Emerging polyanionic and organic compounds for high energy density, non-aqueous potassium-ion batteries
Mingzhe
Chen
 *ab,
Qiannan
Liu
*ab,
Qiannan
Liu
 b,
Yanyan
Zhang
a,
Guichuan
Xing
a,
Shu-Lei
Chou
b,
Yanyan
Zhang
a,
Guichuan
Xing
a,
Shu-Lei
Chou
 *b and
Yuxin
Tang
*b and
Yuxin
Tang
 *a
*a
aInstitute of Applied Physics and Materials Engineering, University of Macau, Macau, China. E-mail: yxtang@um.edu.mo
bInstitute for Superconducting and Electronic Materials, Australian Institute for Innovative Materials, University of Wollongong, Innovation Campus, Squires Way, North Wollongong, NSW 2522, Australia. E-mail: mc800@uowmail.edu.au; shulei@uow.edu.au
First published on 25th November 2019
Abstract
Potassium ion batteries (PIBs) as promising energy storage candidates have attracted increasing attention due to the low-cost and abundant potassium resources. Despite numerous emerging electrode materials, the development of PIBs has consecutively encountered major challenges compared with lithium-ion batteries (LIBs) and sodium-ion batteries (SIBs), such as low reversible specific capacities, inferior cycling stabilities, and unsatisfactory energy densities. In light of this, we provide a state-of-the-art review on the development of emerging classes of cathode materials, especially for polyanionic composites and organic compounds, with the aim of achieving high energy density for non-aqueous PIBs. First of all, we highlight the recent development of electrode materials towards non-aqueous PIBs with the discussion on their existing problems and the potential solutions. Then, the recent advancements in polyanionic and organic compounds towards achieving high energy density of PIBs are provided. We focus on their structure–performance relationships with a comparison of their electrochemical properties, and further promising investigations of these types of cathode materials are identified and discussed as well. Finally, personal perspectives on future optimization on cathode materials towards realizing the real applications in PIBs are constructively discussed.
1. Introduction
In the past several decades, the rapid consumption of traditional fossil fuels has led to severe environmental challenges such as global warming.1 Renewable energy sources are environmentally friendly, as has been witnessed by their fast development in recent years.2 As a result, cost-efficient energy storage and conversion devices are strongly required because of the intermittent nature of renewable energy sources.3 Lithium-ion batteries (LIBs) are the most successful commercialised state-of-the-art power sources for portable electronic devices and electric vehicles (EVs).4,5 In the long run, however, fast consumption of the limited lithium resources leaves an open question as to whether or not LIBs are the most feasible energy storage media. Therefore, seeking alternative energy storage systems in a more cost-efficient way with earth-abundant elements is a preferable approach for large-scale energy storage facilities.6–8Both sodium-ion batteries (SIBs) and potassium-ion batteries (PIBs) have attracted much research attention in recent years, since they both share similar advantages with LIBs while having significantly reduced manufacturing costs.9–11 In particular, compared to SIBs, KIBs are more attractive, since they possess some unique advantages: (i) K+/K has a low standard electrode potential in carbonate ester-based electrolytes (−2.93 V for K+/K vs. standard hydrogen electrode (SHE) and −2.71 V for Na+/Na vs. SHE); (ii) graphite can be utilized as a suitable anode for PIBs with stable performance; (iii) in liquid electrolytes, the charge density of K+ ions is lower than that of Li+ and Na+ ions, which only induces smaller solvated cations and is beneficial for fast ionic conductivity and high power; (iv) less expensive Al current collectors can be adapted for the production of electrodes since K metal will not alloy with Al. These merits make PIBs strong competitors to SIBs and even LIBs.12–15
The development of high-performance cathode materials still remains a major challenge for the practical application of PIBs. Suitable cathode candidates should store more K+ ions with high capacity, high working potential, fast K+ ion transport, and long cycle life, leading to the high energy density of PIBs.16 Since the radius of K+ is much larger than those of Na+ and Li+, the crystal structures of hosts for K+ ions are more vulnerable to extended cycling,17 and the corresponding potential K+ ion hosts possess different working voltages and potassium storage mechanisms from those of their LIB and SIB counterparts. At present, some types of electrodes have already been investigated as potential cathode materials, such us layered transition-metal oxides,18,19 Prussian blue analogues (PBAs),20,21 polyanionic composites,22 and organic compounds.23 Although layered transition-metal oxides have been very successful in commercial LIBs, most K-containing oxides, such as P2-type and P3-type KxCoO2 and KxMnO2, suffer from low working potential and complex phase transitions with unstable crystal structures during cycling,24–26 while for PBAs, they show relatively high working platforms in K half-cells, and their three-dimensional (3D) structures would greatly favour fast K+ ion insertion/extraction. Indeed, PBAs feature small volume change during charge/discharge, although their air stability remains a troublesome issue for real application.27 Similar to PBAs, various types of polyanionic compounds also possess robust 3D open framework structures, endowing fast ionic diffusion.14 In addition, they are usually air-stable, and most of the reported polyanionic compounds have fixed high working potentials with satisfactory cycling stabilities, both of which are critical parameters for reliable energy storage systems. Organic compounds are also considered to be attractive candidates due to their low manufacturing costs and flexible, tunable structures.28 They usually possess relatively good cycling stabilities and high achievable specific capacities, which deserve wider and more in-depth investigations. Among these cathode material candidates, it is necessary to pay special attention to polyanionic and organic compounds since their high voltage platforms as well as high specific capacities are the key routes to achieve high energy density for PIBs.
In this review, we first review the state-of-the-art development of electrode materials (anodes and cathodes) towards non-aqueous potassium storage materials. In recent years, a large volume of various types of potassium hosts with multiple structures have been intensively investigated for both cathode and anode materials. Considering the natural abundance of potassium, many encouraging potassium analogues of PIB electrodes have been used with promising electrochemical properties, as shown in Fig. 1. Then, we systematically summarize the recent progress in the development of polyanionic and organic compounds for non-aqueous PIBs, anticipating future guidance and directions to solve the major disadvantages of polyanionic and organic candidates. Special attention will be given to the key factors such as long-term cycling stability and working potential for practical application concern in the future. Finally, personal perspectives are provided with fruitful strategies for their future optimization towards realizing the real applications of cathode materials in PIBs.
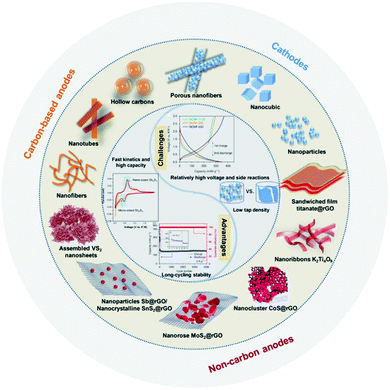 | ||
| Fig. 1 Schematic illustration of the different structured electrodes for PIBs and corresponding advantages and challenges. Reproduced with permission.29 Copyright 2019, AAAS. | ||
2. Overview of the current electrolyte materials for potassium-ion batteries
2.1 Cathode materials
Inspired by the rocking-chair alkali ion working mechanisms, finding potential potassium hosts with rigid structures and high voltage windows to accommodate continuous K+ ion de-/insertion is very important. Considering the large ion size and heavier molecular weight of the K+ ion, a series of great challenges are inevitably encountered with previous recognised structures for LIBs and SIBs, such as inferior reversible capacities and large volume changes, which remain the key obstacles for the high performance of PIBs. Therefore, we initially overview the current states of cathode materials and make comparisons to obtain an in-depth understanding.Layered metal oxides for PIBs are the primary targets of researchers since they have received tremendous success in LIBs and SIBs. Although they usually suffer from large volume changes and structural deformation during cycling, they can not only utilize a variety of redox couples with expected reversible capacities, but also sustain fixed voltage platforms with relatively stable structures. The layered oxides can be traditionally classified with an alpha-numeric expression defined by Delmas and colleagues.30 The alkali site coordination is identified, such as O for octahedral and P for prismatic, and a number represents the oxygen stacking sequence. This structure initially provides a two-dimensional (2D) framework for the rapid transportation of alkali ions, even for the large radius of K+ ions. The mechanism for potassium to be stabilized depends on two key primary characteristics: the compatibility of their interstitial sites where available and the possible coordination environment of potassium ions within specific electronic configurations. This mechanism is also restricted by the degree of electrostatic repulsion between the intercalated cations and the local oxygen atoms.
KxCoO2 and KxMnO2 were introduced as suitable cathodes for PIBs in recent years. K0.3MnO2, with a distorted P2-type structure in orthorhombic symmetry (space group Cmcm), was reported by Vaalma et al. in 2016.31 This material can be recharged in the voltage window of 1.5–4.0 V with a reversible capacity of 125 mA h g−1. A P3-type K0.5MnO2 (Fig. 2a and b) was also demonstrated to exhibit a capacity of 110 mA h g−1 between 1.5 and 3.9 V.18 Both materials can reach higher capacities at elevated cut-off voltage, where there has always been fast capacity fading (Fig. 2c and d). Also, a non-potassium-deficient O3-type KCrO2 reported by Kim et al., as shown in Fig. 2e and f, has shown remarkable progress, since it is the only layered oxide that can host K+ ions without potassium deficiency.32 Nevertheless, its average working platform is relatively low (∼2.7 V) with a reversible capacity of 93 mA h g−1. The participation of the Cr3+/Cr4+ redox couple can minimize the negative effects of K+–K+ interactions, which usually destabilize the layered structure (as shown in Fig. 2g).
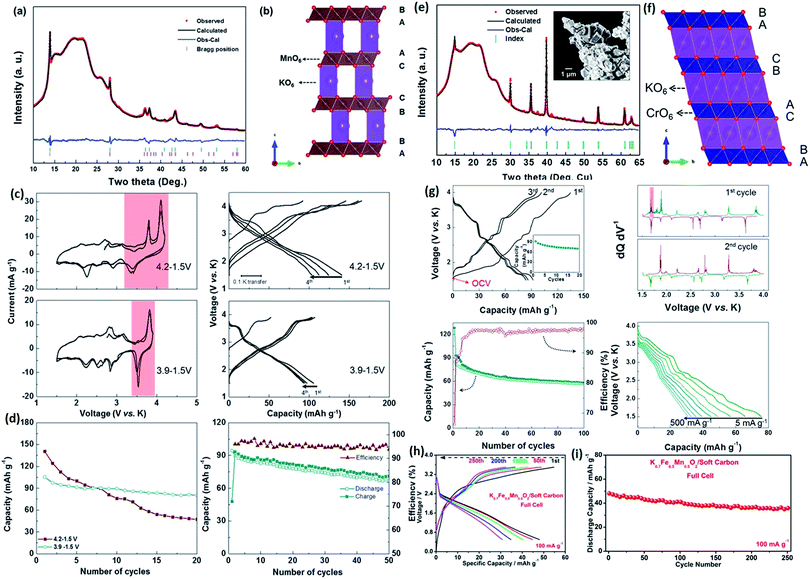 | ||
| Fig. 2 (a) Rietveld refinement results for the as-obtained K0.5MnO2 material. An R3m major phase and a Cmcm minor phase were fitted together to yield the weighted profile reliability factor, Rwp = 3.2%. (b) Schematic illustration of the P3-type K0.5MnO2 structure. (c) Cyclic voltammograms and corresponding charge/discharge profiles of the P3-type K0.5MnO2 material in different voltage windows. (d) Cycling stability in different voltage windows of the P3-type K0.5MnO2 structure. Reproduced with permission.18 Copyright 2017, Wiley-VCH. (e) Rietveld refinement results and scanning electron microscope (SEM) image (inset) for the as-prepared O3-KCrO2. (f) Schematic illustration of the O3-KCrO2 structure. (g) Electrochemical properties in different cycles and at different current densities. Reproduced with permission.32 Copyright 2018, American Chemical Society. (h and i) Full cell performance based on K0.7Fe0.5Mn0.5O2/soft carbon. Reproduced with permission.34 Copyright 2016 American Chemical Society. | ||
Other researchers found that partial replacement of Mn3+ by Fe3+ can effectively suppress the strong Jahn–Teller effect of Mn3+ due to the elevated valence of Mn.33 In addition, the multiple phase transformation can be smoothed, which still remains a major drawback of layered K-oxides. For example, a modified P2-Na2/3[Fe1/2Mn1/2]O2 cathode could reach a high specific capacity of 190 mA h g−1 and retain 150 mA h g−1 after 30 cycles.33 A K0.7Fe0.5Mn0.5O2/soft carbon full PIB cell was fabricated by Mai et al., and high capacity retention of 76% was available after 250 cycles (Fig. 2h and i).34 Its unique stable framework and nanowire morphology both contributed fast kinetics and facile charge transportation. Nevertheless, the upper cut-off voltage still remains a big challenge for all types of K-containing layered oxides, since the deeper discharge states will result in severe structural instability. This phenomenon is still not clear, and it deserves to be investigated in-depth to further utilize potential reversible capacity.
Prussian blue analogues (PBAs) are well-known as a large group of metal–organic frameworks. They possess outstanding merits such as cheap transition metal components, long cycle life, and facile large-scale production. They usually crystallize in cubic symmetry with open 3D frameworks, which can sustain large alkali ions such as Na+ and K+ to de-/insert without structural instability.35 Their general composition can be expressed as KxMA[MB(CN)6]1−δ·nH2O, where MA and MB stand for the N-coordinated and C-coordinated transition metals, and δ represents the contents of [MB(CN)6] vacancies and water residuals.21 Two main features of these PBA materials are highly appreciated for SIBs and PIBs. (i) There are sufficient large 3D channels for sodium/potassium ion diffusion and relatively low diffusion energy barriers. (ii) The contents of [MB(CN)6] vacancies and water residuals can be reduced by a specially designed synthesis process, which is critical for high reversible capacity and long-term cycling stability.
Although K–PBAs show slightly lower specific capacities than that of Na–PBAs due to the heavier K+ ions, their higher voltage platforms lead to competitive high energy densities. Zhu et al. recently employed K0.61Fe[Fe(CN)6]0.91·0.32H2O and K0.22Fe[Fe(CN)6]0.85·0.64H2O as cathode materials to fabricate high-energy-density flexible PIBs (Fig. 3a–e). The Prussian blue (PB) electrodes produced by photographic printing showed good cycling stabilities and excellent rate capabilities (Fig. 3f and g).20 Zhang et al. reported a comprehensive study on K0.22Fe[Fe(CN)6]0.805·4.01H2O nanoparticles, and they found that the carbon-coordinated Fe2+/Fe3+ redox couple was highly active and mainly responsible for the overall structural stability during cycling, while the corresponding excellent rate capability can also be attributed to this reason (Fig. 3h and i).36 High-quality FeFe(CN)6 was also synthesized by Shadike et al. with good electrochemical performances.37 Chong et al. investigated KFeII[FeIII(CN)6] material with various ex situ characterizations, and its excellent electrochemical properties are attributed to its robust structural stability.38
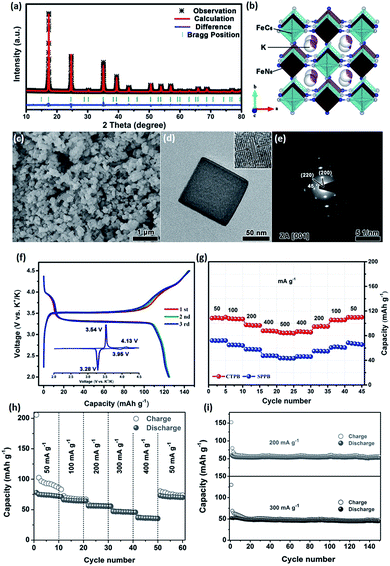 | ||
| Fig. 3 (a) Rietveld refinement results for as-prepared PB nanocubes. (b) Corresponding schematic illustration. (c) SEM, and (d) transmission electron microscope (TEM) images of as-obtained PB nanocubes. (e) Selected area electron diffraction (SAED) pattern of as-obtained PB nanocubes. (f and g) Charge/discharge curves and rate performance of as-obtained PB nanoparticles. Reproduced with permission.20 Copyright 2018 Elsevier Inc. (h) Rate and (i) cycling performances of as-prepared KPBNPs/K half-cell at various current densities. Reproduced with permission.36 Copyright 2017, Wiley-VCH. | ||
Nazar's group recently reported a Prussian white analogue, K1.7Fe[Fe(CN)6]0.9, with suitable crystallite sizes. They found that the particle size plays an important role in its ultimate electrochemical performance.39 Nevertheless, K–PBAs are sensitive to synthetic procedures, air exposure, water content, and particle size, and how these key parameters influence their structure and crystallinity, as well as their electrochemical properties, needs to be systematically investigated. Also, more advanced techniques with in-depth characterization are urgently needed as well.
2.2 Anode materials
In the case of LIBs, graphite has achieved tremendous success in the commercial market, since lithium can be reversibly intercalated via forming graphite intercalation compounds (GICs) with the final product LiC6.40 This process shows a high theoretical capacity of 372 mA h g−1 with excellent cycling stability. However, the application of graphite encountered totally different situations in SIBs and PIBs. Sodium can hardly intercalate into graphite, while GICs can be generated during the potassium intercalation, resulting in a theoretical value of 279 mA h g−1.41 Other carbon-based materials such as soft carbon have also been proved to have the capability to alloy with potassium ions.42,43The mechanism of intercalation of K into graphite is still not completely understood. Compared to Li ions, the large-sized K ions require more associated carbon atoms to accommodate them. Jian et al. reported the initial electrochemical potassium insertion process in graphite in 2015 (Fig. 4a).41 Through ex situ X-ray diffraction (XRD), they found that KC36, KC24, and KC8 are sequentially generated during potassiation, as shown in Fig. 4b and c. Graphite exhibits moderate rate capability and fast capacity fading at high C-rates. Almost at the same time, Luo et al. also made similar discoveries, and decent electrochemical performances were demonstrated (Fig. 4d–g).43 They first performed ab initio calculations, and found that K ions also can be intercalated into reduced graphene oxide (RGO) film with higher capacity (222 mA h g−1).
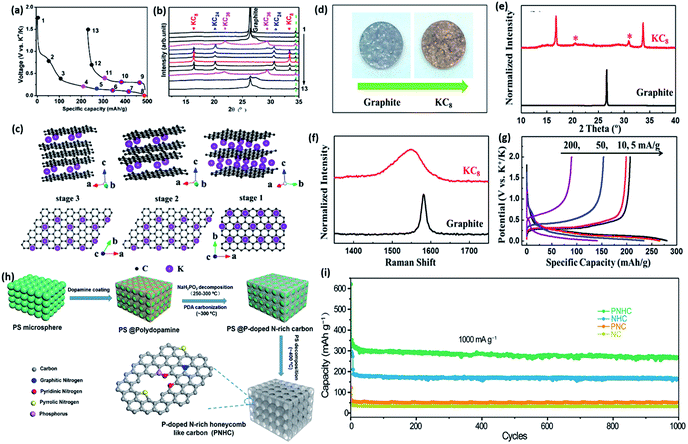 | ||
| Fig. 4 (a) Initial cycle of graphite anode and (b) corresponding ex situ XRD patterns. (c) Schematic illustrations of different K-GICs with side view and top view. Reproduced with permission.41 Copyright 2015 American Chemical Society. Ex situ measurements of KC8 and graphite electrodes: (d) photographs, (e) XRD patterns, (f) Raman spectra, and (g) electrochemical profiles of graphite electrodes at different C-rates. Reproduced with permission.43 Copyright 2015 American Chemical Society. (h) Schematic illustration of the fabrication process for as-prepared P-doped N-rich honeycomb-like carbon. (i) Cycling performances of all prepared samples at 1000 mA g−1. Reproduced with permission.45 Copyright 2019 Elsevier, Ltd. | ||
Similar to SIBs, large volume expansion still remains a big challenge for graphite-based materials for PIBs. The volume expansion of KC8 is around 61%, while LiC6 only undergoes a 10% volume expansion. Therefore, researchers have put great efforts into ameliorating this phenomenon. For example, Ding et al. fabricated sulfur-grafted hollow carbon spheres to accommodate potassium ions for PIBs.44 The specially designed structure could provide a shortened diffusion distance (∼40 nm) and strong C–S bonds to minimize the decay of cycling capacity. He et al. incorporated nitrogen species with phosphorus doping into 3D porous carbon, and this unique structure could provide sufficient pore defects and edges, resulting in rapid interfacial K+ ion absorption reactions. The fabrication process is shown in Fig. 4h.45 Ultrahigh reversible capacity and excellent C-rates were obtained (Fig. 4i). Nitrogen doping of few-layered graphene was also useful for improving the reversible capacity from 278 mA h g−1 to 350 mA h g−1. Share et al. highlighted the importance of nitrogen dopant sites apart from sp3 carbon defects.46In situ Raman spectroscopy demonstrated that there was a slightly different staging sequence for doped and undoped materials. Other researchers also have demonstrated various carbonaceous materials to be potential competitive candidates for PIBs.42,47–49
Recently, graphitizable carbon has aroused great interest among researchers, since their potassium storage capability is outstanding. Soft carbon is a typical example of graphitizable carbon. The degree of graphitization of soft carbon can be easily controlled by using different sintering temperatures (normally above 2000 °C). Some natural resources and raw materials, such as pyrolytic petroleum coke, polypyrrole, and pitch, can be turned into soft carbon after high temperature calcination in a controllable process.50–52 Their potentials for sodium/potassium ion storage, especially with surface defects, have been explored by various techniques.53
By introducing dopant atoms or surface defects, the soft carbon materials can be endowed with great potential for PIBs. For example, Ju et al. investigated few-layer F-doped graphene foam via direct synthesis processes. This material could deliver 165.9 mA h g−1 at 500 mA g−1 for 200 cycles.53 Its high surface area and unique mesoporous membrane structures can offer sufficient active sites to accommodate large potassium ions. N-doping and O-doping also have been demonstrated as useful strategies for improving the electrochemical properties of PIBs. A polyacrylonitrile (PAN) precursor was used as the starting material with an electrospinning method.54 Dual-heteroatom doping, in theory, could offer synergistic effects on soft carbons. Phosphorus and oxygen dual-doped graphene was considered as a superior anode material for PIBs. High reversible capacity of 474 mA h g−1 was achieved at 50 mA g−1 after 50 cycles.55 Cage-like graphitic carbon structures can also effectively reduce anisotropy and ameliorate the large volume expansion during K+ intercalation/deintercalation. Their 3D electrically conducting network also promotes fast electron/ion transport. Other researchers also successfully fabricated various types of carbonaceous materials, such as pre-oxidated microstructures from pitch, nitrogen/sulfur co-doped hollow carbon nanofibers,51 and short-range ordered mesoporous carbon, and superior electrochemical properties were obtained. Further work should explore better ways to increase their initial cycle coulombic efficiency towards their real applications in full PIBs.
Apart from the graphite-based or carbon-based materials, other kinds of materials based on the intercalation reaction mechanism have also been reported recently, such as K2Ti8O17.56 This material shows good rate performance with a micro-flower morphology. What's more, another interesting type of material for PIB anodes comprises alloy-based compounds, including Si, Sb, Sn, Mo, etc.57,58 These types of materials usually have higher theoretical capacities, since these elements can easily alloy with K atoms at various states of charges. Alloy-based materials are promising candidates for LIBs and have received intensive investigations. Since many different alloying compounds will be generated during cycling, however, huge volume expansion is inevitable. Therefore, multiple carbon matrix structures are being created to accommodate the volume shrinkage and improve the electronic/ionic conductivities. Sultana et al. first reported an Sn-based anode in PIBs with a reversible capacity of 150 mA h g−1. Zhang et al. found that concentrated electrolytes could stabilize the Bi-based anode composites in PIBs. The Bi nanoparticles were uniformly coated with a thick carbon layer (Fig. 5a–d). They discovered that a 5 M ether-based electrolyte could sufficiently activate the Bi–C surface. Several GICs (K5Bi4, K3Bi, KBi2, K3Bi2, K3Bi) were found in different states of charge via ex situ XRD, as shown in Fig. 5e and f.59 Phosphorus-based alloys are also important players in high performance PIB anodes. Zhang et al. recently investigated an Sn4P3/C electrode obtained by a ball milling method as a safe anode material.60 They proposed that the final discharge product is possibly a nonstoichiometric K3−xP compound (Fig. 5g). Comparable electrochemical properties are displayed in Fig. 5h. Some of the MoS2, MoSe2 and TiS2 materials also showed promising potassium storage capabilities. Other decent carbon structures have been introduced by other researchers.61–64 Further goals for these kinds of materials should be focused on prolonging their cycling stabilities and achieving higher initial cycle coulombic efficiencies.
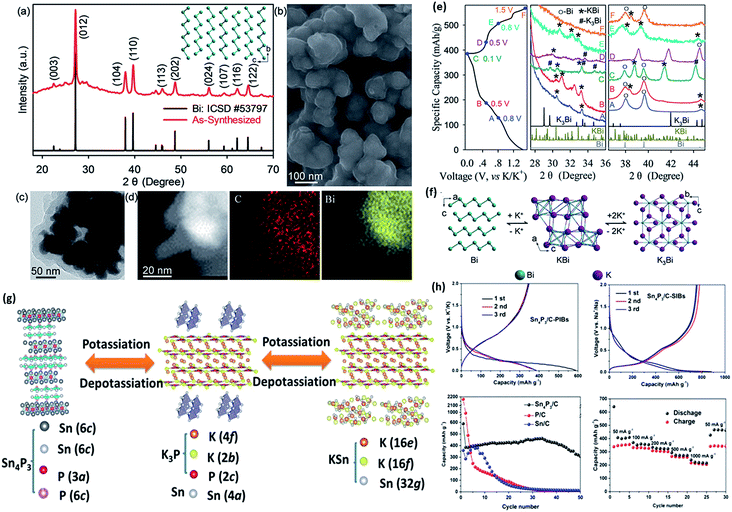 | ||
| Fig. 5 (a) XRD pattern with structure in the inset and (b) SEM image of the as-obtained Bi@C nanocomposite. (c and d) TEM images and energy dispersive spectroscopy (EDS) mapping results for the Bi@C nanocomposite. (e) Ex situ XRD study of the Bi@C anode in 5 M potassium bis(trifluoromethanesulfonyl) imide–diglyme (KTFSI–DEGDME) electrolyte. (f) Schematic illustrations of the different alloying compounds that appear during cycling. Reproduced with permission.59 Copyright 2018, Royal Society of Chemistry. (g) Schematic illustration of the potassiation/depotassiation process in the as-fabricated Sn4P3/C anode. (h) Corresponding electrochemical properties of P/C, Sn/C, and Sn4P3/C materials. Reproduced with permission.60 Copyright 2017, American Chemical Society. | ||
3. Why polyanionic and organic compounds are important for developing potassium storage hosts
Potassium-ion batteries are the most important candidates for emerging energy storage systems due to the limited resources and skyrocketing price of lithium in recent years. Currently, nearly 40% of the total costs can be assigned to the active cathode and anode materials. Stable electrochemical performances of cathode materials are especially important since they are the key factors accounting for the overall energy density and cycling stability. To date, only a few types of cathodes have been reported compared to the anodes, and most of these K-cathodes only possess low energy content in terms of both volumetric energy and gravimetric energy compared to the Li-cathodes and Na-cathodes, as shown in Fig. 6a. Therefore, urgent tasks have been proposed to enable researchers to focus on the discovery of new types of cathodes and the exploration of potential improvements to existing cathodes. Since the large ionic radius of K+ will result in strong K+–K+ interactions, 3D arrangements and special K+ ion hosting mechanisms should first be taken into consideration. As shown in Fig. 6b, most K-containing layered structured oxides possess low voltage platforms, and their 2D arrangements cannot properly accommodate the strong K+–K+ interactions, which may lead to severe capacity degradation during cycling. Prussian blue analogues are promising because they have flatter voltage profiles and high gravimetric energy, although their interstitial water residuals are hard to control, and the mechanisms influencing this remain unclear. In addition, their relatively low volumetric energy density is also a troublesome issue for their further application. Therefore, polyanionic-based and organic compounds are promising candidates to effectively increase the energy density of K-cathodes to compete with Li-cathodes and Na-cathodes with extended cycle life, due to their high working voltage and large specific capacities.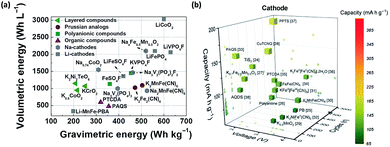 | ||
| Fig. 6 (a) Gravimetric and volumetric energy densities of existing cathodes for PIBs. Reproduced with permission.14 Copyright 2019 Elsevier Inc. (b) Capacity vs. cycle number and working potentials of reported cathodes for PIBs. Reproduced with permission.29 Copyright 2019, Science Publishing Group. | ||
4. Polyanionic materials as advanced potassium hosts
Polyanionic compounds have been intensively investigated as promising cathode materials since the invention of LIBs.65–68 Various types of robust polyanionic structures, such as phosphates, fluorophosphates, pyrophosphates, sulfates, etc., have been explored as alkali ion hosts with specific electrochemical curves. The most representative atomic structures are composed of combinations of tetrahedral XO4 groups (X = P, S, Si, etc.) with corner-sharing MO6 octahedra (M = transition metal elements), and due to their diversified covalent framework, relatively low energy barriers to alkali metal ion migration will ensure satisfactory rate capabilities, while the strong inductive effect generated by adjacent anion groups will offer elevated working potentials as well as reinforced energy densities. Compared to other types of cathode materials, we are delighted to find that recent progress in polyanionic-based materials is showing great promises in PIBs.4.1 Amorphous-FePO4
In the last decades, olivine LiFePO4 has been well studied for LIBs and received great success in the commercial LIB market. Its thermodynamically favoured Na counterpart, maricite NaFePO4, lacks possible Na+ ion diffusion pathways. Therefore, it is regarded to be electrochemically inactive. The feasibility of K+ ion intercalation into olivine and maricite A1−xFePO4 (A = K) is almost non-existent because of the large size of the K ion. The amorphous forms, such as amorphous FePO4, with short range ordering were firstly investigated as potential potassium hosts for PIBs in 2014 by Mathew et al.69 They simply synthesized a dehydrated amorphous FePO4 material by a precipitation–vacuum-drying method. A reversible capacity of 160 mA h g−1 was achieved with a relatively low operating voltage around 2.5 V (Fig. 7a). Ex situ XRD revealed that the short-range ordering was improved after K ion intercalation due to a monoclinic KFe2(PO4)2 product, and this phenomenon was reversible, since KFe2(PO4)2 could be identified again in the second cycle. A schematic illustration of the proposed amorphous-to-crystalline transformation mechanism is shown in Fig. 7b, although the intrinsic amorphous nature of such materials will result in inferior cycling stability and a low working voltage platform, which may hinder their real applications in high energy PIBs in the future.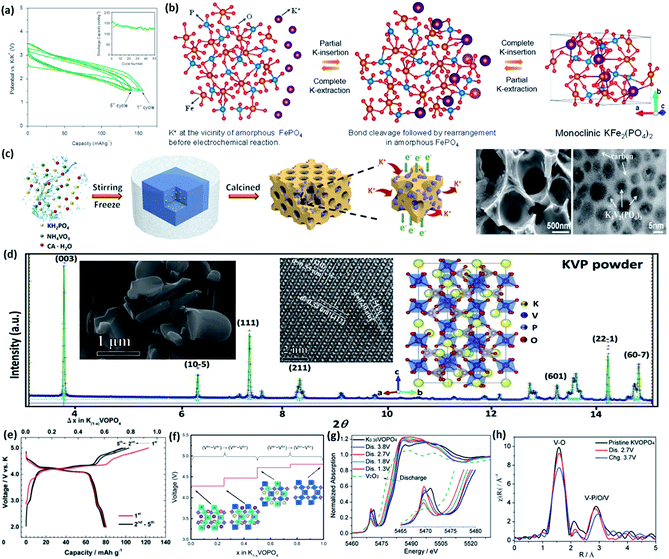 | ||
Fig. 7 (a) The initial five voltage profiles of amorphous FePO4 material. (b) Schematic illustration of the proposed amorphous-to-crystalline transformation of amorphous FePO4 hosting potassium ions. Reproduced with permission.69 Copyright 2014 Nature Publishing Group. (c) Schematic representation of the synthesis process for the 3D porous K3V2(PO4)3/C nanoparticles, and their SEM and TEM images. Reproduced with permission.72 Copyright 2017, Royal Society of Chemistry. (d) Rietveld refinement of the powder diffraction pattern of as-prepared K3V2(PO4)3 particles. The insets are the corresponding SEM and high angle annular dark field (HAADF) images with a schematic illustration of the structure. Reproduced with permission.74 Copyright 2019 Elsevier Ltd. (e) Charging and discharging profiles of KVOPO4 material in the voltage window of 2.0–5.0 V in 1 M KPF6/EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC (1 PC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) electrolyte. Reproduced with permission.78 Copyright 2017, Royal Society of Chemistry. (f) Calculated theoretical voltage profile of KVOPO4 material with associated structures (insets). Reproduced with permission.79 Copyright 2018 Royal Society of Chemistry. (g) V K-edge XANES spectra and (h) EXAFS spectra of pristine KVOPO4 electrode. Reproduced with permission.80 Copyright 2018, Wiley-VCH. 1 v/v) electrolyte. Reproduced with permission.78 Copyright 2017, Royal Society of Chemistry. (f) Calculated theoretical voltage profile of KVOPO4 material with associated structures (insets). Reproduced with permission.79 Copyright 2018 Royal Society of Chemistry. (g) V K-edge XANES spectra and (h) EXAFS spectra of pristine KVOPO4 electrode. Reproduced with permission.80 Copyright 2018, Wiley-VCH. | ||
4.2 Potassium containing phosphates/oxyphosphates
Similar to the situation in SIBs, phosphate-based cathode materials have continued to attract increasing attention for PIBs due to the feasibility of K ion intercalation. A variety of redox couples of different elements are electrochemically active for both SIBs and PIBs. Similar to the well-studied sodium superionic conductor (NASICON)-type Na3V2(PO4)3,70,71 K3V2(PO4)3 with a 3D conductive network was first introduced by Han et al. in 2017.72 A reversible specific capacity of 55 mA h g−1 could be reached at the current density of 20 mA g−1. They specially designed a 3D porous nanostructure with a uniformly covered carbon matrix (as shown in Fig. 7c). It showed good cycling stability up to 100 cycles. Later on, Wang et al. successfully synthesized bundled K3V2(PO4)3 nanowires with ultrahigh cycling stability even after 2000 cycles,73 although its XRD pattern cannot be indexed to any existing JCPDS database based on selected phases. Zhang et al. attempted to analyse its phase constitutions, and they found that its powder diffraction can be assigned to the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group with a defined NASICON structure. The Rietveld refinement of the powder diffraction pattern is displayed in Fig. 7d.74 They also constructed a symmetric full PIB, since K3V2(PO4)3 has two distinct voltage platforms, between 3.5 and 4.0 V and between 1.0 and 2.2 V. Han et al. also studied a nanocubic KTi2(PO4)3 electrode for PIBs for the first time.75 This material has a flat voltage platform of ∼1.6 V, which also can be used as an anode material as well since its ICE is nearly 100%, which is important for the anode part. In addition, the utilization of the second K ion in the K3V2(PO4)3 material is worth further exploration due to the low operation voltage. It can be used as an anode for full cells of KIBs as well. Further investigations should first completely determine the phase constitution and site occupation of each atom, and this will be significantly helpful to rationally optimise this new polyanionic compound towards higher reversible capacity and energy density. In addition, it is worth noting that a non-potassium containing material, VOPO4, has been explored as a potential cathode for PIBs. Peng et al. synthesized 2D VOPO4 nanosheets with remarkable electrochemical performances.76 Hyoung et al. also introduced VOPO4·2H2O as a cathode material for PIBs.77 They explored the reaction mechanism and the role of additive water in structural stabilization, and it is also suitable for wet electrolytes.
c space group with a defined NASICON structure. The Rietveld refinement of the powder diffraction pattern is displayed in Fig. 7d.74 They also constructed a symmetric full PIB, since K3V2(PO4)3 has two distinct voltage platforms, between 3.5 and 4.0 V and between 1.0 and 2.2 V. Han et al. also studied a nanocubic KTi2(PO4)3 electrode for PIBs for the first time.75 This material has a flat voltage platform of ∼1.6 V, which also can be used as an anode material as well since its ICE is nearly 100%, which is important for the anode part. In addition, the utilization of the second K ion in the K3V2(PO4)3 material is worth further exploration due to the low operation voltage. It can be used as an anode for full cells of KIBs as well. Further investigations should first completely determine the phase constitution and site occupation of each atom, and this will be significantly helpful to rationally optimise this new polyanionic compound towards higher reversible capacity and energy density. In addition, it is worth noting that a non-potassium containing material, VOPO4, has been explored as a potential cathode for PIBs. Peng et al. synthesized 2D VOPO4 nanosheets with remarkable electrochemical performances.76 Hyoung et al. also introduced VOPO4·2H2O as a cathode material for PIBs.77 They explored the reaction mechanism and the role of additive water in structural stabilization, and it is also suitable for wet electrolytes.
Potassium-containing oxyphosphates have also been intensively investigated in the last three years. Chihara et al. firstly reported KVOPO4 material as the cathode for PIBs in 2017. A reversible capacity of 84 mA h g−1 could be obtained in the voltage window of 2.0–5.0 V using 1 M KPF6 ethylene carbonate/polypropylene carbonate (EC/PC) as the electrolyte (Fig. 7e).78 The XRD powder pattern was indexed to the Pna21 space group with high purity. In situ XRD revealed that the whole charging process can be assigned to a two-phase reaction. This material possesses a high voltage platform around 4.2 V, which is important for achieving PIBs with high energy density. Lian et al. performed density functional theory (DFT) calculations to further determine the phase transformation, ionic diffusion, and charge transfer mechanism of this promising KVOPO4 cathode material.79 The calculated theoretical voltage profile is exhibited in Fig. 7f with the V4+/V5+ redox couple. From the DFT study, multiple phase transitions are involved during K de/intercalation, which introduced a ∼6.6% volume change. Low active energy barriers were calculated at the same time. In addition, Ding et al. reported that in the KVPO4 material, the V3+ ↔ V4+ ↔ V5+ redox couples are fully electrochemically active in SIBs, and a high specific capacity of 235 mA h g−1 was achieved.80 Further V K-edge X-ray near edge structure (XANES) spectra and extended X-ray absorption fine structure (EXAFS) revealed that the first Na ion occupies the identical crystallographic sites that are emptied by the deintercalated K ion (Fig. 7g and h). Future work can be focused on improvement of the cycling stability of the KVOPO4 material and suitable carbonaceous decoration to upgrade its C-rate performance.81 Other types of potassium-containing oxyphosphates are continuously emerging with distinctive electrochemical profiles, such as K2[(VO)2(HPO4)2(C2O4)] reported by Liao et al.82 More relevant work should be urgently undertaken since these types of materials are likely to be important players in real applications of PIBs in the near future.
4.3 Potassium-containing pyrophosphates
Pyrophosphates are other important candidates as potential high-voltage potassium hosts, since they are common polyanionic compounds and have been widely studied for both LIBs and SIBs.83–85 In the case of PIBs, KMoP2O7 and KVP2O7 exhibited reversible potassium hosting capacities of 25 mA h g−1 and 55 mA h g−1 at 50 °C, respectively. Although their capacity is relatively low according to the reported literature, they have the highest working voltage among all the existing potassium hosts. Higher cathode voltage is very useful for improving the overall energy density of PIBs. Park et al. also proposed an interesting material screening method, inorganic registry, for PIBs.86 They employed DFT calculations during a heuristics-based data-mining process. Six compounds, including KTiP2O7, KVP2O7, KMoP2O7, K2(VO)3(P2O7)2, K2MnP2O7, and KMnVO4 were pinpointed as possessing high voltage platforms, and some of them even had higher platforms than 5.0 V. Park et al. systemically synthesized all the screened materials and collected their cyclic voltammograms, as shown in Fig. 8a. Interestingly, only those electrochemically active materials (in the given voltage window) showed obvious colour changes. In addition, they performed in situ XRD with a full charge and discharge cycle. The enlarged XRD patterns in Fig. 8b exhibit a continuous phase transformation from P21/c to P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a certain type of K+/vacancy rearrangement.
with a certain type of K+/vacancy rearrangement.
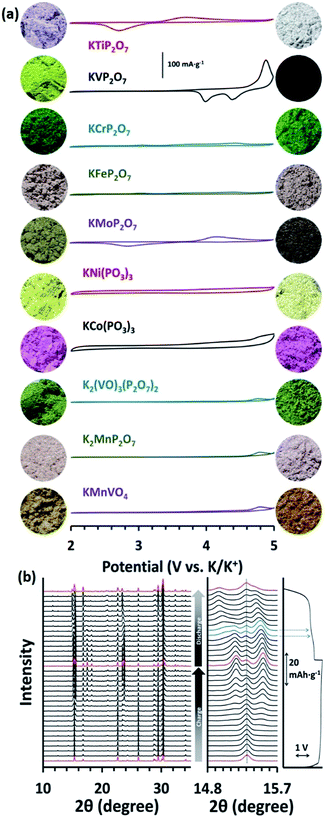 | ||
Fig. 8 (a) Cyclic voltammetry (CV) profiles of ten screened polyanionic compounds in KPF6/EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diethyl carbonate (DEC) at 20 °C and their corresponding colours before and after charging. (b) In situ XRD patterns of KVP2O7 material over one full cycle. Reproduced with permission.86 Copyright 2018, Wiley-VCH. diethyl carbonate (DEC) at 20 °C and their corresponding colours before and after charging. (b) In situ XRD patterns of KVP2O7 material over one full cycle. Reproduced with permission.86 Copyright 2018, Wiley-VCH. | ||
In addition, Fe and Mn-based pyrophosphates, such as KFeP2O7 and K2MnP2O7, possibly have voltage platforms higher than 5.0 V due to the strong inductive effect of P2O74−. Hosaka et al. synthesized K2FeP2O7/C and K2MnP2O7/C electrodes, but only low reversible capacities and low voltage platforms could be achieved.16 They attributed these inferior electrochemical properties to the weak inductive effect that FeO4 tetrahedra can offer (compared to FeO6 octahedral coordination). Although KVP2O7 has a theoretical specific capacity of 102 mA h g−1, the recently achieved capacity is lower than 60 mA h g−1, which means that there is still large room for exploration.
4.4 Potassium-containing fluorophosphates/fluorosulfates
The participation of F in the MO6 (M = V, Fe, Mn, etc.) octahedra and XO4 groups (X = P, S, etc.) can effectively enhance the inductive effect of adjacent transition metal ions, which can offer higher working voltage platforms for various types of polyanionic alkali-metal-ion hosting compounds in LIBs and SIBs.68,87–90 In the case of PIBs, the participation of F in the open framework also provides great advantages in terms of higher voltages, higher rates of ionic diffusion, and enhanced structural stabilization. Chihara et al. first reported a new polyanionic compound, KVPO4F, as a promising cathode material for PIBs.78 The synthesised KVPO4F material crystallized into orthorhombic symmetry with the space group Pna21 (as shown in Fig. 9a). An average voltage platform of ∼4.1 V was achieved with good C-rate performance (Fig. 9b and c). Then, Kim et al. developed a new strategy for the stoichiometric KVPO4F material.91 Two distinctive K ion sites were identified through Rietveld refinements, which are shown in Fig. 9d. The as-obtained material could exhibit a reversible capacity of ∼105 mA h g−1 with a ∼4.2 V voltage platform. Four obvious oxidation/reduction couples were recognised in the voltage range of 4.0 V to 5.0 V (Fig. 9e and f). A high energy density of 450 W h kg−1 was achieved and is displayed in Fig. 9g in comparison with other types of potassium hosts. Formation energy calculations indicated that the lowest energy configuration is less than 6 meV above the convex hull. A XANES study revealed that the pristine sample only contains V3+. Therefore, the oxidation state of fully charged KVPO4F is tetravalent. This K-containing fluoride phosphate is a very important candidate for high energy PIBs and should receive more decent and comprehensive investigations in the near future.92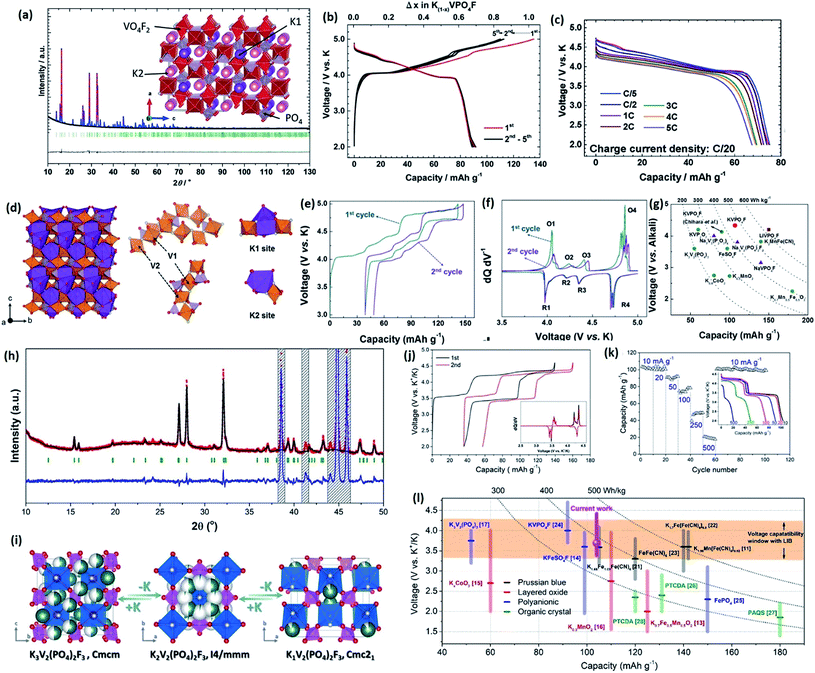 | ||
| Fig. 9 (a) Rietveld refinement of the as-prepared KVPO4F material, with the structure shown in the inset. (b) Electrochemical profiles of the initial five cycles of KVPO4F at 5.0–2.0 V. (c) C-rate capability of KVPO4F. Reproduced with permission.78 Copyright 2017, Royal Society of Chemistry. (d) Schematic illustration and analysis of the structure of KVPO4F with two different K-ion sites. (e) First and second cycle voltage profiles of KVPO4F at 5 mA g−1 and (f) corresponding differential capacity (dQ/dV) plots. (g) Energy density comparison of various potential cathodes for PIBs. Reproduced with permission.91 Copyright 2018, Wiley-VCH. (h) Rietveld refinement result of as-prepared K3V2(PO4)2F3 material. (i) Schematic illustrations of phase transition during K insertion and extraction. (j) Initial two charge and discharge curves of K3V2(PO4)2F3 and the corresponding dQ/dV plot (inset). (k) Rate capabilities. (l) Summary and comparison of capacities and voltages of different types of reported cathode materials. Reproduced with permission.93 Copyright 2018, Elsevier. | ||
Another important candidate for high energy PIBs is K3V2(PO4)2F3. The electrochemically active K3V2(PO4)2F3 cannot be directly synthesized via a sintering method, however, according to Lin et al.,93 K3V2(PO4)2F3 can be obtained from well-crystallized Na3V2(PO4)2F3 through an electrochemical exchange process. The Rietveld refinement of K3V2(PO4)2F3 is displayed in Fig. 9h. Also, the determined phase transition processes for each space group are illustrated in Fig. 9i. Two obvious oxidation/reduction redox couples around 3.5 V and 4.25 V are clearly revealed (Fig. 9j). Relatively good C-rate performance is also displayed in Fig. 9k with a capacity of ∼80 mA h g−1 at 0.1 A g−1. Fig. 9l shows the energy density level that K3V2(PO4)2F3 can achieve, making it highly competitive with the Prussian blue analogues and organic crystals. This work is important in the PIB research field, since other types of cathodes for SIBs can possibly be electrochemically active in PIBs and deserve more intense exploration.
Fluorosulfates have attracted much attention for LIBs and SIBs in the last five years.94–96 The partial substitution of F into the coordinated O sites can increase the inductive effect of adjacent transition metals, resulting in more electrostatic repulsion during the diffusion of alkali ions and leading to elevated de-/intercalation voltage platforms. Recham et al. first investigated the potential performances of KFeSO4F in LIBs, SIBs, and PIBs. Similar structural relationships were identified for the K-based fluorosulfates when K was electrochemically replaced by Li or Na.87 A high voltage platform of ∼3.9 V was achieved. Then, Lander et al. further investigated the structural, electrochemical, and magnetic properties of KFeSO4F polymorphs.97 They found that two main phases can be generated after an annealing process at 310 °C: monoclinic KFeSO4F phase and orthorhombic KFeSO4F. Substitution with Mn and Cu both failed, since no pure phases could be obtained. The bond valence energy landscape (BVEL) revealed that the monoclinic KFeSO4F is a 2D diffusion conductor while orthorhombic KFeSO4F is a 3D diffusion conductor with a low energy barrier of 0.40 eV. A high theoretical energy density of 550 W h kg−1 was proposed once the pure polymorphic phase was ascertained. Hosaka et al. recently has made significant progress in the determination of various phases produced at different sintering temperatures (Fig. 10a and b).16 By adjusting the electrolyte (5.5 mol kg−1 potassium bis(fluorosulfonyl) amide (KFSA)/diglyme vs. 1 M KPF6/EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC), much improved cycling stability was achieved, as shown in Fig. 10c and d. Operando XRD also indicates a reversible structural change from KFeSO4F (space group Pna21) to the FeSO4F space group (Pnna). The operando XRD pattern is displayed in Fig. 10e. A multiphase transition was recognised and should receive more comprehensive investigation, as well as more strategies for improving the electrochemical performance.
PC), much improved cycling stability was achieved, as shown in Fig. 10c and d. Operando XRD also indicates a reversible structural change from KFeSO4F (space group Pna21) to the FeSO4F space group (Pnna). The operando XRD pattern is displayed in Fig. 10e. A multiphase transition was recognised and should receive more comprehensive investigation, as well as more strategies for improving the electrochemical performance.
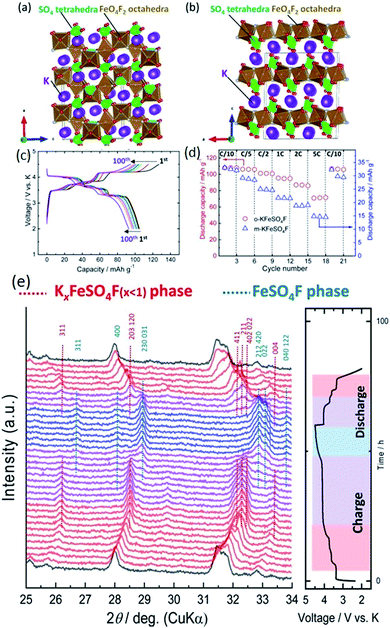 | ||
| Fig. 10 Schematic illustrations of (a) orthorhombic KFeSO4F and (b) monoclinic KFeSO4F phase. (c and d) Their corresponding electrochemical performances in 5.5 mol kg−1 KFSA/diglyme (G2) electrolyte. (e) Operando XRD patterns of orthorhombic KFeSO4F electrode during the initial cycle in a PIB. Reproduced with permission.16 Copyright 2019, Chemical Society of Japan & Wiley-VCH. | ||
In general, most polyanionic compounds possess higher voltage platforms (above 3.5 V vs. K/K+), and therefore, their energy densities are even highly comparable to those of some Li-cathodes. It is important to put an emphasis on these polyanionic K-cathode compounds towards the real application of advanced PIBs. In order to alleviate the existing drawbacks such as low theoretical capacity and sluggish kinetics of polyanionic-type materials for PIBs, it is important to expand the existing crystalline structures with well-designed carbon matrices. In addition, discovering more double redox reaction transition metal ions, such as Mn2+/Mn3+/Mn4+, V3+/V4+/V5+, and Co2+/Co3+/Co4+, is one of the key tasks in the exploration of compounds with increased theoretical capacities.
5. Organic compounds as advanced potassium hosts
Organic compounds have been considered as attractive cathode candidates for SIBs due to their low cost and flexible structures.98–101 In the case of PIBs, their weak intermolecular interactions can benefit the intercalation of large K+ ions. Moreover, the merits of various structures, and the low cost and versatile chemistry of these organic compounds have attracted great interest for PIBs in the last few years. The functional groups that account for electrochemically active oxidation/reduction play key roles and have been subjected to careful characterization. Therefore, it is necessary to provide a detailed summary of their distinctive structures and properties.5.1 Non-metal-containing organic compounds
Non-metal-containing organic compounds are starting materials utilized as electrode materials in LIBs and SIBs due to their low cost, high abundance, environmental friendliness, and amazing structural diversity.102,103 Fan et al. introduced 3,4,9,10-perylene-tetracarboxylic acid-dianhydride (PTCDA) as a potential cathode material for PIBs.104 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in PTCDA can be transformed to C–O–M (M = Li, Na, K) during electrochemical processes. Due to its low electrical conductivity and solubility, however, the electrochemical performance of PTCDA in PIBs is not so satisfactory. They annealed PTCDA at a temperature designed to enhance its electrical conductivity without thermal decomposition (Fig. 11a). A capacity of 131 mA h g−1 in the voltage range of 1.5–3.5 V was achieved.
O bonds in PTCDA can be transformed to C–O–M (M = Li, Na, K) during electrochemical processes. Due to its low electrical conductivity and solubility, however, the electrochemical performance of PTCDA in PIBs is not so satisfactory. They annealed PTCDA at a temperature designed to enhance its electrical conductivity without thermal decomposition (Fig. 11a). A capacity of 131 mA h g−1 in the voltage range of 1.5–3.5 V was achieved.
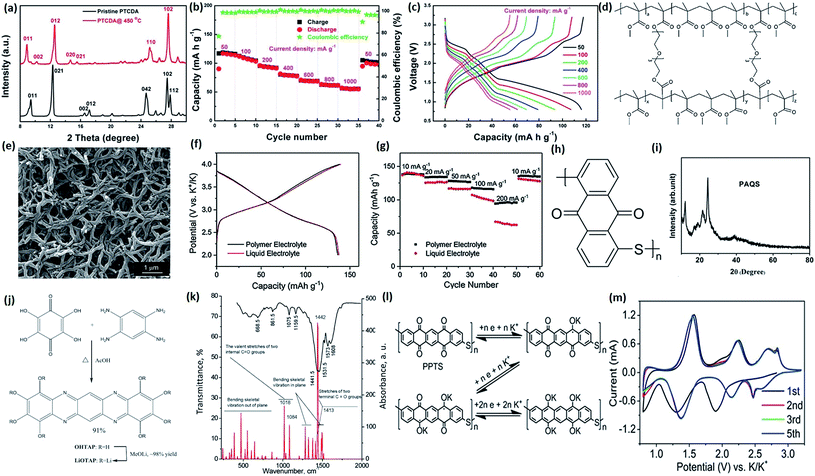 | ||
| Fig. 11 (a) XRD patterns of pristine PTCDA and PTCDA after sintering. (b) Rate performance and (c) charge/discharge profiles at different current densities of sintered PTCDA. Reproduced with permission.104 Copyright 2018, Wiley-VCH. (d) The chemical structure of the cross-linked PMMA. (e) SEM image of the as-obtained PMMA. (f and g) Charge/discharge profiles and C-rate performance of polyaniline cathode. Reproduced with permission.105 Copyright 2018, Wiley-VCH. (h) Chemical structure and (i) XRD pattern of PAQS. Reproduced with permission.23 Copyright 2016, Elsevier. (j) Schematic illustration of the synthesis of OHTAP and tetrahydroxycyclohexa-2,5-diene-1,4-dione (LiOTAP). (k) The experimental and simulated FTIR spectra of LiOTAP. Reproduced with permission.106 Copyright 2019, Elsevier. (l) The theoretical electrochemical reactions of PPTS in PIBs. (m) The initial five CV curves of PPTS. Reproduced with permission.107 Copyright 2019, the Royal Society of Chemistry. | ||
A reversible capacity of 60 mA h g−1 could be obtained at high current density (1 A g−1) (Fig. 11b and c). Ex situ XRD indicated that most peaks could be restored. There are also many other organic compounds under intensive exploration. Gao et al. developed a polyaniline cathode with a polymer-gel electrolyte for high-energy-density PIBs.105 The structure of this cross-linked poly(methyl methacrylate) (PMMA) is shown in Fig. 11d and e. A high reversible voltage platform around 3.0 V was achieved with a high capacity of 130 mA h g−1, so that its energy density is highly competitive with those of other types of cathodes for PIBs, as shown in Fig. 11f and g. Recently, Jian et al. introduced another organic compound, poly(anthraquinonyl sulfide) (PAQS) as a novel cathode material for PIBs.23 Its chemical structure and corresponding XRD patterns are shown in Fig. 11h and i. The PAQS material was prepared by a modified Phillips method. There are two carbonyl groups in one monomer. Upon charging, two K ions can be intercalated with two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O sites, corresponding to two distinctive working platforms in the electrochemical profile. Very recently, Slesarenko et al. reported a new tetraazapentacene-based redox-active material for PIBs.106 The facile synthesis of octahydroxytetraazapentacene (OHTAP) is displayed in Fig. 11j. Detailed analysis of the Fourier transform infrared (FTIR) spectrum is also shown in Fig. 11k, indicating its virtually insoluble property, very different from all common organic solvents. High capacities over 200 mA h g−1 could be achieved in the initial cycles, but its cycling stability is not very satisfactory upon extended cycling. Also, its coulombic efficiency is below 90% for each cycle, which may hinder its further application. Another sulfide-type organic compound, poly(pentacenetetrone sulfide) (PPTS), was successfully synthesized by Tang et al. and recently introduced as an organic cathode.107 It can sustain three K+ ion intercalations with three distinctive oxidation/reduction peaks, which is shown in Fig. 11l and m. A solid-electrolyte interphase (SEI) layer was confirmed via decomposition of dimethoxyethane (DME) and KPF6, and its d-spacing was slightly increased after cycling, which is due to the insertion of K ions. Unexceptional high capacity was achieved (∼260 mA h g−1 at 0.1 A g−1), and even when cycled at 10C, it still had a discharge capacity of 160 mA h g−1. This material shows promising potential for real applications in PIBs since it possesses superior electrochemical properties with acceptable voltage platforms.
O sites, corresponding to two distinctive working platforms in the electrochemical profile. Very recently, Slesarenko et al. reported a new tetraazapentacene-based redox-active material for PIBs.106 The facile synthesis of octahydroxytetraazapentacene (OHTAP) is displayed in Fig. 11j. Detailed analysis of the Fourier transform infrared (FTIR) spectrum is also shown in Fig. 11k, indicating its virtually insoluble property, very different from all common organic solvents. High capacities over 200 mA h g−1 could be achieved in the initial cycles, but its cycling stability is not very satisfactory upon extended cycling. Also, its coulombic efficiency is below 90% for each cycle, which may hinder its further application. Another sulfide-type organic compound, poly(pentacenetetrone sulfide) (PPTS), was successfully synthesized by Tang et al. and recently introduced as an organic cathode.107 It can sustain three K+ ion intercalations with three distinctive oxidation/reduction peaks, which is shown in Fig. 11l and m. A solid-electrolyte interphase (SEI) layer was confirmed via decomposition of dimethoxyethane (DME) and KPF6, and its d-spacing was slightly increased after cycling, which is due to the insertion of K ions. Unexceptional high capacity was achieved (∼260 mA h g−1 at 0.1 A g−1), and even when cycled at 10C, it still had a discharge capacity of 160 mA h g−1. This material shows promising potential for real applications in PIBs since it possesses superior electrochemical properties with acceptable voltage platforms.
In the meantime, other researchers continuously explore novel organic compounds for advanced PIBs. For example, Tian et al. synthesized carbonyl-based polyimide and polyquinoneimide with one-dimensional (1D) and 2D structures for PIBs.108 Among them, polyquinoneimide showed the highest initial capacity because of its multiple carbonyl groups. The cycling stability largely depends on the π-conjugation structure, and the distribution density of carbonyl groups will account for this. Vitamin K also showed potentials in Xue et al.'s work.109 Vitamin K is an essential nutrient for the human body, and it also possesses a high theoretical specific capacity of 313.5 mA h g−1. After hybridization with graphene nanotubes, more stable cycling performance was achieved. This can be ascribed to its enhanced electronic conductivity and soluble nature. Conjugated microporous polymers (CMPs) also came to our attention, since their lowest unoccupied molecular orbitals (LUMOs) are well distributed and easily controlled.110 A possible K storage mechanism was proposed at the same time. An acyclic compound, 2,20-azobis(2-methylpropionitrile) (AIBN), also shows potential potassium hosting capability as an anode.111 It can offer a capacity of over 160 mA h g−1 in the voltage window of 0.01 V to 3.0 V. More importantly, the electrochemical active sites N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N only have trace toxicity.
N only have trace toxicity.
It is satisfying to summarize the non-metal containing compounds for PIBs, since their structural diversity is really impressive, and different functional groups are unique in their distinctive electrochemical profiles. More and more types of organic compounds are continuously being discovered and explored.103,112–117 In addition, there are still some obstacles for these types of compounds that need to be addressed, such us further improving the cycling stability and operating voltage platform, while endowing them with less toxicity, higher coulombic efficiency, etc.
5.2 Metal-containing organic compounds
Organic compound cathodes that contain either transition metal ions or alkali ions are more significant, since the utilization of graphite anode can possibly be achieved for practical application. Therefore, it is necessary to focus on the metal containing organic compounds and continuously explore their further potential. Zhao et al. obtained oxocarbon salts (M2(CO)n) (M = Li, Na, K; n = 4, 5, 6) through a one-pot proton exchange reaction.118 The structures of these organic oxocarbon salts, as well as their preparation procedures, are shown in Fig. 12a. These types of structures are unique and can be similarly expanded. Most oxocarbon salts are soluble in commonly used organic electrolytes. In Fig. 12b, the initial discharge capacity with regard to the available active carbonyls is summarized. High capacities could be obtained when K2C5O5 and K2C6O6 were presented. The authors further explored the possible potassium storage mechanism via in situ Raman spectroscopy (Fig. 12c and d). The ring vibrations remained stable while those of the carbonyl functional groups decreased during charging. High reversibility was confirmed via representations of the vibrations of both rings and carbonyl groups. Zhao et al. reported a novel anthraquinone with substituted sodium sulfonate groups as an organic material for PIBs. Anthraquinone-1,5-disulfonic acid sodium salt was the final product with good capacity retention.119 They also proposed a two-electron transfer process, as shown in Fig. 12e. Wang et al. successfully synthesized potassium perylene-3,4,9,10-tetracarboxylate (K4PTC) as a stable organic anode for PIBs.120 This material was obtained by treating PTCDA with KOH in ethanol solution. The typical charge/discharge profiles in the first three cycles of K4PTC are displayed in Fig. 12f. Its purity and synthesis procedure are introduced in Fig. 12g. Only two H signals can be detected, and no PTCDA signal appeared (Fig. 12g). It also displays excellent C-rate performance, which can be ascribed to the low energy gap between the highest occupied molecular orbital (HOMO) and LUMO levels based on the DFT calculations. They also proposed a two-electron mechanism for K4PTC, as shown in Fig. 12h. Liang et al. developed azobenzene-4,4′-dicarboxylic acid potassium salts (ADAPTS) as a cathode material for high performance PIBs.121 ADAPTS is connected with two N atoms (azo group, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), which is also the main redox centre for the overall charge density distribution. One voltage platform was observed in the charge/discharge profiles and CV curves. Similarly, Li et al. introduced a conjugated dicarboxylate derivative, potassium naphthalene-2,6-dicarboxylate (K2NDC). This material has an aromatic naphthyl skeleton, and they used it as a potential organic anode for PIBs.122 This material possessed a low voltage platform at around 0.55 V and a relatively high specific capacity of 200 mA h g−1. Ex situ FTIR spectra indicated highly reversible two-electron electrochemical behaviour. The feasible chemical structure of this type of compound also makes it possible to introduce cobalt(II) into an organic anode for PIBs.123 A capacity around 200 mA h g−1 was achieved. Its cycling performance and the coulombic efficiency of each cycle were still not sufficient, however. Pre-sodiation can be considered as an efficient approach to ameliorate this problem. Chen et al. introduced para-disodium-2,5-dihydroxy-1,4-benzoquinone (p-Na2C6H2O6), benefiting from salinization (Fig. 12i).124 This material displayed much improved electrochemical performance, with a capacity of 121 mA h g−1 as well as good cycling stability over 50 cycles at 0.1C. The presence of Na in the p-Na2C6H2O6 structure stabilized the energy distribution as well as suppressed the solubility of the electrode (Fig. 12j). Other researchers have also made great efforts to explore the possibilities of these metal-containing organic compounds for PIBs.
N), which is also the main redox centre for the overall charge density distribution. One voltage platform was observed in the charge/discharge profiles and CV curves. Similarly, Li et al. introduced a conjugated dicarboxylate derivative, potassium naphthalene-2,6-dicarboxylate (K2NDC). This material has an aromatic naphthyl skeleton, and they used it as a potential organic anode for PIBs.122 This material possessed a low voltage platform at around 0.55 V and a relatively high specific capacity of 200 mA h g−1. Ex situ FTIR spectra indicated highly reversible two-electron electrochemical behaviour. The feasible chemical structure of this type of compound also makes it possible to introduce cobalt(II) into an organic anode for PIBs.123 A capacity around 200 mA h g−1 was achieved. Its cycling performance and the coulombic efficiency of each cycle were still not sufficient, however. Pre-sodiation can be considered as an efficient approach to ameliorate this problem. Chen et al. introduced para-disodium-2,5-dihydroxy-1,4-benzoquinone (p-Na2C6H2O6), benefiting from salinization (Fig. 12i).124 This material displayed much improved electrochemical performance, with a capacity of 121 mA h g−1 as well as good cycling stability over 50 cycles at 0.1C. The presence of Na in the p-Na2C6H2O6 structure stabilized the energy distribution as well as suppressed the solubility of the electrode (Fig. 12j). Other researchers have also made great efforts to explore the possibilities of these metal-containing organic compounds for PIBs.
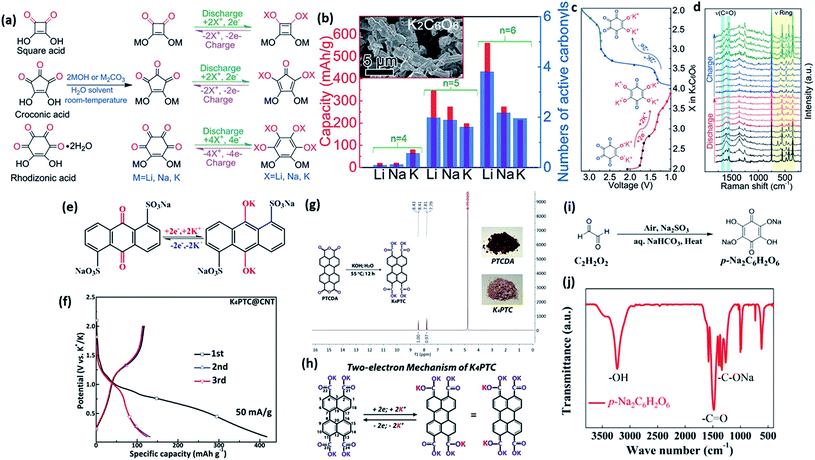 | ||
| Fig. 12 (a) Schematic diagrams of synthesis processes, structures, and theoretical reactions of the obtained oxocarbon salts. (b) The available discharge capacity and corresponding functional carbonyl groups. The inset is an SEM image of K2C6O6. (c) Discharge/charge profile of K2C6O6 with marked points for PIBs and (d) corresponding in situ Raman spectra. Reproduced with permission.118 Copyright 2016, Wiley-VCH. (e) The possible potassium storage mechanism of anthraquinone-1,5-disulfonic acid sodium salt. Reproduced with permission.119 Copyright 2017, Elsevier. (f) The initial three charge/discharge curves of the as-prepared K4PTC@CNT electrode. (g) The synthesis process for the K4PTC@CNT composite and its 1H nuclear magnetic resonance (NMR) spectrum in D2O. (h) The proposed two-electron mechanism of the K4PTC@CNT electrode in PIBs. Reproduced with permission.120 Copyright 2019, the Royal Society of Chemistry. (i) Synthesis process for the as-obtained p-Na2C6H2O6 material. (j) FTIR spectrum of the p-Na2C6H2O6 material. Reproduced with permission.124 Copyright 2019, Elsevier. | ||
Organic compounds have shown promising aspects for the real application of PIBs since their theoretical capacities are the highest among all the types of cathodes. They also possess relatively high voltage platforms, and further discoveries of satisfactory electrodes can be reasonably anticipated due to their feasible and versatile chemical structures. Their distinctive chemical structures also offer great potential for using them as anode materials as well, such as K4PTC mentioned above in Fig. 12f. While using them as anode materials, it is important to pay extra attention to their ICE and reaction mechanisms. Rate performance can be improved with appropriate designs of the carbon matrix. Nevertheless, several problems should be urgently outlined and emphasised. The operation voltages are still not high enough for goals of high energy densities of PIBs. It is important to continuously design and fabricate other types of organic compounds with feasible structures and higher electrostatic repulsion. Focusing on the solubility-related problems and initial coulombic efficiency (ICE) improvement should be first considered. Also, there should be an emphasis on potassium containing organic compounds, so that the use of graphite anode can be applied in practical applications of PIBs.
6. Summary and future perspectives
Intensive attention has been paid to advanced potassium-ion batteries with low-cost, high energy density, and long-cycling life in recent years since they are considered as the alternative energy storage systems to replacing LIBs for portable electronic devices and electric vehicle applications in the near future.125–133 Due to the low cost and abundance of K resources, it is worth making great efforts to investigate the appropriate electrode materials with high energy densities and satisfactory C-rate performance.134,135 Benefiting from the similarities between PIBs and SIBs/LIBs, rapid progress of both cathode and anode materials has been achieved to date. Due to the large radius of K+ ions, however, previously well-recognised electrochemical reaction mechanisms in LIBs/SIBs are different from that of PIBs, so that specific investigations are needed. In addition, since graphite has been proved to be the most successful K+ ion host, the main obstacles are found on the cathode side. Complex electrochemical behaviour, structural evolution, and corresponding reaction mechanisms occur during the K-hosting process. Therefore, we have mainly focused on the cathode materials, especially the polyanionic-based materials and organic materials, which possess the merits of high operating voltages, large theoretical capacities, and versatile chemical structures. These parameters are important for the improvement of the overall energy densities of full PIBs.Polyanionic-based compounds for PIBs have been intensively investigated in recent years. They commonly possess a 3D open framework and 1D to 3D K+ diffusion channels, and almost all the polyanionic-based compounds only suffer from low or at most tiny volume changes during cycling, which is an important factor for long-term cycling stability. The introduction of F and S can effectively enhance the inductive effect between adjacent atoms so that the operating voltage can be elevated. The synthesis processes for polyanionic-based compounds are facile and easy to control. Further studies related to the expansion of composition and structural space are urgently required. Most K-containing polyanionic cathodes are based on vanadium, and their range should be further expanded with other transition metals such as Mn and Fe. In addition, some of the reported materials only exhibit the one-electron transfer mechanism, which represents half of their theoretical capacities. The second platform of these composites is possibly over 5 V, which is out of the range of conventional organic electrolytes. A strategy involving combination with a solid electrolyte is worth the attempt.
Organic compounds are abundant and environmentally friendly with versatile chemical structures, and they are considered as promising cathode materials for PIBs as well. The functional groups that account for electrochemically active oxidation/reduction play key roles and have been subjected to careful characterization. Various carbonyls possess K-hosting capabilities based on different energy distributions. Large theoretical capacities are the main advances that have occurred for organic compounds, which is a key parameter for the improvement of energy density for PIBs. Future work should firstly address the problem of dissolution in conventional organic electrolytes, which will largely ameliorate the poor capacity retention. The operating voltages of organic cathodes for PIBs are also relatively low. The participation of transition metal elements can be considered as an effective way to increase both the electrostatic repulsion and van der Waals interaction forces. Also, the initial cycle coulombic efficiencies should be considered simultaneously, and the K-containing organic compounds should be given priority for further investigations.
In Table 1, we have summarized the achieved specific capacities, mid-working voltages, initial cycle coulombic efficiencies, and cycling stabilities of recently reported polyanionic composites and organic compounds for advanced PIBs. Further investigations should focus on addressing their existing specific limitations. With the obvious merits that we have mentioned above, we expect that the polyanionic composites and organic compounds are better choices for PIB cathodes than existing alternatives. Nevertheless, their manufacturing costs and need for a dry-room and controlled atmosphere should be further taken into consideration. The all-solid-state PIBs can be regarded as a promising way to solve the safety issues. More electrochemically active redox elements in polyanionic composites and more balanced chemical structures in organic compounds are key developing fields. We believe that studies targeted at the full cell configurations will also be taken seriously towards the real application of PIBs in the near future.
| Material | Electrolyte | Mid-working platform | Practical capacity [mA h g−1] | Initial coulombic efficiency | Capacity retention | Ref. |
|---|---|---|---|---|---|---|
| Amorphous FePO4 | 1 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC EMC |
2.3 V | 135 (1.5–3.5 V) | — | 70% (50 cycles) | 69 |
| Ca0.5Ti2(PO4)3@C | 1M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
0.7 V | 270 (0.01–3.0 V, 50 mA g−1) | 42% | 74.6% (1000 cycles, 1A g−1) | 22 |
| K3V2(PO4)3 | 1 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC PC |
3.7 V | 80 (2.0–4.0 V, 25 mA g−1) | 89% | 89% (500 cycles, 25 mA g−1) | 74 |
| K3V2(PO4)3/C nanocomposites | 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
3.6 V | 50 (2.5–4.3 V, 20 mA g−1) | 71% | 96.3% (100 cycles, 20 mA g−1) | 72 |
| KVP2O7 | 0.5 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
4.3 V | 55 (2.0–5.0 V, 25 mA g−1) | 92% | 85% (100 cycles, 25 mA g−1) | 86 |
| KVOPO4 | 1 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC PC |
4.25 V | 80 (2.0–5.0 V) | 65% | 99% (7 cycles) | 78 |
| KVPO4F | 1 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC PC |
4.3 V | 90 (2.0–5.0 V) | 68% | 89.9% (30 cycles) | 78 |
| KVPO4.36F0.64 | 0.7 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
4.3 V | 100 (3.0–5.0 V, 5 mA g−1) | — | 91.7% (10 cycles, 10 mA g−1) | 91 |
| K3V2(PO4)2F3 | 1 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC PC |
3.7 V | 100 (2.0–4.5 V, 10 mA g−1) | — | 95% (180 cycles, 90 mA g−1) | 93 |
| KTi2(PO4)3 | 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
1.7 V | 80 (1.2–2.8 V, 64 mA g−1) | — | 96% (100 cycles, 64 mA g−1) | 75 |
| o-KFeSO4F | 5.5 mol kg−1 KFSA/diglyme | 3.6 V | 105 (2.0–4.5 V, 12.8 mA g−1) | 81% | 86% (100 cycles, 12.8 mA g−1) | 16 |
| K2[(VO)2(HPO4)2(C2O4)] | 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
3.6 V | 82 (2.0–4.5 V, 5.5 mA g−1) | 80% | 83% (200 cycles, 22 mA g−1) | 82 |
| VOPO4·2H2O | 0.6 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
0.5 V | 90 (0.01–1.5 V, 5.5 mA g−1) | 99% | 32% (50 cycles, 20 mA g−1) | 77 |
| K2FeP2O7 | 1 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC PC |
2.5 V | 60 (2.0–4.8 V, 4.35 mA g−1) | 65% | — | 16 |
| Cobalt(II) terephthalate CoC8H4O4 | 1 M KFSI in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC |
0.3 V | 250 (0.005–2.0 V, 60 mA g−1) | 40% | 56% (200 cycles, 60 mA g−1) | 123 |
| Oxocarbon K4C6O6 | 1.25 M KPF6 in DME | 2.4 V | 75 (1.5–3.2 V, 70 mA g−1) | — | — | 118 |
| Oxocarbon K2C6O6 | 1.25 M KPF6 in DME | 1.3 V | 60 (1.0–1.7 V, 25 mA g−1) | 93% | — | 118 |
| 3,4,9,10-Perylene-tetracarboxylicacid-dianhydride (PTCDA) | 0.5 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
2.4 V | 130 (1.5–3.5 V, 25 mA g−1) | 98% | 77% (200 cycles, 10 mA g−1) | 102 |
| Acyclic compound 2,20-azobis(2-methylpropionitrile) (AIBN) | — | 0.8 V | 100 (0.01–2.5 V, 10 mA g−1) | — | 55% (500 cycles, 10 mA g−1) | 111 |
| Anthraquinone-1,5-disulfonic acid sodium (C14H6Na2O8S2, AQDS) | 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
1.8 V | 80 (1.5–3.0 V, 13 mA g−1) | 75% | 98% (100 cycles, 13 mA g−1) | 119 |
| para-disodium-2,5-dihydroxy-1,4-benzoquinone (p-Na2C6H2O6) | 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
1.7 V | 175 (1.0–3.0 V, 25 mA g−1) | 85% | 45% (50 cycles, 24.8 mA g−1) | 124 |
| COF-10@CNTs | 0.8 M KFSI in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
0.4 V | 300 (0.01–3.0 V, 100 mA g−1) | 84% | 96% (500 cycles, 100 mA g−1) | 117 |
| Annealed PTCDA@450 °C | 3 M KFSI in DME | 2.1 V | 120 (1.5–3.5 V, 100 mA g−1) | 79% | 86.7% (1000 cycles, 1 A g−1) | 104 |
| 2-Methyl-1,4-naphthoquinone (vitamin K) | 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
0.8 V | 300 (0.1–2.5 V, 100 mA g−1) | 74% | 84.9% (100 cycles, 100 mA g−1) | 109 |
| Poly(pentacenetetrone sulfide) (PPTS) | 1 M KPF6 in DME | 1.4 V | 250 (0.8–3.2 V, 100 mA g−1) | 97% | 73.7% (3000 cycles, 5 A g−1) | 107 |
| Azobenzene-4,4′-dicarboxylic acid potassium salts (ADAPTS) | 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
1.3 V | 120 (0.5–3.0 V, 15.5 mA g−1) | 42% | 81.4% (100 cycles, 15.5 mA g−1) | 121 |
| Carbonyl-based polyquinoneimide (PQI) | 0.8 M KPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC |
2.3 V | 200 (1.5–3.5 V, 10 mA g−1) | 87% | 60.2% (1000 cycles, 1 A g−1) | 108 |
| Poly(anthraquinonyl sulfide) (PAQS) | 0.5 M KTFSI in DOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DME DME |
1.8 V | 200 (1.2–3.4 V, 20 mA g−1) | 86% | 75% (50 cycles, 200 mA g−1) | 23 |
| Potassium perylene-3,4,9,10-tetracarboxylate (K4PTC) | 1 M KFSI in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC |
0.8 V | 140 (0.1–2.0 V, 20 mA g−1) | 33% | 74% (500 cycles, 50 mA g−1) | 120 |
| Anthraquinone-1,5-disulfonic acid sodium salt | 0.8 M KFSI in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC |
2.0 V | 70 (1.4–3.0 V, 10 mA g−1) | 72% | 80% (1000 cycles, 30 mA g−1) | 114 |
| Potassium naphthalene-2,6-dicarboxylate (K2NDC) | 1 M KFSI in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC |
0.5 V | 120 (0.1–2.0 V, 20 mA g−1) | 47% | 85% (200 cycles, 50 mA g−1) | 122 |
| Tetrahydroxycyclohexa-2,5-diene-1,4-dione (OHTAP) | 1 M KFSI in DOL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DME DME |
1.7 V | 220 (1.1–2.8 V, 20 mA g−1) | 43% | 50% (500 cycles, 200 mA g−1) | 106 |
| Terephthalic acid (H2TP) | 1 M KFSI in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC |
0.4 V | 290 (0.1–2.0 V, 20 mA g−1) | 32% | 90.7% (150 cycles, 50 mA g−1) | 113 |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by The Science and Technology Development Fund, Macau SAR (Grant No. 0057/2019/A1 and 0092/2019/A2).References
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J.-M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS PubMed.
- J. M. Tarascon, Nat. Chem., 2010, 2, 510 CrossRef CAS PubMed.
- Z. P. Cano, D. Banham, S. Ye, A. Hintennach, J. Lu, M. Fowler and Z. Chen, Nat. Energy, 2018, 3, 279–289 CrossRef.
- J. Y. Hwang, S. T. Myung and Y. K. Sun, Chem. Soc. Rev., 2017, 46, 3529–3614 RSC.
- M. Chen, Q. Liu, S.-W. Wang, E. Wang, X. Guo and S.-L. Chou, Adv. Energy Mater., 2019, 9, 1803609 CrossRef.
- M. Chen, E. Wang, Q. Liu, X. Guo, W. Chen, S.-L. Chou and S.-X. Dou, Energy Storage Mater., 2019, 19, 163–178 CrossRef.
- L. Li, Y. Zheng, S. Zhang, J. Yang, Z. Shao and Z. Guo, Energy Environ. Sci., 2018, 11, 2310–2340 RSC.
- Y. Li, Y. Lu, C. Zhao, Y.-S. Hu, M.-M. Titirici, H. Li, X. Huang and L. Chen, Energy Storage Mater., 2017, 7, 130–151 CrossRef.
- Y. Cai, G. Fang, J. Zhou, S. Liu, Z. Luo, A. Pan, G. Cao and S. Liang, Nano Res., 2018, 11, 449–463 CrossRef CAS.
- Q. Zhang, Z. Wang, S. Zhang, T. Zhou, J. Mao and Z. Guo, Electrochem. Energy Rev., 2018, 1, 625–658 CrossRef CAS.
- Y.-H. Zhu, X. Yang, T. Sun, S. Wang, Y.-L. Zhao, J.-M. Yan and X.-B. Zhang, Electrochem. Energy Rev., 2018, 1, 548–566 CrossRef CAS.
- H. Kim, H. Ji, J. Wang and G. Ceder, Trends in Chemistry, 2019, 1, 682–692 CrossRef.
- Y.-S. Xu, S.-Y. Duan, Y.-G. Sun, D.-S. Bin, X.-S. Tao, D. Zhang, Y. Liu, A.-M. Cao and L.-J. Wan, J. Mater. Chem. A, 2019, 7, 4334–4352 RSC.
- T. Hosaka, T. Shimamura, K. Kubota and S. Komaba, Chem. Rec., 2019, 19, 735–745 CrossRef CAS PubMed.
- J. C. Pramudita, D. Sehrawat, D. Goonetilleke and N. Sharma, Adv. Energy Mater., 2017, 7, 1602911 CrossRef.
- H. Kim, D. H. Seo, J. C. Kim, S. H. Bo, L. Liu, T. Shi and G. Ceder, Adv. Mater., 2017, 29, 1702480 CrossRef PubMed.
- N. Naveen, W. B. Park, S. C. Han, S. P. Singh, Y. H. Jung, D. Ahn, K.-S. Sohn and M. Pyo, Chem. Mater., 2018, 30, 2049–2057 CrossRef CAS.
- Y.-H. Zhu, X. Yang, D. Bao, X.-F. Bie, T. Sun, S. Wang, Y.-S. Jiang, X.-B. Zhang, J.-M. Yan and Q. Jiang, Joule, 2018, 2, 736–746 CrossRef CAS.
- B. Wang, Y. Han, X. Wang, N. Bahlawane, H. Pan, M. Yan and Y. Jiang, iScience, 2018, 3, 110–133 CrossRef CAS PubMed.
- Z. Zhang, M. Li, Y. Gao, Z. Wei, M. Zhang, C. Wang, Y. Zeng, B. Zou, G. Chen and F. Du, Adv. Funct. Mater., 2018, 28, 1802684 CrossRef.
- Z. Jian, Y. Liang, I. A. Rodríguez-Pérez, Y. Yao and X. Ji, Electrochem. Commun., 2016, 71, 5–8 CrossRef CAS.
- T. Masese, K. Yoshii, M. Kato, K. Kubota, Z. D. Huang, H. Senoh and M. Shikano, Chem. Commun., 2019, 55, 985–988 RSC.
- H. Zhang, K. Xi, K. Jiang, X. Zhang, Z. Liu, S. Guo and H. Zhou, Chem. Commun., 2019, 55, 7910–7913 RSC.
- X. Wu, D. P. Leonard and X. Ji, Chem. Mater., 2017, 29, 5031–5042 CrossRef CAS.
- X. Jiang, T. Zhang, L. Yang, G. Li and J. Y. Lee, ChemElectroChem, 2017, 4, 2237–2242 CrossRef CAS.
- X. Zou, P. Xiong, J. Zhao, J. Hu, Z. Liu and Y. Xu, Phys. Chem. Chem. Phys., 2017, 19, 26495–26506 RSC.
- W. Zhang, Y. Liu and Z. Guo, Sci. Adv., 2019, 5, eaav7412 CrossRef CAS PubMed.
- C. Delmas, C. Fouassier and P. Hagenmuller, Physica B+C, 1980, 99, 81–85 CrossRef CAS.
- C. Vaalma, G. A. Giffin, D. Buchholz and S. Passerini, J. Electrochem. Soc., 2016, 163, A1295–A1299 CrossRef CAS.
- H. Kim, D.-H. Seo, A. Urban, J. Lee, D.-H. Kwon, S.-H. Bo, T. Shi, J. K. Papp, B. D. McCloskey and G. Ceder, Chem. Mater., 2018, 30, 6532–6539 CrossRef CAS.
- N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada and S. Komaba, Nat. Mater., 2012, 11, 512–517 CrossRef CAS PubMed.
- X. Wang, X. Xu, C. Niu, J. Meng, M. Huang, X. Liu, Z. Liu and L. Mai, Nano Lett., 2017, 17, 544–550 CrossRef CAS PubMed.
- K. Hurlbutt, S. Wheeler, I. Capone and M. Pasta, Joule, 2018, 2, 1950–1960 CrossRef CAS.
- C. Zhang, Y. Xu, M. Zhou, L. Liang, H. Dong, M. Wu, Y. Yang and Y. Lei, Adv. Funct. Mater., 2017, 27, 1604307 CrossRef.
- Z. Shadike, D.-R. Shi, T.-W. Tian-Wang, M.-H. Cao, S.-F. Yang, J. Chen and Z.-W. Fu, J. Mater. Chem. A, 2017, 5, 6393–6398 RSC.
- S. Chong, Y. Chen, Y. Zheng, Q. Tan, C. Shu, Y. Liu and Z. Guo, J. Mater. Chem. A, 2017, 5, 22465–22471 RSC.
- G. He and L. F. Nazar, ACS Energy Lett., 2017, 2, 1122–1127 CrossRef CAS.
- T. A. Y. Mizutani, K. Ikeda, E. Ihara, M. Asano, T. Harada, M. Inaba and Z. Ogumi, Carbon, 1997, 35, 61–65 CrossRef.
- Z. Jian, W. Luo and X. Ji, J. Am. Chem. Soc., 2015, 137, 11566–11569 CrossRef CAS PubMed.
- C. Shen, K. Yuan, T. Tian, M. Bai, J. G. Wang, X. Li, K. Xie, Q. G. Fu and B. Wei, ACS Appl. Mater. Interfaces, 2019, 11, 5015–5021 CrossRef CAS PubMed.
- W. Luo, J. Wan, B. Ozdemir, W. Bao, Y. Chen, J. Dai, H. Lin, Y. Xu, F. Gu, V. Barone and L. Hu, Nano Lett., 2015, 15, 7671–7677 CrossRef CAS PubMed.
- J. Ding, H. Zhang, H. Zhou, J. Feng, X. Zheng, C. Zhong, E. Paek, W. Hu and D. Mitlin, Adv. Mater., 2019, 31, 1900429 CrossRef PubMed.
- H. He, D. Huang, Y. Tang, Q. Wang, X. Ji, H. Wang and Z. Guo, Nano Energy, 2019, 57, 728–736 CrossRef CAS.
- K. Share, A. P. Cohn, R. Carter, B. Rogers and C. L. Pint, ACS Nano, 2016, 10, 9738–9744 CrossRef CAS PubMed.
- Y. Li, Y. Lu, P. Adelhelm, M. M. Titirici and Y. S. Hu, Chem. Soc. Rev., 2019, 48, 4655–4687 RSC.
- W. Cao, E. Zhang, J. Wang, Z. Liu, J. Ge, X. Yu, H. Yang and B. Lu, Electrochim. Acta, 2019, 293, 364–370 CrossRef CAS.
- L. Zhang, S. Peng, Y. Ding, X. Guo, Y. Qian, H. Celio, G. He and G. Yu, Energy Environ. Sci., 2019, 12, 1989–1998 RSC.
- W. Luo, Z. Jian, Z. Xing, W. Wang, C. Bommier, M. M. Lerner and X. Ji, ACS Cent. Sci., 2015, 1, 516–522 CrossRef CAS PubMed.
- Z. Luo, S. Liu, Y. Cai, S. Li, A. Pan and S. Liang, Sci. Bull., 2018, 63, 126–132 CrossRef CAS.
- D. Saurel, B. Orayech, B. Xiao, D. Carriazo, X. Li and T. Rojo, Adv. Energy Mater., 2018, 8, 1703268 CrossRef.
- Z. Ju, S. Zhang, Z. Xing, Q. Zhuang, Y. Qiang and Y. Qian, ACS Appl. Mater. Interfaces, 2016, 8, 20682–20690 CrossRef CAS PubMed.
- R. A. Adams, J. M. Syu, Y. Zhao, C. T. Lo, A. Varma and V. G. Pol, ACS Appl. Mater. Interfaces, 2017, 9, 17872–17881 CrossRef CAS PubMed.
- G. Ma, K. Huang, J.-S. Ma, Z. Ju, Z. Xing and Q.-c. Zhuang, J. Mater. Chem. A, 2017, 5, 7854–7861 RSC.
- J. Han, M. Xu, Y. Niu, G.-N. Li, M. Wang, Y. Zhang, M. Jia and C.-M. Li, Chem. Commun., 2016, 52, 11274–11276 RSC.
- I. Sultana, M. M. Rahman, S. Mateti, V. G. Ahmadabadi, A. M. Glushenkov and Y. Chen, Nanoscale, 2017, 9, 3646–3654 RSC.
- X. Ren, Q. Zhao, W. D. McCulloch and Y. Wu, Nano Res., 2017, 10, 1313–1321 CrossRef CAS.
- R. Zhang, J. Bao, Y. Wang and C. F. Sun, Chem. Sci., 2018, 9, 6193–6198 RSC.
- W. Zhang, J. Mao, S. Li, Z. Chen and Z. Guo, J. Am. Chem. Soc., 2017, 139, 3316–3319 CrossRef CAS PubMed.
- K. Lei, C. Wang, L. Liu, Y. Luo, C. Mu, F. Li and J. Chen, Angew. Chem., Int. Ed., 2018, 57, 4687–4691 CrossRef CAS PubMed.
- K. Huang, Z. Xing, L. Wang, X. Wu, W. Zhao, X. Qi, H. Wang and Z. Ju, J. Mater. Chem. A, 2018, 6, 434–442 RSC.
- Z. Chen, D. Yin and M. Zhang, Small, 2018, 14, 1703818 CrossRef PubMed.
- Q. Zhang, J. Mao, W. K. Pang, T. Zheng, V. Sencadas, Y. Chen, Y. Liu and Z. Guo, Adv. Energy Mater., 2018, 8, 1703288 CrossRef.
- M. Chen, D. Cortie, Z. Hu, H. Jin, S. Wang, Q. Gu, W. Hua, E. Wang, W. Lai, L. Chen, S.-L. Chou, X.-L. Wang and S.-X. Dou, Adv. Energy Mater., 2018, 8, 1800944 CrossRef.
- M. Chen, L. Chen, Z. Hu, Q. Liu, B. Zhang, Y. Hu, Q. Gu, J.-L. Wang, L.-Z. Wang, X. Guo, S.-L. Chou and S.-X. Dou, Adv. Mater., 2017, 29, 1605535 CrossRef PubMed.
- M. Chen, W. Hua, J. Xiao, D. Cortie, W. Chen, E. Wang, Z. Hu, Q. Gu, X. Wang, S. Indris, S. L. Chou and S. X. Dou, Nat. Commun., 2019, 10, 1480 CrossRef PubMed.
- G. Yan, S. Mariyappan, G. Rousse, Q. Jacquet, M. Deschamps, R. David, B. Mirvaux, J. W. Freeland and J. M. Tarascon, Nat. Commun., 2019, 10, 585 CrossRef CAS PubMed.
- V. Mathew, S. Kim, J. Kang, J. Gim, J. Song, J. P. Baboo, W. Park, D. Ahn, J. Han, L. Gu, Y. Wang, Y.-S. Hu, Y.-K. Sun and J. Kim, NPG Asia Mater., 2014, 6, e138 CrossRef CAS.
- E. Wang, W. Xiang, R. Rajagopalan, Z. Wu, J. Yang, M. Chen, B. Zhong, S. X. Dou, S. Chou, X. Guo and Y.-M. Kang, J. Mater. Chem. A, 2017, 5, 9833–9841 RSC.
- E. Wang, M. Chen, X. Liu, Y. Liu, H. Guo, Z. Wu, W. Xiang, B. Zhong, X. Guo, S. Chou and S.-X. Dou, Small Methods, 2019, 3, 1800169 CrossRef CAS.
- J. Han, G. N. Li, F. Liu, M. Wang, Y. Zhang, L. Hu, C. Dai and M. Xu, Chem. Commun., 2017, 53, 1805–1808 RSC.
- X. Wang, C. Niu, J. Meng, P. Hu, X. Xu, X. Wei, L. Zhou, K. Zhao, W. Luo, M. Yan and L. Mai, Adv. Energy Mater., 2015, 5, 1500716 CrossRef.
- L. Zhang, B. Zhang, C. Wang, Y. Dou, Q. Zhang, Y. Liu, H. Gao, M. Al-Mamun, W. K. Pang, Z. Guo, S. X. Dou and H. K. Liu, Nano Energy, 2019, 60, 432–439 CrossRef CAS.
- J. Han, Y. Niu, S. J. Bao, Y. N. Yu, S. Y. Lu and M. Xu, Chem. Commun., 2016, 52, 11661–11664 RSC.
- L. Peng, Y. Zhu, X. Peng, Z. Fang, W. Chu, Y. Wang, Y. Xie, Y. Li, J. J. Cha and G. Yu, Nano Lett., 2017, 17, 6273–6279 CrossRef CAS PubMed.
- J. Hyoung, J. W. Heo, M. S. Chae and S. T. Hong, ChemSusChem, 2019, 12, 1069–1075 CrossRef CAS PubMed.
- K. Chihara, A. Katogi, K. Kubota and S. Komaba, Chem. Commun., 2017, 53, 5208–5211 RSC.
- R. Lian, D. Wang, X. Ming, R. Zhang, Y. Wei, J. Feng, X. Meng and G. Chen, J. Mater. Chem. A, 2018, 6, 16228–16234 RSC.
- J. Ding, Y.-C. Lin, J. Liu, J. Rana, H. Zhang, H. Zhou, I.-H. Chu, K. M. Wiaderek, F. Omenya, N. A. Chernova, K. W. Chapman, L. F. J. Piper, S. P. Ong and M. S. Whittingham, Adv. Energy Mater., 2018, 8, 1800221 CrossRef.
- J. Liao, Q. Hu, B. Che, X. Ding, F. Chen and C. Chen, J. Mater. Chem. A, 2019, 7, 15244–15251 RSC.
- J. Liao, Q. Hu, J. Mu, X. He, S. Wang and C. Chen, Chem. Commun., 2019, 55, 659–662 RSC.
- K.-H. Ha, S. H. Woo, D. Mok, N.-S. Choi, Y. Park, S. M. Oh, Y. Kim, J. Kim, J. Lee, L. F. Nazar and K. T. Lee, Adv. Energy Mater., 2013, 3, 770–776 CrossRef CAS.
- V. M. Kovrugin, J.-N. Chotard, F. Fauth, A. Jamali, R. David and C. Masquelier, J. Mater. Chem. A, 2017, 5, 14365–14376 RSC.
- P. Barpanda, G. Liu, C. D. Ling, M. Tamaru, M. Avdeev, S.-C. Chung, Y. Yamada and A. Yamada, Chem. Mater., 2013, 25, 3480–3487 CrossRef CAS.
- W. B. Park, S. C. Han, C. Park, S. U. Hong, U. Han, S. P. Singh, Y. H. Jung, D. Ahn, K.-S. Sohn and M. Pyo, Adv. Energy Mater., 2018, 8, 1703099 CrossRef.
- N. Recham, G. Rousse, M. T. Sougrati, J.-N. Chotard, C. Frayret, S. Mariyappan, B. C. Melot, J.-C. Jumas and J.-M. Tarascon, Chem. Mater., 2012, 24, 4363–4370 CrossRef CAS.
- P. Serras, V. Palomares, J. Alonso, N. Sharma, J. M. López del Amo, P. Kubiak, M. L. Fdez-Gubieda and T. Rojo, Chem. Mater., 2013, 25, 4917–4925 CrossRef CAS.
- J. S. Ko, V. V. T. Doan-Nguyen, H.-S. Kim, X. Petrissans, R. H. DeBlock, C. S. Choi, J. W. Long and B. S. Dunn, J. Mater. Chem., 2017, 5, 18707–18715 RSC.
- Q. Li, Z. Liu, F. Zheng, R. Liu, J. Lee, G. L. Xu, G. Zhong, X. Hou, R. Fu, Z. Chen, K. Amine, J. Mi, S. Wu, C. P. Grey and Y. Yang, Angew. Chem., Int. Ed., 2018, 57, 11918–11923 CrossRef CAS PubMed.
- H. Kim, D.-H. Seo, M. Bianchini, R. J. Clément, H. Kim, J. C. Kim, Y. Tian, T. Shi, W.-S. Yoon and G. Ceder, Adv. Energy Mater., 2018, 8, 1801591 CrossRef.
- S. S. Fedotov, A. S. Samarin, V. A. Nikitina, D. A. Aksyonov, S. A. Sokolov, A. Zhugayevych, K. J. Stevenson, N. R. Khasanova, A. M. Abakumov and E. V. Antipov, J. Mater. Chem. A, 2018, 6, 14420–14430 RSC.
- X. Lin, J. Huang, H. Tan, J. Huang and B. Zhang, Energy Storage Mater., 2019, 16, 97–101 CrossRef.
- S.-P. Guo, J.-C. Li, Q.-T. Xu, Z. Ma and H.-G. Xue, J. Power Sources, 2017, 361, 285–299 CrossRef CAS.
- R. Tripathi, G. R. Gardiner, M. S. Islam and L. F. Nazar, Chem. Mater., 2011, 23, 2278–2284 CrossRef CAS.
- B. C. Melot, G. Rousse, J. N. Chotard, M. C. Kemei, J. Rodríguez-Carvajal and J. M. Tarascon, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 094415 CrossRef.
- L. Lander, G. Rousse, A. M. Abakumov, M. Sougrati, G. van Tendeloo and J.-M. Tarascon, J. Mater. Chem. A, 2015, 3, 19754–19764 RSC.
- D. Cui, D. Tian, S. Chen and L. Yuan, J. Mater. Chem. A, 2016, 4, 9177–9183 RSC.
- H. Banda, D. Damien, K. Nagarajan, M. Hariharan and M. M. Shaijumon, J. Mater. Chem. A, 2015, 3, 10453–10458 RSC.
- S. Wang, L. Wang, Z. Zhu, Z. Hu, Q. Zhao and J. Chen, Angew. Chem., Int. Ed., 2014, 53, 5892–5896 CrossRef CAS PubMed.
- W. Luo, M. Allen, V. Raju and X. Ji, Adv. Energy Mater., 2014, 4, 1400554 CrossRef.
- Y. Chen, W. Luo, M. Carter, L. Zhou, J. Dai, K. Fu, S. Lacey, T. Li, J. Wan, X. Han, Y. Bao and L. Hu, Nano Energy, 2015, 18, 205–211 CrossRef CAS.
- Z. Xing, Z. Jian, W. Luo, Y. Qi, C. Bommier, E. S. Chong, Z. Li, L. Hu and X. Ji, Energy Storage Mater., 2016, 2, 63–68 CrossRef.
- L. Fan, R. Ma, J. Wang, H. Yang and B. Lu, Adv. Mater., 2018, 30, 1805486 CrossRef PubMed.
- H. Gao, L. Xue, S. Xin and J. B. Goodenough, Angew. Chem., Int. Ed., 2018, 57, 5449–5453 CrossRef CAS PubMed.
- A. Slesarenko, I. K. Yakuschenko, V. Ramezankhani, V. Sivasankaran, O. Romanyuk, A. V. Mumyatov, I. Zhidkov, S. Tsarev, E. Z. Kurmaev, A. F. Shestakov, O. V. Yarmolenko, K. J. Stevenson and P. A. Troshin, J. Power Sources, 2019, 435, 226724 CrossRef CAS.
- M. Tang, Y. Wu, Y. Chen, C. Jiang, S. Zhu, S. Zhuo and C. Wang, J. Mater. Chem. A, 2019, 7, 486–492 RSC.
- B. Tian, J. Zheng, C. Zhao, C. Liu, C. Su, W. Tang, X. Li and G.-H. Ning, J. Mater. Chem. A, 2019, 7, 9997–10003 RSC.
- Q. Xue, D. Li, Y. Huang, X. Zhang, Y. Ye, E. Fan, L. Li, F. Wu and R. Chen, J. Mater. Chem. A, 2018, 6, 12559–12564 RSC.
- C. Zhang, Y. Qiao, P. Xiong, W. Ma, P. Bai, X. Wang, Q. Li, J. Zhao, Y. Xu, Y. Chen, J. H. Zeng, F. Wang, Y. Xu and J. X. Jiang, ACS Nano, 2019, 13, 745–754 CrossRef CAS PubMed.
- Y. Zhu, P. Chen, Y. Zhou, W. Nie and Y. Xu, Electrochim. Acta, 2019, 318, 262–271 CrossRef CAS.
- S.-Y. Yang, Y.-J. Chen, G. Zhou and Z.-W. Fu, J. Electrochem. Soc., 2018, 165, A1422–A1429 CrossRef CAS.
- C. Wang, W. Tang, Z. Yao, Y. Chen, J. Pei and C. Fan, Org. Electron., 2018, 62, 536–541 CrossRef CAS.
- B. Li, J. Zhao, Z. Zhang, C. Zhao, P. Sun, P. Bai, J. Yang, Z. Zhou and Y. Xu, Adv. Funct. Mater., 2019, 29, 1807137 Search PubMed.
- W. Lee, A. Permatasari, B. W. Kwon and Y. Kwon, Chem. Eng. J., 2019, 358, 1438–1445 CrossRef CAS.
- H. Fei, Y. Liu, Y. An, X. Xu, G. Zeng, Y. Tian, L. Ci, B. Xi, S. Xiong and J. Feng, J. Power Sources, 2018, 399, 294–298 CrossRef CAS.
- X. Chen, H. Zhang, C. Ci, W. Sun and Y. Wang, ACS Nano, 2019, 13, 3600–3607 CrossRef CAS PubMed.
- Q. Zhao, J. Wang, Y. Lu, Y. Li, G. Liang and J. Chen, Angew. Chem., Int. Ed., 2016, 55, 12528–12532 CrossRef CAS PubMed.
- J. Zhao, J. Yang, P. Sun and Y. Xu, Electrochem. Commun., 2018, 86, 34–37 CrossRef CAS.
- C. Wang, W. Tang, Z. Yao, B. Cao and C. Fan, Chem. Commun., 2019, 55, 1801–1804 RSC.
- Y. Liang, C. Luo, F. Wang, S. Hou, S.-C. Liou, T. Qing, Q. Li, J. Zheng, C. Cui and C. Wang, Adv. Energy Mater., 2019, 9, 1802986 CrossRef.
- C. Li, J. Xue, J. Ma and J. Li, J. Electrochem. Soc., 2018, 166, A5221–A5225 CrossRef.
- C. Fan, M. Zhao, C. Li, C. Wang, B. Cao, X. Chen, Y. Li and J. Li, Electrochim. Acta, 2017, 253, 333–338 CrossRef CAS.
- L. Chen and Y. Zhao, Mater. Lett., 2019, 243, 69–72 CrossRef CAS.
- Y. Tang, J. Deng, W. Li, O. I. Malyi, Y. Zhang, X. Zhou, S. Pan, J. Wei, Y. Cai, Z. Chen and X. Chen, Adv. Mater., 2017, 29, 1701828 CrossRef PubMed.
- Y. Tang, Y. Zhang, O. I. Malyi, N. Bucher, H. Xia, S. Xi, Z. Zhu, Z. Lv, W. Li, J. Wei, M. Srinivasan, A. Borgna, M. Antonietti, Y. Du and X. Chen, Adv. Mater., 2018, 30, 1802200 CrossRef PubMed.
- Z. Zhu, Y. Tang, W. R. Leow, H. Xia, Z. Lv, J. Wei, X. Ge, S. Cao, Y. Zhang, W. Zhang, H. Zhang, S. Xi, Y. Du and X. Chen, Angew. Chem., Int. Ed., 2019, 58, 3521–3526 CrossRef CAS PubMed.
- Y. Zhang, Y. Tang, J. Deng, W. R. Leow, H. Xia, Z. Zhu, Z. Lv, J. Wei, W. Li, C. Persson, O. I. Malyi, M. Antonietti and X. Chen, ACS Mater. Lett., 2019, 1, 519–525 CrossRef CAS.
- L. Fan, R. Ma, Q. Zhang, X. Jia and B. Lu, Angew. Chem., Int. Ed., 2019, 58, 10500–10505 CrossRef CAS PubMed.
- W. Zhang, X. Sun, Y. Tang, H. Xia, Y. Zeng, L. Qiao, Z. Zhu, Z. Lv, Y. Zhang, X. Ge, S. Xi, Z. Wang, Y. Du and X. Chen, J. Am. Chem. Soc., 2019, 141, 14038–14042 CrossRef CAS PubMed.
- J. Liu, T. Yin, B. Tian, B. Zhang, C. Qian, Z. Wang, L. Zhang, P. Liang, Z. Chen, J. Yan, X. Fan, J. Lin, X. Chen, Y. Huang, K. P. Loh and Z. X. Shen, Adv. Energy Mater., 2019, 9, 1900579 CrossRef.
- J. W. Nai and X. W. Lou, Adv. Mater., 2019, 31, 1706825 CrossRef PubMed.
- Y. Tang, Y. Zhang, X. Rui, D. Qi, Y. Luo, W. R. Leow, S. Chen, J. Guo, J. Wei, W. Li, J. Deng, Y. Lai, B. Ma and X. Chen, Adv. Mater., 2016, 28, 1567–1576 CrossRef CAS PubMed.
- J. Wang, L. Fan, Z. Liu, S. Chen, Q. Zhang, L. Wang, H. Yang, X. Yu and B. Lu, ACS Nano, 2019, 13, 3703–3713 CrossRef CAS PubMed.
- Y. Feng, S. Chen, J. Wang and B. Lu, J. Energy Chem., 2020, 43, 129–138 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |




