactone (PCL), polybenzyl aspartic acid (PBLA), etc.29,30 By taking advantage of these hydrophilic and hydrophobic segments, three blocks of hydrophilic–hydrophobic–hydrophilic copolymers as micellar materials could be designed, such as poloxamer (PEO–PPO–PEO), etc.28 In addition to the variety of materials, polymeric micelles can form a wide variety of shapes, including spherical,15 rod-like,31 worm-like,32 dandelion flower-like,33 and star-like shapes,34 among others, and the shape of a micelle is related to the length or type of its hydrophilic and hydrophobic segments.
As the most significant parameter of micelles, the CMC determines the minimum concentration for micelle structure formation.17 When the polymer concentration is below the CMC, the polymer chains are freely dispersed in the solution as monomers. When the polymer concentration is higher than the CMC, a large number of polymeric micelles can be self-assembled. In the water-based environment inside the body, the drug might be rapidly released once the polymer concentration drops below the CMC, which means that a lower CMC is ideal.
Polymeric micelle formulations have excellent drug delivery capability. For example, drugs with good water solubility mainly solubilize on the surface of micelles, while those with poor water solubility mainly solubilize in the core of micelles. And amphiphilic drugs are mainly solubilized at the interface of hydrophilic and lipophilic micelles.15 It is worth mentioning that polymeric micelles are often used to deliver drugs with strong lipophilic properties to improve their poor water solubility. Because of their similar size to lipoproteins and viruses, they can effectively avoid the phagocytic effect in the body and remain in the blood circulation for a long time, releasing drugs slowly and extending the half-life period of drugs in the body.10,12,25,35
At present, polymeric micelles have a wide range of applications, including anti-tumor, anti-bacterial,23 anti-inflammatory,35 and wound healing activities,36 gene delivery,37 blood–brain barrier penetration and so on.38 Due to their excellent properties, polymeric micelles have many advantages over traditional dosage forms for ocular delivery, in terms of good corneal penetration, improved solubility of insoluble drugs, or increased drug retention, indicating their promising applications in ophthalmic drug delivery.
The micelles applied to the eye can be divided into direct and indirect acting vectors. As the direct carrier of polymeric micelles, most of them are eye drops or injection preparations. These micellar eye drops can increase the loading capacity of insoluble drugs compared to commercial dosage forms. In addition, these polymeric micelles have advanced their overall properties (adhesion, corneal penetrability, stability, safety, etc.) by combining different polymeric fragments in recent years.10,15 Among them, modifying groups on the surface of micelles might impart charge or targeting ability, thus improving the adhesion and specificity of micelles, which has positive significance for improving the efficiency of drug delivery.3,11 In recent years, polymeric micelles, as carriers of indirect action, have mostly existed in hydrogels or contact lenses.39 They can combine the respective characteristics of micelles, hydrogels and contact lenses to improve the ability for drug delivery in the eye. Both of these can improve the retention of micelles in the eye, reducing the drug loss by the tear washing effect. It provides a new idea for the clinical transformation of micelles as drug delivery carriers.13
3. Polymeric micelles that act directly as the delivery vector
In most eye administration systems, the polymeric micelles serve as the primary delivery vehicle for direct ocular drug delivery. In this case, polymeric micelles could function by drug packaging or direct delivery into the eye.11 In the following section, the polymeric micelle formulations which are suitable for ophthalmic drug delivery will be summarized, in terms of polymeric micelles with surface modifications or the ones without surface modifications, and the details are presented in Table 1.| Polymeric micelles | Polymer advantages | Polymers | CMC | Size | Drug encapsulation efficiency EE/drug loading content LC | Drug/polymer weight ratio | Animal model | Ref. |
|---|---|---|---|---|---|---|---|---|
| Polyethylene glycol (PEG) polymerized micelles | Improved micellar adhesion, corneal surface penetration, stability and biocompatibility | PEPP (2% BPOSS) | 0.067 wt% | 115.5 ± 1.5 nm | LC 28%, EE 84.4% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Fungal keratitis C57BL/6 mice | 50 |
| MPEG-b-PAE-g-HA | — | 84.5 nm | LC 0.56% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Isolated rabbit cornea | 51 | ||
| mPEG-b-PLGA | — | 81.3 ± 1.3 nm | LC 8.1 ± 0.2%, EE 80.9 ± 1.6% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
Allogenic penetrating keratoplasty (SD rats to Wistar rats) | 52 | ||
| PEG-b-PGMA | — | ∼200 nm | EE 85.02 ± 3.63%, LC 20.32 ± 0.69% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Fungal keratitis rabbits | 54 | ||
| Soluplus® polymeric micelles | Improved drug solubility, stability, corneal or scleral penetration and drug uptake in ocular cells | Soluplus® (PVCL–PVA–PEG) | 7.6 mg L−1 in 23 °C water | 60.72 ± 1.09 nm | EE 99.5 ± 0.52% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
Eye inflammation rabbits | 56 |
| Solutol polymeric micelles | Excellent solubilization ability, low toxicity, can be delivered to the posterior segment of the eye as an injection | Solutol® HS 15 | 0.40 ± 0.04 mg mL−1 in artificial tears | 12.17 ± 0.73 nm | EE 96.12 ± 0.31% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
Eye inflammation rabbits | 63 |
| Polymeric micelles with surface modifications | Surface modification allows micelles to be charged or targeted | CSO-VV-SA (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
53.70 μg mL−1 | ∼100 nm | EE 91.95 ± 1.86, LC 6.13 ± 0.26 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
New Zealand albino rabbits | 77 |
| SDBS–Ppy (Dex) | 1–2 mM | ∼50 nm | EE 80.5 ± 1.19, LC 6.24 ± 0.04 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (molar ratio) 20 (molar ratio) |
— | 80 | ||
| Micelle-gel platform | Improved corneal adhesion and prolonged drug release time | F127 | 950–1000 ppm | 62.36 ± 3 nm | EE 90.66 ± 10% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Porcine corneal infection injury model in vitro | 86 |
| F68 (100 mg mL−1) | ∼1.3 mg mL−1 | 131 ± 108 nm | NA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Excised bovine cornea | 87 | ||
| Micelle-loaded platform | Improvement of the controlled release effect and stability of micelles | mPEG–PLA | ∼0.0012 mg mL−1 | 20.96 ± 6.050 nm | LC 8.87%, 0.078%; EE 93.2%, 74.8% (timolol and latanoprost, respectively) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (timolol 100 (timolol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) latanoprost latanoprost![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polymer) polymer) |
Rabbit model of high intraocular pressure | 92 |
3.1. Polymeric micelles without surface modifications
Non-surface modified polymeric micelles account for most of the polymeric micelles which are directly used as ocular delivery carriers.40 These micelles exhibit excellent properties without modification. The research progress in this field in the last three years will be summarized by dividing them into polyethylene glycol (PEG) polymerized micelles, Soluplus® polymeric micelles, Solutol polymeric micelles, and other types of polymeric micelle.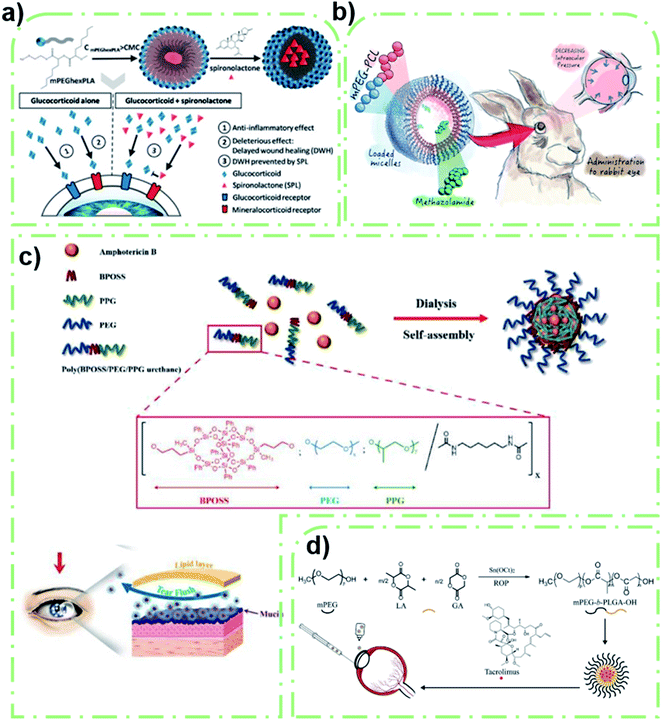 | ||
| Fig. 1 PEG-based polymeric micelles that act directly as the delivery vector. (a) Spironolactone/mPEG–dihexPLA micelle that prevented the delayed effect of glucocorticoids on corneal wound healing. Reproduced from ref. 43 with permission from the American Chemical Society, copyright 2018. (b) MTZ-loaded mPEG–PCL micelle used to treat glaucoma. Reproduced from ref. 47 with permission from Elsevier B.V., copyright 2019. (c) BPOSS–PEG–PPG micelle with high adhesion that prevented the eye clearance mechanism. Reproduced from ref. 50 with permission from Elsevier B.V., copyright 2021. (d) Synthesis and administration methods of a tacrolimus loaded mPEG–PLGA micelle. Reproduced from ref. 52 with permission from Elsevier B.V., copyright 2019. | ||
The other most common micelle is the PEG–PCL polymeric micelle. The negatively charged PEG–PCL micelles can reach deeper during corneal transport than the chitosan-coated micelles.27 PCL has good biocompatibility and biodegradability, which makes PEG–PCL micelles a safe and effective drug delivery carrier.46 As shown by Elmowafy et al., metazolamide (MTZ)-loaded mPEG–PCL micelles were prepared and injected into the corneal surface to treat glaucoma (Fig. 1b).47 The micelle could effectively encapsulate MTZ (EE was 93%) and improve its bioavailability. In vitro release experiments and animal models also verified the sustained-release effect of micelles and their ocular tolerance. This indicates that PEG–PCL micelles can be used as a good delivery vector for insoluble drugs. In Shi et al.'s study, mPEG–PCL micelles were also used for the delivery of axitinib, a tyrosine kinase inhibitor.48 This micelle effectively increases drug solubility, shows good histocompatibility, and can effectively penetrate the cornea against angiogenesis. It provides a new treatment method for corneal neovascularization related corneal diseases in ophthalmology. These polymeric micelles exhibit excellent corneal transport performance of PEG–PCL, but the disadvantage is that it’s hard to reach the deeper segment (i.e., retina).
In addition to the two hydrophobic fragments mentioned above, there are many other hydrophobic blocks copolymerized with PEG to form polymeric micelles. PEG shells are extensively utilized, and play an important role in enhancing the corneal penetration and adhesion of micelles.49 Han et al. designed a novel organic–inorganic hybrid micelle by linking a bi-polyhedral oligomeric silsesquioxane (BPOSS) group to a polyethylene glycol–polypropylene glycol aminoester (PEG–PPG) copolymer (BPEP).50 POSS could enhance the intercellular adhesion, and the addition of the PEG shell obviously improved the overall adhesion of polymeric micelles. Therefore, the AMB-loaded polymeric micelle could be effectively released and retained in the cornea, thus greatly improving the drug loss problems associated with conventional preparations (Fig. 1c). Animal models have verified that the polymeric micelles have longer corneal retention and better penetration compared to commercial dosage forms. However, the improvement of corneal permeability by the POSS system was limited by the large volume of AMB and corneal epithelial tight junction barrier. This design has a positive effect on the development of ocular drugs in the future. A hyaluronic acid (HA) modified mPEG-b-PAE block copolymer micelle prepared by Li et al. also improved the penetration ability of genistein (the cumulative penetration rate was 17%) to rabbit corneas in vitro compared with free genistein (11%). The solubility of the hydrophobic drug was improved and the formation of new blood vessels was successfully inhibited.51 Similar experiments have been demonstrated by Liu et al.52 A methoxy poly(ethylene glycol)-block-poly(D,L)-lactic-co-glycolic acid (mPEG-b-PLGA) micelle was synthesized to improve the corneal penetration of tacrolimus (Fig. 1d). The result of cumulative permeability is obviously better than that of commercial eye drops. In vivo immunofluorescence analysis further verified that the drugs could effectively reduce the immune rejection after corneal transplantation. The PEG hydrophilic shell can help the drug-loaded micelles pass through the hydrophilic corneal stroma and improve drug absorption. But in the initial stage, tacrolimus was rapidly released at pH 7.4, which was related to dissociation of surface-attached tacrolimus. Another preparation based on PLGA–PEG was developed by Hiroki et al.53 This study optimized the formulation of non-inflammatory biodegradable polymer containing sunitinib, which realized self-aggregation in depot and continuous inhibition of VEGF signals through vitreous injection. The preparation could maintain the treatment level in the retinal pigment epithelium/choroid and retina for more than 6 months. However, focal yellow vitreous humor and lens discoloration have been observed in some animals, which has become an important obstacle to clinical application.
Slow or controlled release has always been one of the most prominent functions of micelles. Guo et al. developed a poly(ethylene glycol)-block-poly(glycidyl methacrylate) (PEG-b-PGMA) micelle.54 This micelle had slow and controlled release properties due to the hydrolysis of PGMA that could generate a hydrophilic hydroxyl group. The self-assembled micelles were highly antifungal and the frequency of drug administration in animal experiments was reduced from eight to three times per day. This indicates that the sustained release ability of polymer micelles will play a positive role in future clinical practice.
As demonstrated above, polymeric micelles with PEG shells have not only good biocompatibility and water solubility, but also good corneal penetration and sustained-release properties, and could serve as a hydrophilic block with significant clinical value. However, the drawback is that it cannot reach deeper areas, which requires injection.
Studies have shown that PVCL–PVA–PEG polymeric micelles can significantly improve the solubility of drugs and have a high encapsulation rate.56 The low water solubility and stability of myricetin (Myr) were significantly improved with the polymeric micelle eye preparation, thereby reducing the loss of the drug. Myr-micelles were labeled with Cou6 and the micelle uptake effect of human corneal epithelial (HCE) cells was observed by fluorescence microscopy. It was found that the fluorescence intensity increased gradually from 5 to 90 min. Myr micelles were well tolerated in rabbit eyes after injection. In addition, the corneal permeability of micelles at 30 min was more than 4 times that of free drugs, and it also showed a good penetrating effect on the corneal epithelium under a fluorescence microscope. This micelle greatly enhances the properties of Myr. Different antioxidants have also been studied. For example, Li et al. have used Soluplus® micelles to improve the solubility and stability of resveratrol.57 The Sol-Res micelle had a strong HCE cell uptake effect at 60 min. The inhibitory effect of micelles on inflammatory mediators was verified by a rabbit corneal wound model, and the results showed complete healing at 72 h. The excellent anti-inflammatory effect of these micelles may be related to the long-term existence of micelles in the circulatory system and their aggregation in the inflammatory region, which was illustrated in research conducted by Mehra and co-workers.58 They chose everolimus as the treatment for uveitis after extraocular treatment, and the Soluplus® micelle was used as a partial delivery medium to prepare the Soluplus-everolimus nanomicelle. This micelle has low CMC (7.2 μg mL−1), high encapsulation efficiency (97.4 ± 1.1%), high corneal permeability (compared to free drugs), good ocular tolerance and improved stability. It was proven that the polymeric micelles may be potential nanocarriers for the treatment of uveitis (Fig. 2a). In addition to enhancing corneal penetration, Soluplus® micelles significantly increase the amount of drugs that penetrates the sclera. This kind of study was conducted by Varela-Garcia et al. who used Soluplus® to increase the solubility of acyclovir.59 The results showed that the addition of drugs had no obvious effect on the size of micelles. In addition, increasing the temperature could improve Soluplus's adhesion to the eye. The final measurement of permeability revealed that the amount of drug penetrating the sclera was nearly 10 times greater than the amount penetrating the cornea, which may be related to the porous structure of the sclera.60 This indicates that this micelle is expected to be an important carrier for drug delivery in the posterior ocular segment. However, the drawback is that different drugs encapsulated in Soluplus® have different behaviors in corneal and scleral penetration, which is related to the drug's diffusion manner in the eye.
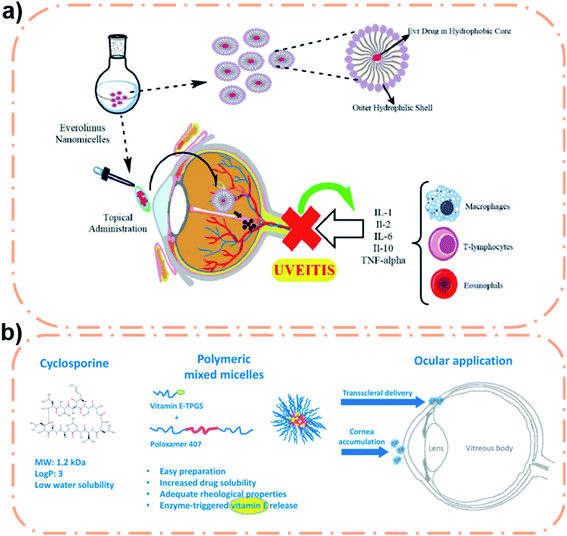 | ||
| Fig. 2 (a) Scheme of Soluplus® nanomicelle for uveitis treatment. Reproduced from ref. 58 with permission from Elsevier B.V., copyright 2021. (b) Scheme of the F127/TGPS polymeric micelle for high permeability drug delivery. Reproduced from ref. 67 with permission from the American Chemical Society, copyright 2018. | ||
Soluplus® micelles are used as delivery vehicles for most ocular drugs, and they not only improve solubility, stability, and bioavailability, but also increase corneal and scleral penetration. To a certain extent, their application makes up for the shortage of the existing carrier materials, presenting good application prospects for ocular drug delivery.
For example, Younes et al. prepared streptozotocin (STZ)-loaded Solutol binary hybrid micelles (with Pluronic® F68 (F68), Brij® 58 (B58), and Pluronic® L121 (L121)) using thin-film hydration.62 The solubility of STZ was improved by nearly 339 times. As a topical antifungal agent, the very low water solubility of STZ limits its ocular application. But this micelle not only significantly improved its solubility, but also explored the two optimal formulations: B58-Transcutol® P at the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of HS 15
1 ratio of HS 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) drug, and B58-propylglycol at the 20
drug, and B58-propylglycol at the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of HS 15
1 ratio of HS 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) drug. It may be due to the addition of these dressings that it has a high adhesion. Instead of this binary hybrid micelle, Hou et al. developed an HS 15 polymeric micelle that coated Myr.63 The micelle has an extremely small particle size (∼12 nm), which is conducive to corneal penetration and eye absorption.64 The experimental results showed that micelles have good short-term stability and rabbit eye tolerance. Fluorescence microscopy showed that the micelle had higher corneal penetration than free Myr and its anti-inflammatory effect was also verified in a rabbit eye model.
drug. It may be due to the addition of these dressings that it has a high adhesion. Instead of this binary hybrid micelle, Hou et al. developed an HS 15 polymeric micelle that coated Myr.63 The micelle has an extremely small particle size (∼12 nm), which is conducive to corneal penetration and eye absorption.64 The experimental results showed that micelles have good short-term stability and rabbit eye tolerance. Fluorescence microscopy showed that the micelle had higher corneal penetration than free Myr and its anti-inflammatory effect was also verified in a rabbit eye model.
In summary, HS 15 has the characteristics of high solubilization ability, low toxicity, and good tolerance, and can form ultra-small micelles in ocular delivery and improve corneal permeability. The disadvantage of this preparation is that it’s often used as an injection, which causes clinical inconvenience.65
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio), a micellar polymeric micelle for encapsulation of CsA.66 This novel ocular micelle formulation offers superior stability and solubility compared to the commercially available Ikervis®. This micelle is different from other micelles in that its TPGS segment can release vitamin E and play an antioxidant role, and the presence of polypropylene oxide (PPO) blocks in this micelle can interfere with the accumulation of bilayer lipids in epithelial cells to increase drug penetration (Fig. 2b).67 Because the micelles contained no oil phase, delivery of CsA didn't not cause irritation to the eye. The final results showed that the mixed micelles dispersed into the sclera and maintained CsA release (the CsA accumulation reached 145 ± 27 μg cm−2 after 48 h). Therefore, this CsA eye preparation, with a good performance improvement compared with commercial preparations, has good clinical significance. However, non-surface modified micelles cannot achieve satisfactory results in some aspects (i.e., adhesion, cell uptake, and targeting performance). Therefore, to improve these properties, surface modification is necessary.
1 molar ratio), a micellar polymeric micelle for encapsulation of CsA.66 This novel ocular micelle formulation offers superior stability and solubility compared to the commercially available Ikervis®. This micelle is different from other micelles in that its TPGS segment can release vitamin E and play an antioxidant role, and the presence of polypropylene oxide (PPO) blocks in this micelle can interfere with the accumulation of bilayer lipids in epithelial cells to increase drug penetration (Fig. 2b).67 Because the micelles contained no oil phase, delivery of CsA didn't not cause irritation to the eye. The final results showed that the mixed micelles dispersed into the sclera and maintained CsA release (the CsA accumulation reached 145 ± 27 μg cm−2 after 48 h). Therefore, this CsA eye preparation, with a good performance improvement compared with commercial preparations, has good clinical significance. However, non-surface modified micelles cannot achieve satisfactory results in some aspects (i.e., adhesion, cell uptake, and targeting performance). Therefore, to improve these properties, surface modification is necessary.
3.2. Polymeric micelles with surface modifications
Traditional polymeric micelles only need to design appropriate proportion and type of polymer blocks according to drug usage requirements, without the need to modify functional groups on the surface of the micelle formulation. However, recent reports have shown that specific tissue or organ targeting could be achieved by modifying a specific antibody or ligand on the polymeric micelle surface, with enhanced bioavailability or reduced systemic toxicity.68,69 In case of the eye's complex environment, the physiological barrier of the cornea and conjunctiva at the front of the eye is one of the biggest difficulties in drug delivery.70 The structure and function of polymeric micelles can greatly affect the bioavailability of therapeutic molecules in vivo. Appropriate surface modifications of polymeric micelle formulations can not only enhance drug delivery, but also increase corneal penetration and improve target efficiency.As a typical example, Lin et al. developed a positive nanomicelle loaded with tacrolimus (FK 506), which is formed from an amphiphilic polyethylene glycol–polyglutamic acid benzyl ester block copolymer (PEP–PEG–PBG).71 The surface of the micelle is modified with hexapeptides with rich basic amino acids, in order to give the micelle its positive electrical power in tears (Fig. 3a). In this case, the unique formulation can interact with the negatively charged mucin layer which is present on the surface of the eye. Furthermore, PEP–PEG–PBG micelles with strong positive electrical properties have a good retention effect on the eye surface, compared with the FK 506 suspension or commercial FK 506 formulation in terms of the drug's corneal permeability. Experimental results of the in vivo dry eye disease model have proven that the surface modification of polymeric micelles could flexibly utilize the ocular environment to enhance the retention of the drug-carrying micelles, which achieved a better therapeutic effect.
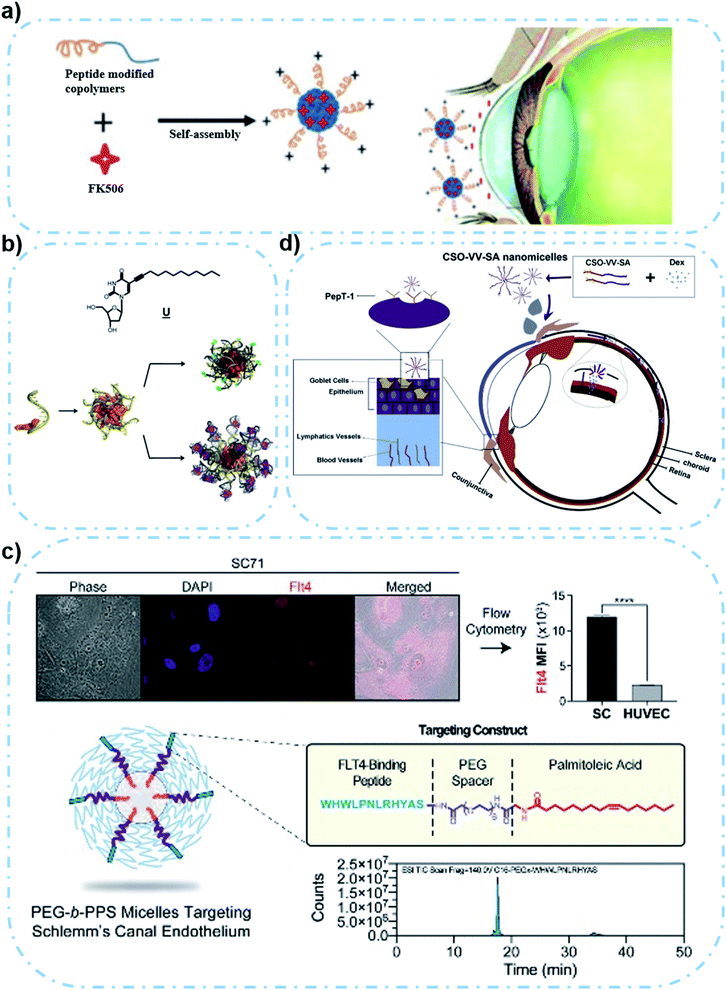 | ||
| Fig. 3 Scheme of polymeric micelles with surface modification. (a) Hexapeptide-modified FK 506/PEP–PEG–PBG micelle with positive charge that can bind the negatively charged mucin layer. Reproduced from ref. 71 with permission from the American Chemical Society, copyright 2019. (b) Structure of a lipid-modified nucleoside. Orange represents hydrophobic groups, and yellow represents DNA. Micelles can be functionalized by hybridization with complementary DNA or RNA strands (gray), which can then covalently bind to a fluorescent dye (green) or noncovalently bind to an aptamer or drug molecule (purple). Reproduced from ref. 72 with permission from Elsevier Ltd, copyright 2017. (c) Peptide-modified LatA/PEG-b-PPS micelle with enhanced uptake of SC cells and reduced uptake of HUVECs. Reproduced from ref. 74 with permission from Wiley-VCH GmbH, copyright 2020. (d) CSO-VV-SA micelle can target PepT-1 to increase drug retention. Reproduced from ref. 78 with permission from Elsevier Ltd, copyright 2019. | ||
A similar functional micelle designed by Herrmann et al. to improve corneal adhesion was formed by using lipid modified deoxyribonucleic acid (DNA) strands. In detail, the dodecyne modification on deoxyuracil was conducted to endow the hydrophilic DNA strands with hydrophobicity,72 which allows the spontaneous formation of modified DNA strand based micelles. This micelle can then be hybridized with complementary DNA or ribonucleic acid (RNA) strands to obtain a functional micelle shell. It is worth mentioning that the electronegative micelle surface is capable of non-covalently binding to aptamers carrying drug molecules (Fig. 3b). Although the adhesion mechanism of the nanoparticles has not been clearly revealed, the results showed that the micelles had better adhesion to the porcine cornea than monomer drugs. The ability of the polymeric micelles to deliver kanamycin B and the therapeutic effectiveness were also demonstrated in porcine corneal germicidal experiments in a keratitis mouse model. In short, surface modification to improve micelle adhesion is of great significance. However, the limitation of this study is lack of relevant experiments on biocompatibility and safety, so it is unknown whether the preparation has negative effects on human tissues or cells.
Besides structural modifications for micelle formation, surface modification could also be an important method to improve functionality. For example, Song et al. synthesized a linolenic acid (LNA) modified methoxy polyethylene glycol (mPEG) and oligochitosan (CS) conjugate (mPEG–CS–LNA), which showed the significantly improved solubility of amphotericin B (AMB).73 The introduction of hydrophobic LNA to the backbone of mPEG–CS conjugates could lead to the formation of a stable polymeric micelle, with enhanced cellular uptake of encapsulated drugs thanks to the membrane permeability of LNA. Fortunately, this micelle has a good sustained-release effect, and low nephrotoxicity or hemolysis. However, the deficiency of this study is that there is no ophthalmic in vivo experiment, which may be related to the size of the drug-loaded micelle (257.94 ± 10.42 nm) that might affect penetration ability. On the other hand, tissue or cellular targeting ability is another important purpose of surface modification of polymeric micelles. For example, Stack et al. developed a polymeric micelle that targets the Schlemm's canal (SC), in order to address the off-target side effect of the actin depolymerizer latrunculin A (tLatA-MC).74 High-affinity peptides modified on poly(ethylene glycol)-block-poly(propylene sulfide) (PEG-b-PPS) micelles could bind to vascular endothelial growth factor-3 (VEGFR3) receptors that are highly expressed in secondary (SC) cells. Studies have shown that the increased intraocular pressure in glaucoma is related to the high hardness of SC endothelial cells, and the polymeric micelle can effectively deliver LatA with the ability of softening cells to SC cells.75 It is worth mentioning that in this study, the introduction of peptides increased the micelle uptake by SC cells, but human umbilical vein endothelial cell (HUVEC) uptake was unexpectedly decreased at the same time (Fig. 3c), which may be related to the altered membrane interactions induced by palmitic acid in the micelles.76 And this decrease in intake was alleviated after loading with LatA. But it is still unclear whether this micelle has a similar effect in other cells. In short, these uncertainties make clinical translation of polymeric micelle formulations remain a challenge. In conclusion, this micelle effectively delivers LatA and shows a significant intraocular pressure reduction effect (30–50%) in vivo. In another case, Xu et al. designed a nanomicellar preparation prepared in chitosan oligosaccharide-valylvaline-stearic acid (CSO-VV-SA) loaded with dexamethasone (Dex) for partial ocular delivery.77 The slow turnover of chitosan-oligosaccharides in the mucous layer increased drug retention on the surface of the eye.78 Modification of the surface of the micelle by valylvaline (VV) enhanced the recognition between the nanomicelle and peptide transporter-1 (PepT-1), which ultimately promoted the penetration of Dex into the anterior and posterior segments, thereby enhancing the bioavailability of the drug (Fig. 3d). The fluorescence of the eyes of rats showed that the fluorescence was mainly distributed in the corneal epithelium, indicating that it was hard to penetrate the cornea. However, the conjunctival epithelium and stroma had stronger fluorescence, while the same phenomenon was also observed in the sclera, choroid and retina, indicating that the gelatin reached the posterior segment through the conjunctival pathway.
Last but not least, responsive drug delivery systems, such as light response,79 pH response, temperature response, etc., provide a targeted drug release platform, which can better exert the efficacy of the drug. For example, Uppalapati et al. designed an electrically responsive sodium dodecyl benzene sulfonate (SDBS)–polypyrrole (Ppy) nanomicelle to investigate its drug delivery mechanism to dexamethasone and its phosphate (DexP).80 As a medium to improve drug solubility, Ppy was modified into the micellar shell to give it electrical responsiveness. The results showed that the electrically driven release of negative ion Dexp was more efficient than that of uncharged Dex, but the cycle of alternating potential could cause a sudden release of both drugs. In addition, the preparation lacked characterization in vivo and is difficult to be directly applied in clinical study due to its color.
Many benefits of surface modification of polymeric micelles are obvious as mentioned above, such as improved adhesion and specific targeting. In the case of ocular drug delivery, most of the polymeric micelles used for the eye only need to penetrate the cornea to meet the needs of therapeutic drugs. However, there are very few micelle related studies that can be delivered to the posterior segment of the eyeball by topical administration, which remains a challenge.
4. Polymeric micelles that act indirectly as the delivery vector
Polymeric micelles can also play the role of auxiliary materials on which they do not act as direct delivery carriers, but become part of the delivery carrier, for example, wrapping micelles in hydrogels or wrapping micelles in other materials like contact lenses, and so on.2,21 However, the role of micelles remains the same: to encapsulate drugs to improve the solubility of insoluble drugs.13 Next, we will discuss this part from micellar-gel systems and micellar loading systems.4.1. Micelle-gel platform
In micelle-gel systems, micelles can be encapsulated in hydrogels. Micelles can encapsulate drugs, and gels can improve overall adhesion and reduce drug loss due to factors such as tears.21,81,82 However, different polymers also exhibit different characteristics. Some polymers have the properties of gelling because of their special fragments. They can undergo sol–gel transformation when temperature,83 pH and other factors change.84 Micellar loaded hydrogel systems are also promising because they combine the best of both micelles and hydrogels.For example, Wang et al. prepared an FK 506-coated PEG–PBG polymeric micelle (with a size of 292 nm), which was then co-encapsulated in a temperature-sensitive hydrogel with ciliary neurotrophic factor (CNTF).85 The hydrogel was CS based and could gel at 37 °C under physiological conditions. On the one hand, the hydrogel reduced the release rate of FK 506 compared with the micelles alone. On the other hand, the system could also protect the retinal ganglion cells (RGCs) and had the potential to repair the optic nerve in a rabbit optic nerve injury model. It was considered to be a novel combination.
Furthermore, this system can also be used for antimicrobial applications. Annabi et al. developed an antimicrobial adhesive hydrogel system containing a ciprofloxacin (CPX)-loaded micelle.86 They used F127 to form CPX loaded micelles, which were then combined with GelCORE hydrogel formed by gelatin methacryloyl (GelMA) and a photoinitiator to form the system. The hydrogel had good adhesion and antibacterial effects on the cornea, and can supplement corneal defects and promote healing (Fig. 4a). Compared with free CPX, this system had a better sustained release effect. The inhibitory effect of the micellar loaded hydrogel on both Gram-negative and Gram-positive bacteria was verified by using Staphylococcus aureus and Pseudomonas aeruginosa. This system provides a new idea for drug delivery in other eye diseases. A micellar-gel system also used to repair corneal wounds was utilized by Puglisi et al.87 They encapsulated the antioxidant ferulic acid in an F68 micelle and added hyaluronic acid to the micelle, which was then cross-linked with ε-polylysine to constitute the system (Fig. 4b). The particle size of the micelle-gel system was about 300 nm, but the particle size increased after freeze-drying. Compared with micellar preparation, this system significantly prolonged the drug release duration (2 days). The system has been shown to promote the growth of fibroblasts and can accumulate up to 100 μg cm−2 in damaged cornea. Notably, they found that the accumulation of ferulic acid was higher in the intact cornea than in the damaged cornea, possibly due to the increased reactive oxygen species (ROS) in the hot-brined eyeball, which degraded the amount of ferulic acid.88
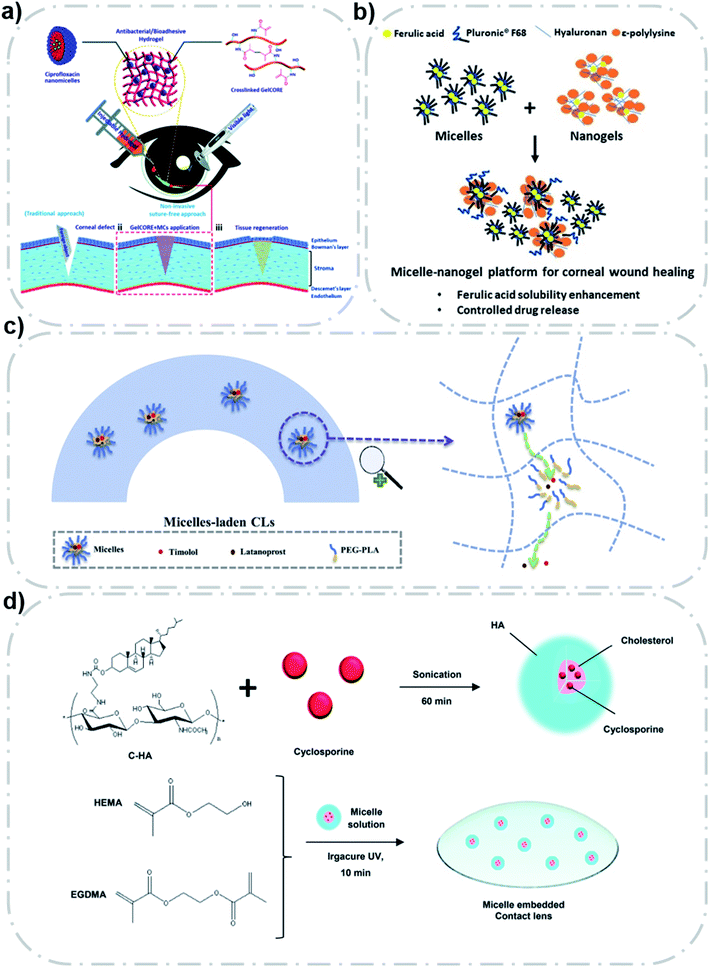 | ||
| Fig. 4 Polymeric micelles that act indirectly as the delivery vector. (a) F127 and GelMA based micelle-gel systems can adhere and fill corneal lesions. Reproduced from ref. 86 with permission from the Royal Society of Chemistry, copyright 2020. (b) System of ferulic acid/F68 micelle and HA–ε-polylysine nanogel for corneal wound healing. Reproduced from ref. 87 with permission from Elsevier B.V., copyright 2019. (c) CL-M with long time drug delivery of timolol and latanoprost. Reproduced from ref. 92 with permission from Elsevier B.V., copyright 2019. (d) The synthesis process of cyclosporine/C–HA micelle-embedded HEMA–EGDMA CLs. Reproduced from ref. 93 with permission from Royal Society of Chemistry, copyright 2019. | ||
Therefore, as a novel strategy for ocular drug delivery, micelle-gel systems on the one hand can increase the solubility of insoluble drugs through micelles and on the other hand can improve ocular adhesion by using hydrogels.89 In addition, compared with micelles, the system can further improve the sustained-release ability of drugs.
4.2. Micelle-loaded platform
Contact lenses (CLs) have been studied extensively as ocular drug delivery devices because of portability, high adhesion, and so on.39 The introduction of polymer micelles can not only increase the drug loading, but also enhance the slow release properties of the drug. However, the direct introduction of some insoluble drugs can reduce the transmittance and swelling properties of contact lenses.90 The introduction of micelles can improve this problem.91Xu et al. designed a kind of micelle-loaded contact lens (CL-M) to achieve the sustained-release of timolol and latanoprost (144 h and 120 h, respectively, in simulated tears, and 120 h and 96 h, respectively, in rabbit eyes).92 The CL-M was fabricated by free radical polymerization of mPEG–PLA and monomer hydroxyethyl methacrylate (HEMA). Compared with eye drops, the CL-M remarkably increased the average retention time in the eye (by 79.6 and 122.2 times, respectively) and bioavailability (by 2.2 and 7.3 times, respectively). The potential therapeutic effect of glaucoma has also been demonstrated in animal models (Fig. 4c).
Mun et al. designed a micellar embedded contact lens consisting of a cholesterol–hyaluronic acid (C–HA) and ethylene glycol dimethacrylate (EGDMA)–(HEMA) copolymer CL (Fig. 4d).93 The particle size of the C–HA micelle was about 290 nm. The CL could extend the slow-release effect of cyclosporine for more than 12 days. In vitro and in vivo experiments proved that it has a good therapeutic effect on dry eye disease. The clinical feasibility of this strategy was proven.
This micellar loaded contact lens can greatly enhance the slow and controlled release of the drug because the drug has to go through two steps: first, it diffuses through the micellar structure into the contact lens hydrogel, and then it diffuses out through the contact lens hydrogel.
5. Future challenges of polymeric micelles
As a multifunctional nanotechnology for hydrophobic drug encapsulation, polymeric micelles still face many serious challenges despite their excellent drug delivery capabilities, as shown in Fig. 5.The innovative development of polymer blocks faces major challenges. Since polymeric micelles are formed by self-assembly of amphiphilic copolymers, the arrangement and chemistry of hydrophilic and hydrophobic fragments determine the chemical properties of the resulting micelles.94,95 Two-block or three-block micelles are the most commonly used copolymer types at present.22 It is a new challenge whether to break through and innovate new types. In addition, the change of the block will affect all properties of micelles such as CMC, encapsulation efficiency, degradation rate and so on.11,15 In this case, this field still remains largely unexplored.
The clinical application of polymeric micelles faces significant challenges. Despite their potential, polymeric micelles have been a tough sell. At present, few micellar products are used in clinical application.12 Due to the special structure of the eye, higher biocompatibility and stability are required, which makes the clinical application more difficult. Posterior administration is also a problem, such as vitreous implantation, which requires not only skilled and specialized procedures, but also frequent administration.11 In addition, there are many biological barriers to the eye, and these biological barriers may differ from species to species.1 All of these challenges make clinical trials more complicated. Furthermore, the use of nanomedicines in clinical trials depends on ensuring the quality of the final product through implementation and validation of good manufacturing practice (GMP). The production method of nanomicelles is not reproducible or scalable, which makes it difficult for it to pass the requirements of GMP. Besides, the fact that the regulation of nanomedicines is still immature is a major barrier to the clinical use of polymeric micelles, indicating that regulations on manufacturing processes and process control, quality characterization, drug quality, product specification and stability might need to be improved.96 Furthermore, there are many variable unknowns such as chemical structure (i.e., the chemical composition of micelles can cause changes on the surface of cell membranes such as palmitic acid changes membrane interactions74), charge (i.e., the slow degradation of micelles will reduce the electrical properties, thus reducing the adhesion to the mucin layer71) and surface (i.e., the surface of the hydrophilic micelles may adhere to several other substances including drugs, which can affect the release properties of the micelles52) that need to be considered for polymeric micelles, which hinder their clinical development.22
The use of polymeric micelles is limited by the anatomy of the eye, which is divided into four parts: the anterior corneal region, the cornea, the anterior segment, and the posterior segment.10,11 The size and charge of the micelles determine the transmittance of the anterior part, and the blood–eye barrier as well as blood–retinal barrier are factors that hinder drug access to the posterior segment.1 These physical barriers are also the biggest impediments to drug delivery. Therefore, the development road of polymeric micelles might still be full of difficulties.
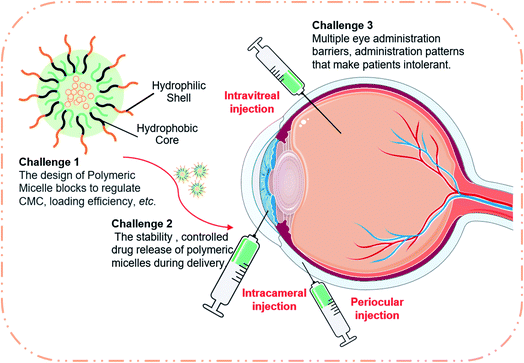 | ||
| Fig. 5 Potential challenges for polymeric micelle formulations in future ophthalmic drug delivery applications. | ||
6. Advances of polymeric micelles
Over the past few years, the development of polymeric micelles for ocular drug delivery has improved while more and more micellar preparations with unique properties have been emerging, which have been described above.In terms of structure, polymeric micelles have been further innovated. The organic–inorganic hybrid structure formed by connecting POSS to the PEG–PPG polymer makes the micelle adhesion better and can effectively prevent the scouring by eye tears.50 The introduction of PGMA into PEG–PGMA micelles endows them with the properties of slow and controlled release because PGMA can be hydrolyzed to produce hydrophilic hydroxyl groups at neutral pH, while previous studies could only do it at non-neutral pH.54 In respect of structural modification, the modification of PEP–PEG–PBG with peptides and the DNA chain with a hydrophobic structure can make the formed micelles charged and improve their corneal adhesion.71,72 In addition, the modification of PEG-b-PPS with targeted peptides can effectively target SC cells and increase their uptake, which is a new strategy for the treatment of glaucoma.74
In terms of formulation, the polymeric micelles have explored advanced formulations. Micelle formulations (i.e., PEG–PCL, PEG–PLA, PEG–PLGA, and HS 15) are not innovative in structure, and this has been improved in ocular application, significantly improving various data including drug loading rate, corneal penetration, safety, and stability compared with previous traditional preparations or current commercial preparations.41–43,46–48 For example, two optimal formulations (B58-Transcutol® P at the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of HS 15
1 ratio of HS 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) drug; B58-propylglycol at the 20
drug; B58-propylglycol at the 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of HS 15
1 ratio of HS 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) drug) were developed to improve the solubility of STZ by nearly 339 times.62
drug) were developed to improve the solubility of STZ by nearly 339 times.62
In terms of function, polymeric micelles have a more progressive delivery capability in ocular drug delivery. The CSO-VV-SA micelle improves the bioavailability of the posterior segment through the conjunctival pathway, and achieves topical drug delivery in the deep part of the eye.77 The design of some responsive eye nanomicelles has also made progress (i.e., photoresponse and magnetic response).80 In addition, the introduction of drug-loaded micelles in contact lenses can further confer drug delivery function, providing a new reference for clinical application.90–93 Both drug release and biocompatibility of these micelles have excellent results.
In summary, in the last three years, a number of polymeric micelles have been developed for ocular drug delivery, which have different functions and characteristics that have been improved in different ways. However, frequent administration and shallow delivery distance remain the biggest problems, so overcoming the ocular physiological barrier to improve the drug delivery depth and bioavailability remains challenging.
7. Conclusion and prospects
Over the past few decades, polymeric micelles have proven to be an effective nanotechnology for ocular drug delivery. It can significantly improve the solubility of insoluble drugs, so as to solve the problem of insoluble drugs in ocular applications. But due to the special anatomy and physical barrier of the eye, the drug delivery process is difficult. In recent years, more and more micelles with excellent properties have been reported. These polymeric micelles use various polymer blocks to improve the loading and release capacity and stability of drugs, and effectively overcome some ocular barriers through topical administration, achieving effective treatment in the anterior or posterior. These novel polymeric micelles overcome the vital issue of drug loss and significantly improve the bioavailability of drugs compared to traditional dosage forms and formulations currently on the market. In the future, more and more new micelles will be developed to solve the existing problems. High biocompatibility, drug loading, bioavailability and stability of the micelles will be expected, which requires more systematic studies of the size, shape, charge, adhesion and other capabilities of the polymer micelles.Conflicts of interest
The authors declare no conflict of interest.References
- M. L. Occhiutto, F. R. Freitas, R. C. Maranhao and V. P. Costa, Pharmaceutics, 2012, 4, 252–275 CrossRef CAS PubMed
.
- F. A. Maulvi, T. G. Soni and D. O. Shah, Drug Delivery, 2016, 23, 3017–3026 CrossRef CAS PubMed
.
- V. Gote, M. Ansong and D. Pal, Expert Opin. Drug Metab. Toxicol., 2020, 16, 885–906 CrossRef CAS PubMed
.
- L. Ke, P. Cai, Y. L. Wu and X. Chen, Adv. Ther., 2020, 3, 1900213 CrossRef CAS
.
- Y.-L. Wu and Z. Li, Ther. Delivery, 2021, 12, 423–425 CrossRef CAS PubMed
.
- Y. Liu, C. Xu, X. Fan, X. J. Loh, Y. L. Wu and Z. Li, Mater. Sci. Eng., C, 2020, 108, 110464 CrossRef CAS PubMed
.
- X. J. Loh and Y. L. Wu, Chem. Commun., 2015, 51, 10815–10818 RSC
.
- M. Kahlweit, Science, 1988, 240, 617–621 CrossRef CAS PubMed
.
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145–160 CrossRef CAS PubMed
.
- S. Liu, L. Jones and F. X. Gu, Macromol. Biosci., 2012, 12, 608–620 CrossRef CAS PubMed
.
- A. Mandal, R. Bisht, I. D. Rupenthal and A. K. Mitra, J. Controlled Release, 2017, 248, 96–116 CrossRef CAS PubMed
.
- A. Sosnik and M. Menaker Raskin, Biotechnol. Adv., 2015, 33, 1380–1392 CrossRef CAS
.
- R. D. Vaishya, V. Khurana, S. Patel and A. K. Mitra, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2014, 6, 422–437 CAS
.
- Y. L. Wu and J. Li, Angew. Chem., Int. Ed. Engl., 2009, 48, 3842–3845 CrossRef CAS
.
- M. E. Durgun, S. Gungor and Y. Ozsoy, J. Ocul. Pharmacol. Ther., 2020, 36, 323–341 CrossRef CAS PubMed
.
- S. Eetezadi, S. N. Ekdawi and C. Allen, Adv. Drug Delivery Rev., 2015, 91, 7–22 CrossRef CAS PubMed
.
- H. Su, F. Wang, W. Ran, W. Zhang, W. Dai, H. Wang, C. F. Anderson, Z. Wang, C. Zheng, P. Zhang, Y. Li and H. Cui, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 4518–4526 CrossRef CAS PubMed
.
- H. Cheng, X. Fan, X. Wang, E. Ye, X. J. Loh, Z. Li and Y. L. Wu, Biomacromolecules, 2018, 19, 1926–1938 CrossRef CAS PubMed
.
- H. Cheng, Z. Wu, C. Wu, X. Wang, S. S. Liow, Z. Li and Y. L. Wu, Mater. Sci. Eng., C, 2018, 83, 210–217 CrossRef CAS
.
- I. Bravo-Osuna, M. Noiray, E. Briand, A. M. Woodward, P. Argueso, I. T. Molina Martinez, R. Herrero-Vanrell and G. Ponchel, Pharm. Res., 2012, 29, 2329–2340 CrossRef CAS PubMed
.
- G. Feng, Z. Zha, Y. Huang, J. Li, Y. Wang, W. Ke, H. Chen, L. Liu, Y. Song and Z. Ge, Adv. Healthcare Mater., 2018, 7, e1800623 CrossRef
.
- X. Liu, X. Fan, L. Jiang, X. J. Loh, Y. L. Wu and Z. Li, J. Mater. Chem. B, 2018, 6, 5488–5498 RSC
.
- Y. Zheng, L. Ke, Y. Lu, Q. Zuo, G. Deng, H. Wang and X. Zeng, Front. Chem., 2020, 8, 273 CrossRef CAS PubMed
.
- L. Dai, X. Li, M. Yao, P. Niu, X. Yuan, K. Li, M. Chen, Z. Fu, X. Duan, H. Liu, K. Cai and H. Yang, Biomaterials, 2020, 241, 119901 CrossRef CAS
.
- A. H. Salama and R. N. Shamma, Int. J. Pharm., 2015, 492, 28–39 CrossRef CAS
.
- K. Song, M. Xin, F. Zhang, W. Xie, M. Sun and X. Wu, Int. J. Pharm., 2020, 577, 119035 CrossRef CAS PubMed
.
- A. De Campos, Eur. J. Pharm. Sci., 2003, 20, 73–81 CrossRef CAS
.
- A. Rey-Rico and M. Cucchiarini, Int. J. Mol. Sci., 2018, 19, 775 CrossRef PubMed
.
- C. Zheng, H. Gao, D. P. Yang, M. Liu, H. Cheng, Y. L. Wu and X. J. Loh, Mater. Sci. Eng., C, 2017, 74, 110–116 CrossRef CAS
.
- S. Chang, Y. Wang, T. Zhang, X. Pu, L. Zong, H. Zhu, L. Zhao and B. Feng, Front. Oncol., 2019, 9, 823 CrossRef PubMed
.
- Y. Wu, Y. Xiao, Y. Huang, Y. Xu, D. You, W. Lu and J. Yu, Biomacromolecules, 2019, 20, 1167–1177 CrossRef CAS PubMed
.
- Z. Chu, C. A. Dreiss and Y. Feng, Chem. Soc. Rev., 2013, 42, 7174–7203 RSC
.
- Y. Yao, D. Xu, Y. Zhu, X. Dai, Y. Yu, J. Luo and S. Zhang, Chem. Sci., 2020, 11, 757–762 RSC
.
- X. Fan, X. Wang, M. Cao, C. Wang, Z. Hu, Y.-L. Wu, Z. Li and X. J. Loh, Polym. Chem., 2017, 8, 5611–5620 RSC
.
- Q. Lin, M. Qu, B. Zhou, H. K. Patra, Z. Sun, Q. Luo, W. Yang, Y. Wu, Y. Zhang, L. Li, L. Deng, L. Wang, T. Gong, Q. He, L. Zhang, X. Sun and Z. Zhang, J. Controlled Release, 2019, 311–312, 104–116 CrossRef CAS PubMed
.
- J. Qu, X. Zhao, Y. Liang, T. Zhang, P. X. Ma and B. Guo, Biomaterials, 2018, 183, 185–199 CrossRef CAS PubMed
.
- C. Xu, Y.-L. Wu, Z. Li and X. J. Loh, Mater. Chem. Front., 2019, 3, 181–192 RSC
.
- A. R. Khan, M. Liu, M. W. Khan and G. Zhai, J. Controlled Release, 2017, 268, 364–389 CrossRef CAS PubMed
.
- Z. Li, H. Cheng, L. Ke, M. Liu, C. G. Wang, X. Jun Loh, Z. Li and Y. L. Wu, ChemNanoMat, 2021, 7, 564–579 CrossRef CAS
.
- L. Ke, Y.-L. Wu and Z. Li, Ther. Delivery, 2020, 11, 537–540 CrossRef CAS
.
- M. Kasper, D. Gabriel, M. Moller, D. Bauer, L. Wildschutz, H. Courthion, M. Rodriguez-Aller, M. Busch, M. R. R. Bohm, K. Loser, S. Thanos, R. Gurny and A. Heiligenhaus, Mol. Pharm., 2018, 15, 2539–2547 CrossRef CAS PubMed
.
- Y. Yu, D. Chen, Y. Li, W. Yang, J. Tu and Y. Shen, Drug Delivery, 2018, 25, 888–899 CrossRef CAS
.
- N. Dahmana, T. Mugnier, D. Gabriel, V. Kaltsatos, T. Bertaim, F. Behar-Cohen, R. Gurny and Y. N. Kalia, Mol. Pharm., 2018, 15, 1192–1202 CrossRef CAS PubMed
.
- D. Liu, Q. Wu, W. Chen, H. Lin, Y. Zhu, Y. Liu, H. Liang and F. Zhu, Int. J. Pharm., 2019, 562, 1–10 CrossRef CAS PubMed
.
- M. Dvorakova, E. Rollerova, S. Scsukova, A. Bujnakova Mlynarcikova, L. Laubertova and I. Zitnanova, Oxid. Med. Cell. Longevity, 2017, 2017, 7430435 Search PubMed
.
- Z. Li, Z. Guo, D. Chu, H. Feng, J. Zhang, L. Zhu and J. Li, Drug Delivery, 2020, 27, 358–366 CrossRef CAS PubMed
.
- E. Elmowafy, H. Gad, F. Biondo, L. Casettari and M. E. Soliman, Int. J. Pharm., 2019, 566, 573–584 CrossRef CAS PubMed
.
- S. Shi, F. Peng, Q. Zheng, L. Zeng, H. Chen, X. Li and J. Huang, Int. J. Pharm., 2019, 560, 19–26 CrossRef CAS PubMed
.
- F. Bongiovi, C. Fiorica, F. S. Palumbo, G. Di Prima, G. Giammona and G. Pitarresi, Mol. Pharm., 2018, 15, 5031–5045 CrossRef CAS
.
- Y. Han, C. Xu, H. Shi, F. Yu, Y. Zhong, Z. Liu, X. J. Loh, Y.-L. Wu, Z. Li and C. Li, Chem. Eng. J., 2021, 421, 129734 CrossRef CAS
.
- C. Li, R. Chen, M. Xu, J. Qiao, L. Yan and X. D. Guo, Drug Delivery, 2018, 25, 1258–1265 CrossRef CAS PubMed
.
- D. Liu, Q. Wu, W. Chen, H. Lin, Y. Liu, H. Liang and F. Zhu, Eur. J. Pharm. Sci., 2019, 133, 104–114 CrossRef CAS PubMed
.
- H. Tsujinaka, J. Fu, J. Shen, Y. Yu, Z. Hafiz, J. Kays, D. McKenzie, D. Cardona, D. Culp, W. Peterson, B. C. Gilger, C. S. Crean, J. Z. Zhang, Y. Kanan, W. Yu, J. L. Cleland, M. Yang, J. Hanes and P. A. Campochiaro, Nat. Commun., 2020, 11, 694 CrossRef CAS PubMed
.
- Y. Guo, F. Karimi, Q. Fu, G. Qiao and H. Zhang, Expert Opin. Drug Delivery, 2020, 17, 407–421 CrossRef CAS
.
- Z. K. Nagy, A. Balogh, B. Vajna, A. Farkas, G. Patyi, A. Kramarics and G. Marosi, J. Pharm. Sci., 2012, 101, 322–332 CrossRef CAS PubMed
.
- F. Sun, Z. Zheng, J. Lan, X. Li, M. Li, K. Song and X. Wu, Drug Delivery, 2019, 26, 575–585 CrossRef CAS PubMed
.
- M. Li, L. Zhang, R. Li and M. Yan, Drug Dev. Ind. Pharm., 2020, 46, 1960–1970 CrossRef CAS PubMed
.
- N. Mehra, M. Aqil and Y. Sultana, Eur. J. Pharm. Sci., 2021, 159, 105735 CrossRef PubMed
.
- A. Varela-Garcia, A. Concheiro and C. Alvarez-Lorenzo, Int. J. Pharm., 2018, 552, 39–47 CrossRef CAS PubMed
.
- I. Ahmed, R. D. Gokhale, M. V. Shah and T. F. Patton, J. Pharm. Sci., 1987, 76, 583–586 CrossRef CAS PubMed
.
- A. W. Alani, D. A. Rao, R. Seidel, J. Wang, J. Jiao and G. S. Kwon, J. Pharm. Sci., 2010, 99, 3473–3485 CrossRef CAS PubMed
.
- N. F. Younes, S. A. Abdel-Halim and A. I. Elassasy, Drug Delivery, 2018, 25, 1706–1717 CrossRef CAS PubMed
.
- Y. Hou, F. Zhang, J. Lan, F. Sun, J. Li, M. Li, K. Song and X. Wu, Drug Delivery, 2019, 26, 158–167 CrossRef CAS
.
- K. Song, M. Xin, H. Yu, Z. Zheng, J. Li, M. Li, H. Guo, Y. Tan and X. Wu, Int. J. Pharm., 2018, 552, 265–276 CrossRef CAS PubMed
.
- R. G. Strickley, Pharm. Res., 2004, 21, 201–230 CrossRef CAS
.
- M. A. Grimaudo, S. Pescina, C. Padula, P. Santi, A. Concheiro, C. Alvarez-Lorenzo and S. Nicoli, Mol. Pharm., 2018, 15, 571–584 CrossRef CAS PubMed
.
- V. Y. Erukova, O. O. Krylova, Y. N. Antonenko and N. S. Melik-Nubarov, Biochim. Biophys. Acta, 2000, 1468, 73–86 CrossRef CAS
.
- X. Fan, H. Cheng, Y. Wu, X. J. Loh, Y.-L. Wu and Z. Li, Macromol. Mater. Eng., 2018, 303, 1800255 CrossRef
.
- H. Xiang, H. Chen, H. P. Tham, S. Z. F. Phua, J. G. Liu and Y. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 27553–27562 CrossRef CAS
.
- C. Di Tommaso, J. L. Bourges, F. Valamanesh, G. Trubitsyn, A. Torriglia, J. C. Jeanny, F. Behar-Cohen, R. Gurny and M. Moller, Eur. J. Pharm. Biopharm., 2012, 81, 257–264 CrossRef CAS PubMed
.
- S. Lin, C. Ge, D. Wang, Q. Xie, B. Wu, J. Wang, K. Nan, Q. Zheng and W. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 39603–39612 CrossRef CAS
.
- J. Willem de Vries, S. Schnichels, J. Hurst, L. Strudel, A. Gruszka, M. Kwak, K. U. Bartz-Schmidt, M. S. Spitzer and A. Herrmann, Biomaterials, 2018, 157, 98–106 CrossRef CAS PubMed
.
- Z. Song, Y. Wen, P. Deng, F. Teng, F. Zhou, H. Xu, S. Feng, L. Zhu and R. Feng, Carbohydr. Polym., 2019, 205, 571–580 CrossRef CAS
.
- T. Stack, M. Vincent, A. Vahabikashi, G. Li, K. M. Perkumas, W. D. Stamer, M. Johnson and E. Scott, Small, 2020, 16, e2004205 CrossRef PubMed
.
- D. R. Overby, E. H. Zhou, R. Vargas-Pinto, R. M. Pedrigi, R. Fuchshofer, S. T. Braakman, R. Gupta, K. M. Perkumas, J. M. Sherwood, A. Vahabikashi, Q. Dang, J. H. Kim, C. R. Ethier, W. D. Stamer, J. J. Fredberg and M. Johnson, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 13876–13881 CrossRef CAS PubMed
.
- G. Sahay, E. V. Batrakova and A. V. Kabanov, Bioconjugate Chem., 2008, 19, 2023–2029 CrossRef CAS PubMed
.
- X. Xu, L. Sun, L. Zhou, Y. Cheng and F. Cao, Carbohydr. Polym., 2020, 227, 115356 CrossRef CAS
.
- C. Muanprasat and V. Chatsudthipong, Pharmacol. Ther., 2017, 170, 80–97 CrossRef CAS PubMed
.
- Z. Luo, L. Jiang, S. Yang, Z. Li, W. M. W. Soh, L. Zheng, X. J. Loh and Y. L. Wu, Adv. Healthcare Mater., 2019, 8, e1900406 CrossRef PubMed
.
- D. Uppalapati, M. Sharma, Z. Aqrawe, F. Coutinho, I. D. Rupenthal, B. J. Boyd, J. Travas-Sejdic and D. Svirskis, Int. J. Pharm., 2018, 543, 38–45 CrossRef CAS PubMed
.
- L. Jiang, Z. Luo, X. J. Loh, Y.-L. Wu and Z. Li, ACS Appl. Bio Mater., 2019, 2, 3591–3600 CrossRef CAS
.
- B. Hu, C. Owh, P. L. Chee, W. R. Leow, X. Liu, Y. L. Wu, P. Guo, X. J. Loh and X. Chen, Chem. Soc. Rev., 2018, 47, 6917–6929 RSC
.
- Z. Luo, K. Xue, X. Zhang, J. Y. C. Lim, X. Lai, D. J. Young, Z. X. Zhang, Y. L. Wu and X. J. Loh, Biomater. Sci., 2020, 8, 1364–1379 RSC
.
- Y. Han, S. Liu, H. Mao, L. Tian and W. Ning, Polymers, 2016, 8, 367 CrossRef PubMed
.
- D. Wang, M. Luo, B. Huang, W. Gao, Y. Jiang, Q. Li, K. Nan and S. Lin, Drug Delivery, 2020, 27, 556–564 CrossRef CAS PubMed
.
- I. A. Khalil, B. Saleh, D. M. Ibrahim, C. Jumelle, A. Yung, R. Dana and N. Annabi, Biomater. Sci., 2020, 8, 5196–5209 RSC
.
- M. A. Grimaudo, G. Amato, C. Carbone, P. Diaz-Rodriguez, T. Musumeci, A. Concheiro, C. Alvarez-Lorenzo and G. Puglisi, Int. J. Pharm., 2020, 576, 118986 CrossRef CAS PubMed
.
- A. Shoham, M. Hadziahmetovic, J. L. Dunaief, M. B. Mydlarski and H. M. Schipper, Free Radical Biol. Med., 2008, 45, 1047–1055 CrossRef CAS PubMed
.
- X. Liu, Z. Li, X. J. Loh, K. Chen, Z. Li and Y. L. Wu, Macromol. Rapid Commun., 2019, 40, e1800117 CrossRef PubMed
.
- F. A. Maulvi, R. J. Parmar, M. R. Shukla, A. R. Desai, D. T. Desai, K. M. Ranch, S. A. Shah, S. Sandeman and D. O. Shah, Int. J. Pharm., 2019, 566, 513–519 CrossRef CAS
.
- F. A. Maulvi, R. J. Parmar, A. R. Desai, D. M. Desai, M. R. Shukla, K. M. Ranch, S. A. Shah and D. O. Shah, Int. J. Pharm., 2020, 581, 119279 CrossRef CAS PubMed
.
- J. Xu, Y. Ge, R. Bu, A. Zhang, S. Feng, J. Wang, J. Gou, T. Yin, H. He, Y. Zhang and X. Tang, J. Controlled Release, 2019, 305, 18–28 CrossRef CAS
.
- J. Mun, J. w. Mok, S. Jeong, S. Cho, C.-K. Joo and S. K. Hahn, RSC Adv., 2019, 9, 16578–16585 RSC
.
- W. Li, C. Xu, S. Li, X. Chen, X. Fan, Z. Hu, Y. L. Wu and Z. Li, Mater. Sci. Eng., C, 2019, 105, 110047 CrossRef CAS PubMed
.
- X. Liu, X. Chen, M. X. Chua, Z. Li, X. J. Loh and Y. L. Wu, Adv. Healthcare Mater., 2017, 6, 1700159 CrossRef PubMed
.
- E. B. Souto, G. F. Silva, J. Dias-Ferreira, A. Zielinska, F. Ventura, A. Durazzo, M. Lucarini, E. Novellino and A. Santini, Pharmaceutics, 2020, 12, 146 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2021 |
