Efficacious fluorescence turn-on probe for high-contrast imaging of human cells overexpressing quinone reductase activity†
Quinn A.
Best
,
Bijeta
Prasai
,
Alexandra
Rouillere
,
Amanda E.
Johnson
and
Robin L.
McCarley
 *
*
Department of Chemistry, Louisiana State University, Baton Rouge, Louisiana 70803-1804, USA. E-mail: tunnel@LSU.edu
First published on 12th December 2016
Abstract
We report a new turn-on substrate probe whose intense fluorescent reporter signature is selectively provided upon probe activation by the cancer-associated oxidoreductase, hNQO1. The extremely high fluorescence turn-on of the probe was utilized to generate fluorescence microscope images of hNQO1-expressing, tumor-derived colorectal and ovarian cancer cells with unprecedented positive signal-to-negative background ratios (PNRs), a key step toward probe application in guided surgical removal of diseased tissues.
Detection of cancer cells by pro-fluorescent molecules whose emission is turned on by disease-associated enzymes is a promising technology1 that has the potential to aid in the diagnosis and treatment of cancers via fluorescence-guided surgery (FGS),1–4 as well as in the design and assessment of novel anticancer drugs that leverage the presence of the target enzyme.5–7 A characteristic of activatable probes that is key to their successful application is the ability to respond to cells containing the target enzyme, and in a highly selective fashion, allowing such target-positive cells to be readily differentiated from enzyme-lacking “background” cells, as well as those cells possessing different enzyme activity levels. The associated figures of merit for such cellular discrimination are based on a ratio of the measured target (positive) fluorescence signal and background (negative) response, namely the target-to-background contrast (TBC) of diseased/normal tissues, and the positive-to-negative ratio (PNR) for cell cultures containing target enzyme versus those not having elevated levels of the enzyme. PNR determination for an activatable probe in traditional two-dimensional cultures of tumor-derived cells is of great value to studies that attempt to correlate drug efficacy with cellular enzyme activity.5,8 Furthermore, high PNR values should translate to successful differentiation of diseased and healthy tissues in pre-clinical animal studies, which is the case when the target-to-background contrast (TBC) is 3 or greater for a given probe under investigation,9 an exceedingly desirable but challenging outcome.10–12
A recent strategy to increase the signal-to-background values for probe/reporter systems in cancer cell imaging is to not only increase the signal associated with production of reporter from enzyme-activation of probe, but importantly, decrease the background associated with the presence of unactivated probe.9,13–15 Typically, with this approach, a fluorophore has covalently attached to it a substrate that can be cleaved by enzyme action, and presence of the substrate results in a probe having significantly reduced/virtually undetectable absorption and fluorescence versus its reporter version. In such turn-on substrate probes (TOSPs) for cancer cell imaging, fluorescence emission is restricted by either the arrangement of bonds within the fluorophore or electron-/energy-transfer processes between the substrate moiety and the fluorescent reporter. Enzyme action on cancer-cell-associated substrates10,13–20 attached to the reporter results in release/formation of reporter, yielding a fluorescent signal whose intensity is a measure of target enzyme activity.
Despite these valuable efforts, there exists an unmet demand for TOSPs that are selectively and rapidly activated by a variety of specific cancer-related enzymes, so as to yield exceptionally large turn-on values, i.e. large PNRs. A main path of attack to address the challenge of TOSP development is the creation of reporters having large fluorescence quantum yield (Φ) and high molar absorptivity (ε) values, and the ability to undergo covalent attachment of a single enzyme substrate, with the additional requirement such a structural modification yields probes with immeasurably low fluorescence emission, small absorptivity, or both. To date, such strategies have provided TOSPs that yield very large turn-on values (ca. 1400) in the presence of purified β-galactosidase16 and PNRs up to ∼500 for tumor cells overexpressing the cell-protective NAD(P)H:quinone oxidoreductase isozyme 1 (hNQO1).14 This reductase is upregulated 15–1300 fold in the cytosol of numerous human tumor cells,21–23 making it an important biomarker for identifying cancerous tissue. Also, hNQO1 plays a major role in shielding cancer cells from therapies,24 and as a result, probes that allow for high-fidelity measurement of hNQO1 activity and its correlation with efficacy of drug candidates are highly desirable.25
To overcome the shortcomings of modest PNR-yielding probes that target overexpressed hNQO1 activity in cancer cells,19,20,26 we have made a probe whose fluorescence at the energy of its free naphthalimide reporter is efficiently decreased as a result of electron-transfer quenching between hNQO1 substrate and reporter,14 as well as a probe whose corresponding rhodamine-based reporter fluorescence is diminished due to alteration of its bonding by attachment of the hNQO1 substrate.15 The latter approach is preferable—because such probes are not prone to environment-induced increases in their background response—but they require reporters with high Φ and ε that are not significantly affected by the cellular environment. One such fluorophore that fits these criteria is a rhodamine 110 (R110) analogue, hydroxymethylrhodamine green (HMRG),13 which in addition to having outstanding optical properties (Φ = 0.84; ε = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1; pH independent from pH 2 to 7),13 it possesses a hydroxymethyl functionality at the HMRG 2′-position that results in locking of the colorless and non-fluorescent spiro-cyclized rhodamine structure upon amide-linkage of a single enzyme substrate, as opposed to the requisite two substrates in the case of R110.27,28 In addition, our recent report15 points to a superior chemical stability of HMRG in the presence of cellular reductant NADH versus urea-modified, R110-based probes that have lower quantum yields (Φ ∼ 0.5),29–31 supporting successful use of HMRG-based probes to measure peptidase activity.10,11,13,32
000 M−1 cm−1; pH independent from pH 2 to 7),13 it possesses a hydroxymethyl functionality at the HMRG 2′-position that results in locking of the colorless and non-fluorescent spiro-cyclized rhodamine structure upon amide-linkage of a single enzyme substrate, as opposed to the requisite two substrates in the case of R110.27,28 In addition, our recent report15 points to a superior chemical stability of HMRG in the presence of cellular reductant NADH versus urea-modified, R110-based probes that have lower quantum yields (Φ ∼ 0.5),29–31 supporting successful use of HMRG-based probes to measure peptidase activity.10,11,13,32
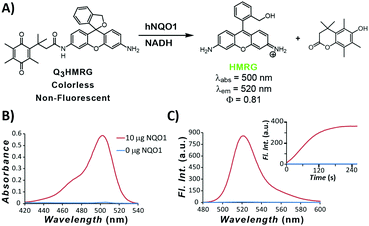
To move toward attaining a high PNR probe for hNQO1 activity, we have used HMRG as the reporter and made it optically unresponsive by coupling to it an hNQO1-selective,15,33 permethylated quinone propionic acid trigger group. As we have shown in previous work, the Q3-responsive moiety is highly selective toward hNQO1 versus other oxidoreductases, including carbonyl reductase, NRH:quinone oxidoreductase 2, and cytochrome P450 reductase.15 The resulting Q3HMRG probe (Fig. 1A) is subsequently locked into its spiro-cyclic form at pH ≥ 6 (pKcyclization ∼ 5; Fig. S1, ESI†), and as a result, has miniscule absorptivity and fluorescence. Sensing of hNQO1 activity by the probe was initially measured by addition of 10 μg purified, recombinant hNQO1 to an aqueous solution containing 5 μM Q3HMRG and 200 μM NADH cofactor, resulting in an almost instantaneous appearance of a bright-green color. Spectroscopic measurement of this solution yielded an absorption spectrum with a λmax = 500 nm and fluorescence response centered at λmax = 520 nm (Fig. 1B and C) that are both characteristic of the HMRG fluorophore. Kinetics of the enzyme-initiated, turn-on process were measured under these conditions; the reaction between probe and enzyme is extraordinarily rapid and near complete within two minutes, as noted by the temporal-dependence of fluorescence intensity at 520 nm, Fig. 1C inset.34 A turn-on ratio of the activated/unreacted probe solutions was found to be 212, the highest measured to date for any hNQO1-specific probe.14,15,19,20
To evaluate the kinetics for HMRG reporter generation upon probe activation by hNQO1, we utilized the fluorescent product formation technique. Solutions containing 1–20 μM Q3HMRG probe were incubated with hNQO1 (0.5 μg, 87 U; one unit (U) will reduce 1 nmol of cytochrome C per min in the presence of menadione substrate at 37 °C), with the reaction initiated by addition of 100 μM NADH. A steady increase in fluorescence was measured, and the initial rate of HMRG reporter formation, V (μmol min−1 mg hNQO1−1), was calculated and then plotted as a function of Q3HMRG concentration, Fig. 2. Apparent kinetic parameters were obtained by fitting the data in Fig. 2 to Michaelis–Menten kinetics,35 namely, the Michaelis constant (Km) = 3.9 ± 1.3 μM, and maximum velocity (Vmax) = 0.517 ± 0.059 μmol min−1 mg hNQO1−1. From these were calculated the catalytic constant (kcat) = 1.6 min−1, and catalytic efficiency (kcat/Km) = 6.5 × 103 M−1 min−1. The Q3HMRG/hNQO1 kinetic characteristics were found to be comparable to recently reported probes (5–34 × 103 M−1 min−1),14,15,19,20 as expected for a probe having the trigger group attached directly to the reporting moiety.33 With 10 μM Q3HMRG and various amounts of hNQO1 (Fig. S2, ESI†), we investigated the linear working range of the probe–enzyme response (30 s incubation) and determined the limit of detection (LOD) and limit of quantification (LOQ) to be 0.058 μg mL−1 (1.0 × 101 U mL−1) and 0.17 μg mL−1 (3.0 × 101 U mL−1).36
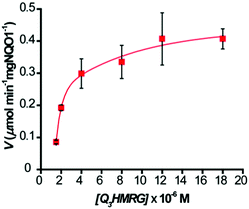 | ||
| Fig. 2 Kinetics of hNQO1 (0.5 μg, 87 U) with Q3HMRG (1.5–20 μM) in pH 7.4, 0.1 M PBS at 25 °C. Values reported are the average (n = 3) ± one standard deviation. Curve is the best fit to the data. | ||
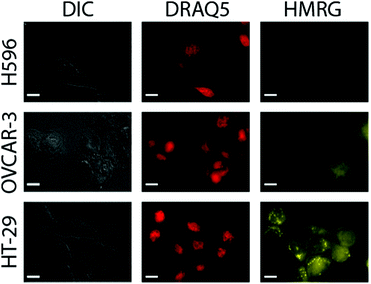
Next, probe response to the presence of hNQO1 within live cells and its sensitivity to different enzyme levels were measured by examining a series of human cell lines possessing varying hNQO1 activity. To do so, we examined a cancer cell line that expresses high levels of hNQO1 (colorectal: HT-29, 488 nmol min−1 mg protein−1, 761 U mL−1; 1-day-old cells) and a cell line with low levels of hNQO1 (ovarian serous adenocarcinoma: OVCAR-3, 7 nmol min−1 mg protein−1, 12 U mL−1; 1-day-old cells); the cell volume was assumed to be 1 pL.37 As a negative control to demonstrate that the Q3HMRG probe is selective for hNQO1 and also stable in the cellular environment, the human lung cell carcinoma line H596 was used, for it is known to have no measurable hNQO1 activity due to its expressing solely an hNQO1 polymorph (proline to serine change at position 187) that makes it incapable of catalysed NADH reduction of quinones.38 It is important to note the OVCAR-3 cell line provides a testbed scenario for the lowest hNQO1 activity reported in a given tumor-derived cell line, while the HT-29 cell line possesses one of the highest recorded hNQO1 activities.22,39 It was anticipated that production of HMRG reporter fluorescence signal within hNQO1-positive cells would scale with increasing hNQO1 activity levels, while little or no fluorescence would be observed in the hNQO1-negative H596 cells. For the hNQO1-positive HT-29 and OVCAR-3 cells, a statistically significant fluorescence signal characteristic of the HMRG reporter (540–580 nm) was found after their dosing with Q3HMRG for times as little as 10 min, Fig. 3. H596 cells (negative control) that lack active hNQO1 have virtually no measurable fluorescence after treatment with Q3HMRG, thereby demonstrating the requirement of hNQO1 activity for probe activation, as well as providing evidence for the intracellular stability of the probe on the experimental timescale. Importantly, HMRG fluorescence is observed throughout the hNQO1-positive cells, including the cytosol and the nucleus, as confirmed by colocalization studies using the nuclear stain DRAQ5. These outcomes are in agreement with hNQO1 having been found mostly in the cytosol,40 but also in the nucleus.41 The additional punctate fluorescence observed in the HT-29 cells is suggested to be due to accumulation of reporter in endosomes/lysosomes, a result of the large amount of reporter produced in a short time in the high activity HT-29 cells;20 a similar observation has been found for HeLa cells, wherein protease-generated HMRG within the cytosol was found to accumulate in the lysosomes.13 As expected from the lower expression level of hNQO1 in OVCAR-3 cells, the fluorescence intensity is markedly lower in OVCAR-3 cells versus that in HT-29 cells. Overall, the results support Q3HMRG responding to the presence of active intracellular hNQO1, as well as providing a reporter signal that is a function of the expression level of the reductase enzyme.42
Quantification of the probe response to hNQO1 in the presence of hNQO1-positive cells vs. hNQO1-negative cells (positive-to-negative ratio, PNR) was determined by measuring the fluorescence intensities of 51 HT-29 and 59 OVCAR-3 cells, and 33 H596 cells in widefield microscopy images, such as those in Fig. 3. From this analysis, a stark contrast between the hNQO1-positive cell lines and the hNQO1-negative cell line was found; exceedingly high PNR values of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 for HT-29/H596 and 2580 for OVCAR-3/H596 were determined (Table S1, ESI†). These PNR values are the highest to date for hNQO1-specific probes—dwarfing the previous record of 510 for a naphthalimide-based probe14—and can be attributed to the extremely low fluorescence background signal associated with Q3HMRG that results from its lack of light absorption and subsequent emission.
300 for HT-29/H596 and 2580 for OVCAR-3/H596 were determined (Table S1, ESI†). These PNR values are the highest to date for hNQO1-specific probes—dwarfing the previous record of 510 for a naphthalimide-based probe14—and can be attributed to the extremely low fluorescence background signal associated with Q3HMRG that results from its lack of light absorption and subsequent emission.
In summary, we have developed a novel fluorescence-based probe capable of detecting the cancer-linked enzyme hNQO1, and it offers differentiation of hNQO1-positive cells from those devoid of active enzyme with unprecedented figures of merit. The operating circumstances that provide the “off-state” of the Q3HMRG probe differ from that of previously reported naphthalimide-based hNQO1 probes,14,19,20 namely, spiro-cyclization versus photoinduced electron-transfer quenching. Furthermore, the reporter has a fluorescence quantum efficiency 3–4 times greater than in previous systems.14,15,19,20 In sum, the overall characteristics of the Q3HMRG probe/HMRG reporter system provide for the highest known turn-on ratio (∼200-fold) upon probe interaction with hNQO1 target. As a result, the probe/reporter system has a significant sensitivity to enzyme activity level in hNQO1-positive cell lines, and its use leads to ready differentiation of cultured hNQO1-positive cancer cells from those that do not have the enzyme, as judged by the extremely large PNR values. These outcomes suggest that Q3HMRG or its variants may be of practical use in medical applications, such as FGS, as well as studies of drugs targeting hNQO1 and its role in cancer metastasis. Our future plans include investigating the relationship between the PNR of hNQO1 probes possessing different excitation/emission energies in cell cultures and the TBC in xenografts/tissues, as to date, we know of no study that rigorously addresses this topic for any target enzyme. Thus, the future is bright for Q3HMRG and its derivatives in a variety of applications, especially in the detection of cancerous and diseased tissue, where it will complement and augment the capabilities of other HMRG-based fluorescent probes, such as those directed at detecting membrane-bound18,32 and intracellular10 peptidases and non-protein thiols.43Q3HMRG stands out in that it responds to the specific intracellular enzyme hNQO1; a large variety of tumor-derived cells in the NCI cell line database possess a high activity level of this important oxidoreductase22,39 that is known to protect cancer cells from chemotherapeutic agents and anoikis.25,44
Notes and references
- A. Razgulin, N. Ma and J. Rao, Chem. Soc. Rev., 2011, 40, 4186–4216 RSC.
- H. J. Handgraaf, F. P. Verbeek, Q. R. Tummers, L. S. Boogerd, C. J. van de Velde, A. L. Vahrmeijer and K. N. Gaarenstroom, Gynecol. Oncol., 2014, 135, 606–613 CrossRef PubMed.
- Q. T. Nguyen and R. Y. Tsien, Nat. Rev. Cancer, 2013, 13, 653–662 CrossRef CAS PubMed.
- M. Garland, J. J. Yim and M. Bogyo, Cell Chem. Biol., 2016, 23, 122–136 CrossRef CAS PubMed.
- A. B. Pardee, Y. Li and C. J. Li, Curr. Cancer Drug Targets, 2002, 2, 227–242 CrossRef CAS PubMed.
- E. I. Parkinson and P. J. Hergenrother, Acc. Chem. Res., 2015, 48, 2715–2723 CrossRef CAS PubMed.
- J. J. Pink, S. M. Planchon, C. Tagliarino, M. E. Varnes, D. Siegel and D. A. Boothman, J. Biol. Chem., 2000, 275, 5416–5424 CrossRef CAS PubMed.
- L. S. Li, E. A. Bey, Y. Dong, J. Meng, B. Patra, J. Yan, X. J. Xie, R. A. Brekken, C. C. Barnett, W. G. Bornmann, J. Gao and D. A. Boothman, Clin. Cancer Res., 2011, 17, 275–285 CrossRef CAS PubMed.
- P. L. Choyke and H. Kobayashi, IEEE J. Sel. Top. Quantum Electron., 2012, 18, 1140–1146 CrossRef CAS.
- T. Fujii, M. Kamiya and Y. Urano, Bioconjugate Chem., 2014, 25, 1838–1846 CrossRef CAS PubMed.
- H. Onoyama, M. Kamiya, Y. Kuriki, T. Komatsu, H. Abe, Y. Tsuji, K. Yagi, Y. Yamagata, S. Aikou, M. Nishida, K. Mori, H. Yamashita, M. Fujishiro, S. Nomura, N. Shimizu, M. Fukayama, K. Koike, Y. Urano and Y. Seto, Sci. Rep., 2016, 6, 26399 CrossRef CAS PubMed.
- G. M. van Dam, G. Themelis, L. M. Crane, N. J. Harlaar, R. G. Pleijhuis, W. Kelder, A. Sarantopoulos, J. S. de Jong, H. J. Arts, A. G. van der Zee, J. Bart, P. S. Low and V. Ntziachristos, Nat. Med., 2011, 17, 1315–1319 CrossRef CAS PubMed.
- M. Sakabe, D. Asanuma, M. Kamiya, R. J. Iwatate, K. Hanaoka, T. Terai, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2013, 135, 409–414 CrossRef CAS PubMed.
- S. U. Hettiarachchi, B. Prasai and R. L. McCarley, J. Am. Chem. Soc., 2014, 136, 7575–7578 CrossRef CAS PubMed.
- Q. A. Best, A. E. Johnson, B. Prasai, A. Rouillere and R. L. McCarley, ACS Chem. Biol., 2016, 11, 231–240 CrossRef CAS PubMed.
- D. Asanuma, M. Sakabe, M. Kamiya, K. Yamamoto, J. Hiratake, M. Ogawa, N. Kosaka, P. L. Choyke, T. Nagano, H. Kobayashi and Y. Urano, Nat. Commun., 2015, 6, 6463 CrossRef CAS PubMed.
- H. Matsuzaki, M. Kamiya, R. J. Iwatate, D. Asanuma, T. Watanabe and Y. Urano, Bioconjugate Chem., 2016, 27, 973–981 CrossRef CAS PubMed.
- Y. Urano, M. Sakabe, N. Kosaka, M. Ogawa, M. Mitsunaga, D. Asanuma, M. Kamiya, M. R. Young, T. Nagano, P. L. Choyke and H. Kobayashi, Sci. Transl. Med., 2011, 3, 110–119 Search PubMed.
- B. Prasai, W. C. Silvers and R. L. McCarley, Anal. Chem., 2015, 87, 6411–6418 CrossRef CAS PubMed.
- W. C. Silvers, B. Prasai, D. H. Burk, M. L. Brown and R. L. McCarley, J. Am. Chem. Soc., 2013, 135, 309–314 CrossRef CAS PubMed.
- X. Cui, L. Li, G. Yan, K. Meng, Z. Lin, Y. Nan, G. Jin and C. Li, BMC Cancer, 2015, 15, 244 CrossRef PubMed.
- S. A. Fitzsimmons, P. Workman, M. Grever, K. Paull, R. Camalier and A. D. Lewis, J. Natl. Cancer Inst., 1996, 88, 259–269 CrossRef CAS PubMed.
- P. J. O'Dwyer, R. P. Perez, K.-S. Yao, A. K. Godwin and T. C. Hamilton, Biochem. Pharmacol., 1996, 52, 21–27 CrossRef.
- G. Pani, E. Giannoni, T. Galeotti and P. Chiarugi, Antioxid. Redox Signaling, 2009, 11, 2791–2806 CrossRef CAS PubMed.
- B. Madajewski, M. A. Boatman, G. Chakrabarti, D. A. Boothman and E. A. Bey, Mol. Cancer Res., 2016, 14, 14–25 CrossRef CAS PubMed.
- S. T. Huang and Y. L. Lin, Org. Lett., 2006, 8, 265–268 CrossRef CAS PubMed.
- S. S. Chandran, K. A. Dickson and R. T. Raines, J. Am. Chem. Soc., 2005, 127, 1652–1653 CrossRef CAS PubMed.
- S. P. Leytus, W. L. Patterson and W. F. Mangel, Biochem. J., 1983, 215, 253–260 CrossRef CAS PubMed.
- L. D. Lavis, T. Y. Chao and R. T. Raines, ACS Chem. Biol., 2006, 1, 252–260 CrossRef CAS PubMed.
- S. X. Cai, H. Z. Zhang, J. Guastella, J. Drewe, W. Yang and E. Weber, Bioorg. Med. Chem. Lett., 2001, 11, 39–42 CrossRef CAS PubMed.
- Z. Q. Wang, J. Liao and Z. Diwu, Bioorg. Med. Chem. Lett., 2005, 15, 2335–2338 CrossRef CAS PubMed.
- Y. Shinden, H. Ueo, T. Tobo, A. Gamachi, M. Utou, H. Komatsu, S. Nambara, T. Saito, M. Ueda, H. Hirata, S. Sakimura, Y. Takano, R. Uchi, J. Kurashige, S. Akiyoshi, T. Iguchi, H. Eguchi, K. Sugimachi, Y. Kubota, Y. Kai, K. Shibuta, Y. Kijima, H. Yoshinaka, S. Natsugoe, M. Mori, Y. Maehara, M. Sakabe, M. Kamiya, J. W. Kakareka, T. J. Pohida, P. L. Choyke, H. Kobayashi, Y. Urano and K. Mimori, Sci. Rep., 2016, 6, 27525 CrossRef CAS PubMed.
- M. F. Mendoza, N. M. Hollabaugh, S. U. Hettiarachchi and R. L. McCarley, Biochemistry, 2012, 51, 8014–8026 CrossRef CAS PubMed.
- Because Q3HMRG has a propensity to form in polar solvent systems an hNQO1-inactive intramolecular spirolactam between the amide bond and one of the quinone carbon sites, solutions of Q3HMRG were used in vitro or in cellulo within 15 minutes of their preparation.
- W. W. Cleland, Methods Enzymol., 1979, 63, 103–138 CAS.
- We thank Z. Shen for performing these experiments.
- S. Kössler, C. Nofziger, M. Jakab, S. Dossena and M. Paulmichl, Toxicology, 2012, 292, 123–135 CrossRef PubMed.
- R. D. Traver, D. Siegel, H. D. Beall, R. M. Phillips, N. W. Gibson, W. A. Franklin and D. Ross, Br. J. Cancer, 1997, 75, 69–75 CrossRef CAS PubMed.
- G. P. Doherty, M. K. Leith, X. Wang, T. J. Curphey and A. Begleiter, Br. J. Cancer, 1998, 77, 1241–1252 CrossRef CAS PubMed.
- L. Ernster, Chem. Scr., 1987, 27A, 1–13 CAS.
- S. L. Winski, Y. Koutalos, D. L. Bentley and D. Ross, Cancer Res., 2002, 62, 1420–1424 CAS.
- We have noted variability in widefield microscope fluorescence signal from hNQO1-positive cells treated with Q3HMRG that we posit are due to differences in hNQO1 activity that are linked to cell cycle and culturing age, as well as variations in amount of spirolactam present, which we minimize by using solutions of Q3HMRG within 15 minutes of their preparation.
- M. Yoshida, M. Kamiya, T. Yamasoba and Y. Urano, Bioorg. Med. Chem. Lett., 2014, 24, 4363–4366 CrossRef CAS PubMed.
- Y. Yang, Y. Zhang, Q. Wu, X. Cui, Z. Lin, S. Liu and L. Chen, J. Exp. Clin. Cancer Res., 2014, 33, 1–9 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; synthesis of Q3HMRG; 1H and 13C NMR spectra; mass spectra; PNR values. See DOI: 10.1039/c6cc08306d |
| This journal is © The Royal Society of Chemistry 2017 |