Open Access Article
Open Access ArticleTuning temperature responsive poly(2-alkyl-2-oxazoline)s by supramolecular host–guest interactions†
Victor R.
de la Rosa
a,
Werner M.
Nau
b and
Richard
Hoogenboom
*a
aSupramolecular Chemistry Group, Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281 S4, 9000 Ghent, Belgium. E-mail: richard.hoogenboom@ugent.be
bDepartment of Life Sciences and Chemistry, Jacobs University Bremen, Campus Ring 1, D 28759 Bremen, Germany
First published on 15th January 2015
Abstract
A poly[(2-ethyl-2-oxazoline)-ran-(2-nonyl-2-oxazoline)] random copolymer was synthesized and its thermoresponsive behavior in aqueous solution modulated by the addition of different supramolecular host molecules. The macrocycles formed inclusion complexes with the nonyl aliphatic side-chains present in the copolymer, increasing its cloud point temperature. The extent of this temperature shift was found to depend on the cavitand concentration and on the strength of the host–guest complexation. The cloud point temperature could be tuned in an unprecedented wide range of 30 K by supramolecular interactions. Since the temperature-induced breakage of the inclusion complexes constitutes the driving force for the copolymer phase transition, the shift in cloud point temperature could be utilized to estimate the association constant of the nonyl side chains with the cavitands.
Introduction
The synergetic combination of polymer and supramolecular chemistry is leading to the development of adaptive smart materials with properties typical of those of natural systems. In virtue of the reversibility of intermolecular forces, besides offering a high level of control on the polymer conformation, supramolecular chemistry also offers the ability to switch the polymer conformation by using stimuli-responsive host–guest systems.1 Since the seminal work of Harada et al.2 cyclodextrins, featuring a hydrophilic outer shell and a hydrophobic cavity, have been extensively utilized as building blocks in supramolecular systems in combination with polymers. A wide variety of architectures in solution has been developed by combination of cyclodextrins and other macrocycles in combination with polymers,3 impacting fields such as drug delivery,4 supramolecular polymers,5 self-healing materials6 or artificial muscles.7Thermoresponsive polymers, with the ability to respond to changes in temperature, are being exploited in a vast number of applications in areas spanning construction,8,9 water management,10 separation sciences,11,12 shape memory materials,13 and biomedicine,14 and allow the development of smart soluble materials or smart fluids inspired by natural systems. Most of such polymeric materials that undergo a solubility phase transition in response to a change in temperature exhibit a lower critical solution temperature (LCST), i.e. the polymer solution phase separates upon heating, and can be used to measure the temperature in solution.15,16 The temperature at which this transition takes place for a specific polymer composition and concentration in solution is regarded as cloud-point temperature (TCP). These solution nanosensors have shown their effectiveness in fields such as cellular research, imaging and microscopy.17–21
The combination of supramolecular hosts with thermoresponsive polymers allows to modulate the hydrophilic–hydrophobic balance of the polymer and thus to finely tune the polymer transition temperature. Many examples have been reported on the complexation of supramolecular cavitands with end-group functionalized thermoresponsive polymers, mostly using poly(N-isopropylacrylamide) (PNIPAm),22 and other poly(N-susbtituted-acrylamide)s.23 These systems are interesting to control the architecture of the polymer ensembles in solution.24–26 However, the change in TCP upon polymer complexation with the cavitand is usually restricted to ca. 5–10 K, which makes these systems rather limited for their use as tunable sensors. The incorporation of hydrophobic host units throughout the polymer backbone multiplies the hydrophilicity gain upon host–guest complex formation thus potentially leading to a broader tunability of the polymer TCP. Recently, a system comprising copolymers based on N,N-dimethylacrylamide and 2-methacrylamido-caprolactam in combination with methylated-β-cyclodextrin was reported, reaching a TCP increment of up to 13 K.27 Another example was recently reported by us based on a system comprising PNIPAm functionalized with electron rich naphthalene side chains. A solution of this polymer was titrated with increasing amounts of the electron deficient cyclobis(paraquat-p-phenylene) macrocycle, producing a stepwise increase in the copolymer TCP. Interestingly, a memory function was observed in this system, arising from the breakage of the host–guest complexes upon the temperature-induced collapse of the polymer chains and the formation of kinetically-trapped polymer-cavitand ensembles.28
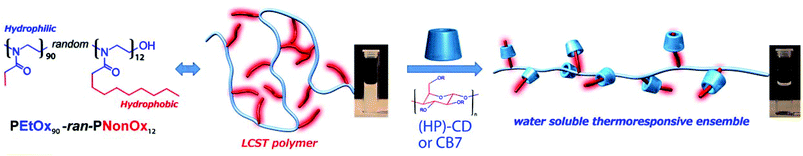
In this context, we have very recently achieved the extension of the thermal memory function to near indefinite time-scales using a water insoluble random copoly(2-oxazoline) based on 2-ethyl-2-oxazoline (EtOx) (hydrophilic) and 2-nonyl-2-oxazoline (NonOx) (hydrophobic) in combination with cyclodextrins.29
Besides the growing number of publications in the field,30 to the best of our knowledge there is no systematic study on the effect of cavitand type and its concentration in solution on the thermoresponsive behavior of water soluble thermoresponsive polymers. In addition, although the modulation of the TCPvia the establishment of multiple supramolecular interactions has been broadened, to date it is still restricted to 10–15 K. Finally, the widespread use of broadly dispersed copolymers synthesized by free-radical polymerization complicates the establishment of structure–property relationships.
This situation triggered us to synthesize a well-defined amphiphilic copolymer by cationic ring opening polymerization of EtOx and NonOx, to obtain insights on the interplay between the polymer and the supramolecular host. This relatively simple PEtOx-ran-PNonOx random copolymer containing 12 mol% nonyl side chains allowed us to study the dynamics of the host–guest complex formation with a variety of cyclodextrins and cucurbit[7]uril as supramolecular host molecules (see Fig. 1). Furthermore, an unprecedentedly broad tunability of the TCP was achieved of up to 30 K by using stoichiometric amounts of the supramolecular cavitands. In addition, we found that the TCP variation was dictated by the strength of the polymer-cavitand host–guest interactions, allowing us to estimate the binding constants of the investigated supramolecular host molecules with the PEtOx-ran-PNonOx nonyl chains.
Results and discussion
A well-defined PEtOx90-ran-PNonOx12 random copolymer with a Mn,SEC of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a dispersity of 1.08 was prepared by living cationic ring-opening polymerization following a recently reported microwave-assisted polymerization protocol.31 This copolymer was utilized to study the effect of the addition of different cavitands on the TCP.
000 g mol−1 and a dispersity of 1.08 was prepared by living cationic ring-opening polymerization following a recently reported microwave-assisted polymerization protocol.31 This copolymer was utilized to study the effect of the addition of different cavitands on the TCP.
Alpha-cyclodextrin (αCD)
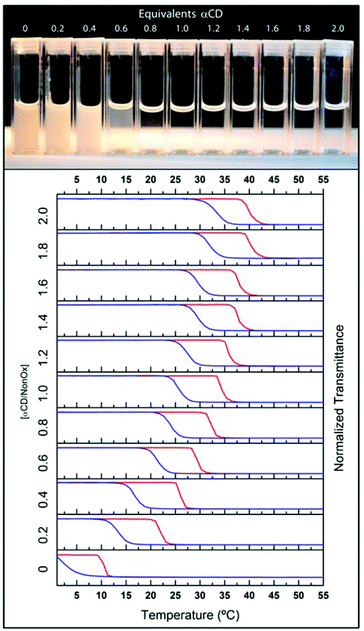
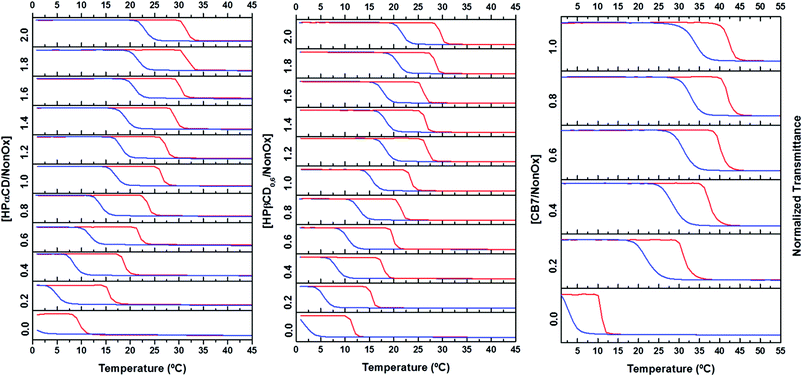
Due to its high water solubility and ability to form host–guest complexes with linear aliphatic chains,32,33 we first studied the self-assembly behavior of native alpha-cyclodextrin (αCD) with the PEtOx90-ran-PNonOx12 copolymer, to determine the influence on the thermoresponsive property of the copolymer.A 5 mg mL−1 solution of the PEtOx90-ran-PNonOx12 random copolymer was prepared in an ice bath, becoming opaque when brought to room temperature. Different aliquots of this solution were titrated with a 120 mg mL−1 solution of αCD. The polymer solution became transparent at room temperature upon addition of 1.0 equivalent of the cyclodextrin host (Fig. 2, top picture). The PEtOx90-ran-PNonOx12 random copolymer exhibited a cloud-point solubility phase transition at ca. 10 °C, passing from a clear transparent solution to a white opaque solution when heated beyond this temperature. This translated into a sharp drop in the % transmittance, from ca. 100% to ca. 0%, as measured by temperature-dependent turbidimetry. As seen in Fig. 2, the addition of increasing amounts of αCD to the copolymer solution resulted in a progressive increase of the TCP.
The TCP increased from 10 °C for the free copolymer to ca. 40 °C in the presence of 2 equivalents of αCD in relation to the nonyl chains. The TCP did not linearly increase with the amount of cyclodextrin added but, instead, it followed a logarithmic trend towards higher temperatures. This is in agreement with the evolution of any observable shift due to a supramolecular association, leading to a plateau value at full complexation.34,35 It thus seems that the fully complexed copolymer-αCD ensemble exhibits a phase transition around 40 °C. When considering the length of a fully stretched nonyl alkylic chain, of ca. 1.1 nm, and the longitudinal size of the cyclodextrin cavity (0.79 nm), a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry is proposed for the association, which will be further on supported by non-linear fitting of the TCP values against the corresponding cyclodextrin/nonyl ratios (vide infra), that yielded a good fitting when applying a 1
1 stoichiometry is proposed for the association, which will be further on supported by non-linear fitting of the TCP values against the corresponding cyclodextrin/nonyl ratios (vide infra), that yielded a good fitting when applying a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry model.
1 binding stoichiometry model.
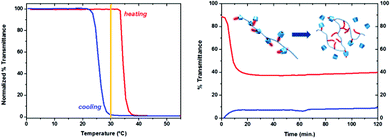
All the turbidimetry measurements exhibited a marked solubility hysteresis of ca. 10 K. This hysteresis between the heating and cooling curves is ascribed to kinetic reasons, due to the relatively fast heating/cooling rate applied (1 K min−1), that does not allow sufficient time for the copolymer-cavitand inclusion complexes to break/re-form and equilibrate. This is also applicable to the free copolymer, whose aggregates require a certain amount of time to re-hydrate from the collapsed globular conformation and to break the hydrophobic association of the nonyl-side chains. To prove this hypothesis, and rule out the possibility of a thermodynamically stable hysteresis, isothermal turbidimetry experiments were performed on the sample containing 1.0 equivalent of αCD. The solution was heated at 1 K min−1 to 30 °C, which is in the middle of the hysteresis window.
Temperature was then maintained constant and the evolution of the solution transmittance was followed during 2 hours. In analogy, the solution was heated to 55 °C, provoking the collapse of the copolymer and the appearance of a white opaque solution, and subsequently cooled at 1 K min−1 to 30 °C, where the transmittance was monitored in time. As seen in Fig. 3, the previously heated solution (blue curve) remained mostly opaque. On the other hand, the previously cooled transparent solution became turbid, quickly loosing 50% transmittance during 20 minutes, as a result of the breakage of the copolymer-αCD inclusion complexes and the consequent collapse of the copolymer chains.
Although the decay in transmittance occurred rapidly, it stabilized at ca. 40% transmittance, indicating partial but not total breakage of the copolymer-αCD inclusion complexes. Nonetheless, these isothermal measurements indicate that the hysteresis observed mostly arises from the experimental conditions, due to the relatively fast heating/cooling rate of 1 K min−1 applied, and partially from thermodynamic reasons, i.e. the need of applying a different temperature to fully break the inclusion complexes (higher) than to re-form them (lower).
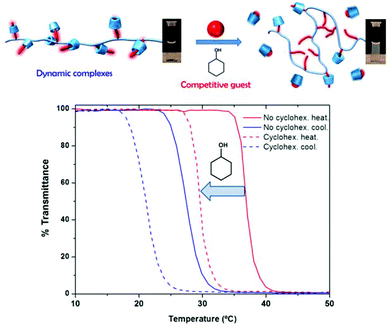
To understand the dynamics of the cyclodextrin-PEtOx-ran-PNonOx host guest complexation, and answer the question of whether the nonyl–cyclodextrin inclusion complexes are kinetically trapped or involved in a constant exchange, a competitive guest experiment was performed. Cyclohexanol was selected as a competitive guest, as it is known that it has a slightly lower affinity for αCD (Ka ≈ 101 M−1) and can effectively compete with the alkyl chains (Ka ≈ 102 M−1).33 A solution of PEtOx90-ran-PNonOx12 was therefore prepared in the presence of 2 equivalents of αCD. The TCP of this solution was ca. 38 °C. Four equivalents of cyclohexananol were then added to this solution as a competitive guest for binding to αCD, lowering the TCP to ca. 32 °C as a result of their complexation with part of the αCD in solution (see Fig. 4).
The low binding constant of cyclohexanol with αCD, thus, only led to a TCP decrease of 7 K, as there was still enough free host available for complexation with the copolymer nonyl chains. The addition of indole, a stronger guest for αCD (Ka ≈ 107 M−1),36 was also attempted and led to the immediate precipitation of the copolymer resulting in a white opaque solution. Both observations thus indicate that the cyclodextrin–nonyl inclusion complexes are dynamic, and a continuous exchange of cyclodextrin in solution takes place.
To rule-out the formation of cyclodextrin–ethyl side chain complexes as well as non-specific interactions between the polymer and the cavitands, a control experiment was envisioned, in which the TCP variation of a PEtOx homopolymer would be measured upon addition of an excess of host. However, PEtOx does not exhibit an LCST behavior below 100 °C under these conditions. Therefore, the control experiment was performed with a 5 mg mL−1 solution of poly(2-npropyl-2-oxazoline)100 (PnPrOx) revealing a TCP of 30 °C. In the presence of 1.0 equivalent of hydroxypropyl-alpha-cyclodextrin (HPαCD) as a host per propyl side chain, the TCP of the solution increased by ca. 2.5 K. This change is very small considering the concentration of host (one HPαCD per propyl side chain), and can be most likely ascribed to changing the solvent polarity. In fact, ten times less host was found to produce a shift of more than 15 K in TCP of the PEtOx90-ran-PNonOx12 copolymer (vide infra). These results, thus, prove that the specific cyclodextrin–nonyl interactions are responsible for the large increase in TCP observed.
To summarize, αCD formed dynamic inclusion complexes with the nonyl side chains borne by the PEtOx90-ran-PNonOx12 copolymer, rendering them hydrophilic and consequently producing a shift of up to 30 K in its TCP. The extent of the temperature shift was correlated with the ratio of αCD to nonyl chains present in solution. Once this good understanding of the host–guest complexation between the PEtOx90-ran-PNonOx12 nonyl side chains and the αCD host molecule was achieved, the attention was focused on evaluating the influence of different hosts on the supramolecular association and its effect on the copolymer thermoresponsive properties.
Effect of the supramolecular host: hydroxypropyl-alpha-cyclodextrin (HPαCD), hydroxypropyl-beta-cyclodextrin (HPβCD) and cucurbit[7]uril (CB7)
In this part of the study, we first investigated the effect of partial substitution of the cyclodextrin hydroxyl groups by hydroxypropyl units on the host–guest complex formation, by using hydroxypropyl-alpha-cyclodextrin (HPαCD). The average degree of hydroxypropyl substitution per glucose unit was of 0.6 (average of 3.6 HP groups per αCD molecule) as determined by 1H-NMR spectroscopy. Subsequently, the effect of an extended cavity size of the host was assessed by employing HPβCD, which features one extra glycopyranose unit, with the same hydroxypropyl degree of substitution. Finally, a different host, cucurbit[7]uril (CB7), a relatively rigid host known to establish strong inclusion complexes with hydrophobic molecules, was tested.37,38 Unlike cyclodextrins, that exhibit a tapered cylinder shape with one opening wider than the other, CB7 is symmetrical with both portals having the same size. CB7 is slightly more voluminous than βCD, being also larger in the longitudinal axis (9.1 Å instead of 7.9 Å for cyclodextrins)39 and exhibits a relatively low solubility in water, similar to that of βCD. In contrast with the slightly positively polarized cyclodextrins, CB7 shows a negative electrostatic potential around the portals, increasing its tendency to bind with cationic guests and enabling the formation of strong charge-dipole interactions with the guest. CB7 has been found to exhibit a remarkably high binding affinity towards a variety of hydrophobic guests, including linear aliphatic chains,40,41 therefore becoming a promising candidate to establish strong host–guest complexes with the PEtOx-ran-PNonOx copolymer nonyl chains.In analogy to the protocol performed earlier for αCD, a 5 mg mL−1 solution of the PEtOx90-ran-PNonOx12 random copolymer was prepared in an ice bath, and different aliquots of this solution were titrated with a stock solution of each cavitand. Temperature-dependent turbidimetry experiments were subsequently performed. As seen in Fig. 5, all the tested supramolecular hosts produced an increase in the solubility phase-transition temperature of the PEtOx90-ran-PNonOx12 copolymer, indicating the formation of host–guest complexes. Several studies have explored the impact of chemical modification on the cyclodextrin guest-binding abilities. In particular, partial substitution of the cyclodextrin hydroxyl groups by hydroxypropyl is proposed to affect complexation in two opposite ways.42–45 First, a negative effect has been ascribed to the steric blockage of the cyclodextrin cavity entrance by hydroxypropyl groups, sterically hindering inclusion complex formation. Secondly, as previously described, chemical modification disrupts the cyclodextrin intramolecular hydrogen-bond network resulting in the extension of the hydrophobic cavity. The larger hydrophobic surface can then lead to increased interactions with the hydrophobic guest, leading to stronger host–guest complexation.46–50
Analysis of the results in Fig. 5 indicates that, even though the hydrophilicity of HPαCD is much higher than that of native αCD, host–guest complexation with the copolymer has a lower impact on its TCP. This is illustrated by the variation on TCP obtained upon addition of 1.0 equivalent of the cavitand, which increased by 30 K with αCD and only by 20 K with HPαCD. The lower impact of HPαCD on the TCP is ascribed to a lower association constant between HPαCD and nonyl chains. This is possibly related to the steric hindrance exerted by the hydroxypropyl groups that partially block the cyclodextrin cavity entrance, sterically hindering inclusion complex formation.42,44,48 Considering the tight fit between alkyl chains and αCD, in combination with the large polymer structure, it is reasonable to assume a relatively large impact of steric effects on the inclusion complex formation.
On the other hand, the negative contribution of the hydroxypropylation due to steric reasons should have a minor effect on the larger cavity of HPβCD. This is demonstrated by the turbidimetry results, showing a displacement of the copolymer TCP comparable to that produced by HPαCD. Therefore, in the case of HPβCD, the positive contribution of the extended hydrophobic cavity seems to constructively affect its binding affinity bringing it close to HPαCD, even though it is known that βCD undergoes rather weak binding with small alkyl chains due to loose host–guest fitting.32
Both modified cyclodextrins seem, therefore, to form weaker host–guest complexes with the PEtOx-ran-PNonOx copolymer nonyl side chains than the native αCD. On the other hand, addition of CB7 to the copolymer solution exerted a remarkably steep displacement of its TCP.
Whereas at least 1.2 equivalents of hydroxypropylated cyclodextrins were necessary to maintain the copolymer in solution at 25 °C, the same result was achieved with less than 0.2 equivalents of CB7. Considering the relatively low hydrophilicity of CB7, these observations indicate that the stability of host guest complexes between the copolymer pendant nonyl chains and CB7 largely surpasses that of those formed with any of the cyclodextrins tested. The symmetry of the CB7 molecule, that features the same size in both cavity entrances, may relate to a higher tendency to thread along the nonyl chains forming pseudorotaxanes. In addition, CB7's higher rigidity in comparison to cyclodextrins is expected to translate into a higher association constant, as has been reported for a variety of hydrophobic guests.37 The titration of the PEtOx-ran-PNonOx solution could not be continued beyond 1.0 equivalent due to the relatively low water solubility of CB7, of ≈5 mM at 25 °C.
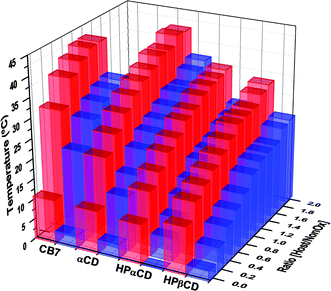
Fig. 6 summarizes the obtained TCP's obtained during the titrations performed with all cavitands, where CB7 and αCD stand out over the hydroxypropylated cyclodextrins with regard to increase of the TCP upon host–guest complexation. Importantly, at high cavitand concentrations, the TCP of the copolymer-cavitand solution was found to be highest for CB7 and αCD that are, especially in the case of CB7, the least hydrophilic supramolecular hosts of the series. This indicates that the temperature-triggered phase transitions of the supramolecular polymer-cavitand assemblies is not driven by the TCP of the ensemble, but rather occurs as a result of host–guest complex breakage. If the TCP of the copolymer-cavitand ensemble would be responsible for the observed phase separation, the observed order of TCP increase should be reversed, being the highest for hydroxypropylated-cyclodextrins, which truly constitute the most hydrophilic molecules of the series.
Isothermal titration calorimetry was explored as a method to determine the thermodynamic parameters associated with the host–guest complexation. However, the low temperatures needed to keep the free copolymer in solution complicated the measurements and led to inconclusive results. Therefore, since the TCP increase was found to be directly correlated to host–guest complex stability, we evaluated the possibility of using the variation of TCP as a measure to calculate the binding constant of the investigated supramolecular host molecules with the PEtOx-ran-PNonOx nonyl chains. TCP, as a property proportional to the concentration of nonyl-cavitand inclusion complexes, seemed to be suitable to perform these calculations.51 It should be noted, however, that the association constant is temperature-dependent, as it is related to the molar Gibbs free energy of the process (ΔG).
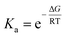 | (1) |
Nevertheless, considering that the temperature range at which the TCP variations occur is restricted to ≈20–30 K, a minor effect of temperature over the association constant values may be assumed. Being aware of the limitations of this method, and the approximations made, the calculated values should be taken for comparative reasons and as an indication of the order of magnitude for the actual binding constants rather than as absolute values.
Once the method constraints have been stated, let us consider the equilibrium between the copolymer nonyl chains and the supramolecular host molecule. For simplicity, the copolymer solution will be modelled as a solution containing individual nonyl chains.
Considering, as has been discussed earlier, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equilibrium between nonyl chain guest (G) and supramolecular host (H) species,
1 equilibrium between nonyl chain guest (G) and supramolecular host (H) species,
| H + G ↔ HG | (2) |
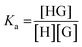 | (3) |
In the equilibrium, the fraction of nonyl chains complexed with a host molecule is defined as p, according to the expression
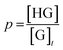 | (4) |
Denoting the TCP property as X, and considering that the observed TCP (Xobs) corresponds to the TCP inherent to the copolymer (uncomplexed nonyl chains) and a contribution from the copolymer-host ensemble (complexed nonyl chains), the observed TCP can be described as
| Xobs = XHG + (XG − XHG) (1 − p) | (5) |
Where XG corresponds to the TCP inherent to the copolymer, p is the fraction of complexed nonyl chains, and XHG is the TCP of the copolymer where all the nonyl chains are complexed.
The expression for p was derived from [HG] similarly as previously reported52,53 (eqn (6)).
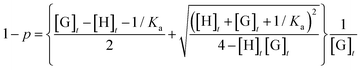 | (6) |
The TCP values found for each turbidimetry titration, containing constant concentration of nonyl guest units, were represented against the corresponding total host concentration. Non-linear curve fitting of the data using eqn (6) allowed to calculate the binding constant (Ka) for each supramolecular host investigated and also estimate the TCP value for complete copolymer complexation (XHG) (see Fig. 7 and Table 1).
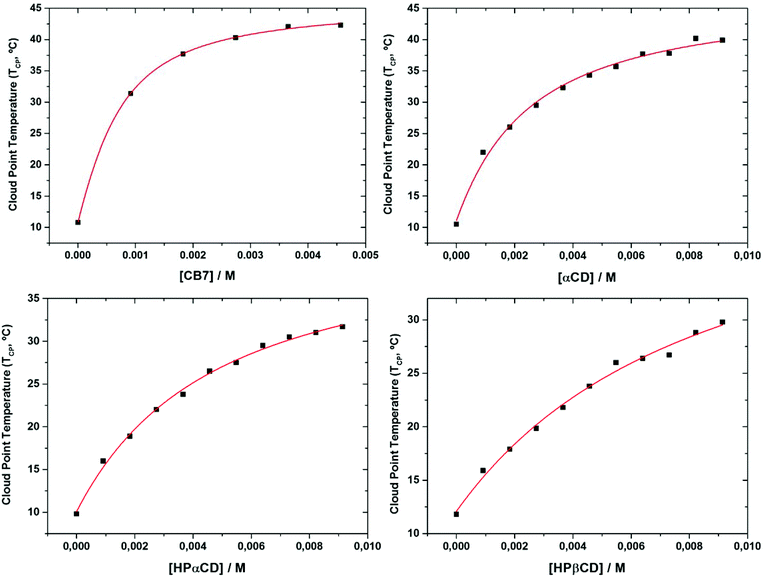 | ||
| Fig. 7 Turbidimetry titration curves corresponding to 5 mg mL−1 solutions of PEtOx90-ran-PNonOx12, and non-linear curve fitting based on eqn (6). The average stability constants between the copolymer nonyl chains and cavitands follow the order: CB7 ≫ αCD > HPαCD > HPβCD. | ||
| ID | Cyclodextrin | Non-linear fittinga | ||
|---|---|---|---|---|
| R 2 | K a [M−1] | T CP (HG) [°C] | ||
| a Calculated using eqn (6). | ||||
| αCD | Alpha-cyclodextrin | 0.992 | 443 ± 60 | 47 ± 1 |
| HPαCD | Hydroxypropyl-alpha-cyclodextrin | 0.996 | 224 ± 25 | 43 ± 1 |
| HPβCD | Hydroxypropyl-beta-cyclodextrin | 0.991 | 120 ± 26 | 46 ± 4 |
| CB7 | Cucurbit[7]uril | 0.999 | 2280 ± 133 | 45.9 ± 0.3 |
The calculated binding constant for αCD-nonyl complexation is Ka ≈ 443 ± 60 M−1, twice the value as found for HPαCD which in turn exhibits a value 90% higher than that of HPβCD. CB7 clearly forms the strongest host–guest complexes, with a Ka ≈ 2280 M−1.
In conclusion, a wide range of supramolecular hosts has been shown to form host–guest complexes with the aliphatic nonyl chains present in a thermoresponsive PEtOx90-ran-PNonOx12 copolymer. These inclusion complexes are dynamic and maintain the copolymer in solution as a random coil. The temperature-induced breakage of the inclusion complexes constitutes the driving force for the copolymer phase transition, which is thus tuned by the stability of the host–guest complexes. Titration of a copolymer solution with the supramolecular host, followed by temperature dependent turbidimetry allowed the estimation of the average binding constant of the nonyl chains with each cavitand. The order in affinity between nonyl chains and the investigated cavitands was found to be: CB7 ≫ αCD > HPαCD > HPβCD. The large association constant of nonyl chains with CB7 is ascribed to its rigidity and cylindrical shape, that is thought to facilitate threading through the alkylic chain resulting in pseudo-rotaxane formation. αCD forms relatively strong host–guest complexes with alkyl chains, due to its tight fit with the nonyl alkyl chains, as has been previously reported. On the other hand, HPαCD binding is possibly penalized by the steric hindrance associated to the introduction of hydroxypropyl groups at the cavity entrances. HPβCD is less affected due to its larger cavity diameter, and advantages from an extended hydrophobic surface, which partially compensates its worse fit with the small nonyl alkyl chains.
Conclusions
A range of supramolecular hosts has been shown to form host–guest complexes with the aliphatic nonyl chains present in amphiphilic PEtOx-ran-PNonOx copolymers. These inclusion complexes are dynamic and allow tuning of the copolymer phase transition temperature over an unprecedentedly large 30 K range. The temperature-induced breakage of the inclusion complexes constitutes the driving force for the copolymer phase transition, which is tuned by the strength of the nonyl-cavitand association. Analysis of the TCP variation upon addition of different cavitands allowed to estimate the binding constant for each supramolecular host, resulting in the following order of binding affinity towards nonyl chains: CB7 ≫ αCD > HPαCD > HPβCD. The least hydrophilic of the cavitands tested (CB7) resulted in the strongest association while partial substitution of the native αCD hydroxyl groups by hydroxypropyl units decreased by a factor of 2 the association constant with nonyl chains. We are currently investigating the effect of polymer chain length on the thermoresponsive behavior of these supramolecular systems.Experimental
Materials
Solvents and reagents were purchased from Sigma Aldrich, and used as received unless otherwise specified. Cucurbit[7]uril was synthesized as reported.54,55 Methyl tosylate (MeOTs) was distilled twice under vacuum prior to use. 2-Ethyl-2-oxazoline (EtOx), 2-nonyl-2-oxazoline (NonOx, Henkel), and 2-npropyl-2-oxazoline (nPrOx, synthesized as previously reported56) were distilled over barium oxide (BaO). Acetonitrile (CH3CN, Acros Organics) was dried over molecular sieves (3 Å). All reagents were stored and handled under a dry argon or nitrogen atmosphere.Deionized (Milli-Q) water was obtained from a Sartorius Arium 611 with a Sartopore 2 150 (0.45 + 0.2 μm pore size) cartridge filter (resistivity ≥18.2 MΩ cm).
Instrumentation
The polymerization was performed in a Biotage initiator sixty microwave synthesizer utilizing capped microwave vials. The vials were heated to 120 °C for 24 hours and cooled down to room temperature under vacuum prior to use. The polymerization was performed with temperature control (IR sensor).1H-NMR spectroscopy was performed in CDCl3 on a Bruker Avance 300 MHz spectrometer. Spectra were processed using TOPSPIN 3.0.
Size exclusion chromatography (SEC) measurements were performed on an Agilent 1260-series equipped with a 1260 ISO-pump, a 1260 Diode Array Detector (DAD), a 1260 Refractive Index Detector (RID), and two Mixed-D columns and a Mixed-D guard column (Agilent) in series inside a 1260 Thermostated Column Compartment (TCC) at 50 °C using dimethylacetamide containing 50 mM of LiCl (flow rate of 0.6 mL min−1) as solvent. Molar mass and dispersity were calculated against poly(methyl methacrylate) standards.
Turbidimetry and dynamic light scattering studies
Turbidimetry measurements were performed in a CARY Bio 100 UV-VIS spectrophotometer equipped with a temperature controller, at a wavelength of 700 nm. Heating/cooling cycles were performed at a rate of 1 K min−1 with stirring. The polymer concentration was kept at 5 mg mL−1 in deionized water. The equivalents of cyclodextrin and cucurbit[7]uril added were calculated in relation to the equivalents of nonyl side chains, or n-propyl side chains, in the case of the control experiment.Poly[(2-ethyl-2-oxazoline)-ran-(2-nonyl-2-oxazoline)] synthesis
The polymerization was performed as previously reported.31 The microwave vial was loaded in a glove box (Vigor Gas Purification Technologies Inc.) with MeOTs (0.0335 g, 1.0 equivalent), and the monomers EtOx (1.606 g, 90 equivalents) and NonOx (0.355 g, 10 equivalents) in the desired molar ratio, maintaining a total monomer concentration of 4 M in acetonitrile (2.491 mL). The polymerization was run for 15 minutes at 140 °C. The polymer was terminated with KOH in methanol, yielding hydroxyl-terminated polymers. The solvent was evaporated under reduced pressure, and the polymer was subsequently precipitated in diethyl ether from dichloromethane. The pure polymer was then dried in a vacuum oven at 50 °C for 24 h.
1H NMR spectroscopic analysis revealed the polymer composition to be PEtOx90-ran-PNonOx12. Size-exclusion chromatography yielded a number-average molecular weight (Mn) of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and a dispersity (Đ) of 1.08.
000 and a dispersity (Đ) of 1.08.
General protocol followed for the copolymer titrations with cavitands
For all the titrations with a cavitand stock solution, PMMA cuvettes for Vis-spectroscopy (Karl-Roth), each equipped with a stirring bar, were filled with 2 mL of copolymer solution. To calculate the amount of cavitand stock solution necessary to add to each cuvette, the following calculations were performed.First, the weight fraction of NonOx (fNonOx) in the copolymer was calculated, according to the following equation:
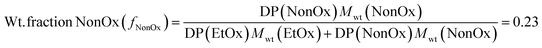 | (7) |
where DP is the degree of polymerization i.e. the number of repeating units of each monomer in the copolymer.
Then, the mass of cavitand necessary to equal the number of NonOx groups contained in 2 mL (cuvette) of 5 mg mL−1 copolymer solution is calculated.
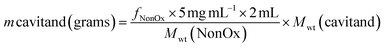 | (8) |
Finally, the volume of cavitand stock solution required to add to each copolymer solution is calculated:
 | (9) |
In the present study, the number of equivalents ranged from 0.2 to 2.0. The aliquots were measured and dispensed with a micropipette.
Since PEtOx90-ran-PNonOx12 is water soluble at low temperatures, a 5 mg mL−1 stock solution was prepared. PMMA cuvettes were filled with 2 mL of the copolymer solution. Subsequently, increasing amounts of a cavitand stock solution were added to each cuvette, obtaining the desired cavitand concentration. Cavitand stock solution concentrations ranged from 120 mg mL−1 for αCD to 150 mg mL−1 for HPαCD and HPβCD. The dilution of the copolymer dilution by addition of cavitand was always kept below 10%.
Due to the relatively low solubility of CB7, a 4 mM solution of the cavitand was prepared (4.83 mg mL−1). To avoid high dilution effects upon titration, the copolymer was directly weighted in the cuvette, and the necessary amounts of water and CB7 stock solution added to obtain a 5 mg mL−1 concentration of copolymer.
Acknowledgements
R. H. and V. R. R. would like to thank Ghent University for financial support through the Concerted Research Actions (project BOF11/GOA/023). The authors also thank the COST Action CM 1005 “Supramolecular Chemistry in Water”.Notes and references
- R. Sakai and T. Kakuchi, Macromol. Symp., 2007, 249–250, 81–85 CrossRef.
- A. Harada, H. Adachi, Y. Kawaguchi and M. Kamachi, Macromolecules, 1997, 30, 5181–5182 CrossRef CAS.
- B. V. K. J. Schmidt, M. Hetzer, H. Ritter and C. Barner-Kowollik, Prog. Polym. Sci., 2014, 39, 235–249 CrossRef CAS PubMed.
- J. Zhou and H. Ritter, Polym. Chem., 2010, 1, 1552–1559 RSC.
- A. Harada, Y. Takashima and H. Yamaguchi, Chem. Soc. Rev., 2009, 38, 875–882 RSC.
- S. Tamesue, Y. Takashima, H. Yamaguchi, S. Shinkai and A. Harada, Angew. Chem., Int. Ed., 2010, 49, 7461–7464 CrossRef CAS PubMed.
- Y. Takashima, S. Hatanaka, M. Otsubo, M. Nakahata, T. Kakuta, A. Hashidzume, H. Yamaguchi and A. Harada, Nat. Commun., 2012, 3, 1270 CrossRef PubMed.
- J. Zhang, G. Pu, M. R. Dubay, Y. Zhao and S. J. Severtson, J. Mater. Chem. C, 2013, 1, 1080–1086 RSC.
- A. C. Rotzetter, C. M. Schumacher, S. B. Bubenhofer, R. N. Grass, L. C. Gerber, M. Zeltner and W. J. Stark, Adv. Mater., 2012, 24, 5352–5356 CrossRef CAS PubMed.
- H. Yang, H. Zhu, M. M. R. M. Hendrix, N. J. H. G. M. Lousberg, G. de With, A. C. C. Esteves and J. H. Xin, Adv. Mater., 2013, 25, 1150–1154 CrossRef CAS PubMed.
- A. Kikuchi and T. Okano, Prog. Polym. Sci., 2002, 27, 1165–1193 CrossRef CAS.
- I. Tan, F. Roohi and M.-M. Titirici, Anal. Methods, 2011, 4, 34–43 RSC.
- T. Defize, R. Riva, J.-M. Raquez, P. Dubois, C. Jérôme and M. Alexandre, Macromol. Rapid Commun., 2011, 32, 1264–1269 CrossRef CAS PubMed.
- M. A. Ward and T. K. Georgiou, Polymers, 2011, 3, 1215–1242 CrossRef CAS PubMed.
- R. Hoogenboom, in Complex Macromolecular Architectures, John Wiley & Sons (Asia) Pte Ltd, 2011, pp. 685–715 Search PubMed.
- X.-d. Wang, O. S. Wolfbeis and R. J. Meier, Chem. Soc. Rev., 2013, 42, 7834–7869 RSC.
- T. Tsuji, S. Yoshida, A. Yoshida and S. Uchiyama, Anal. Chem., 2013, 85, 9815–9823 CrossRef CAS PubMed.
- C.-Y. Chen and C.-T. Chen, Chem. Commun., 2011, 47, 994–996 RSC.
- F. Ye, C. Wu, Y. Jin, Y.-H. Chan, X. Zhang and D. T. Chiu, J. Am. Chem. Soc., 2011, 133, 8146–8149 CrossRef CAS PubMed.
- R. J. Meier, L. H. Fischer, O. S. Wolfbeis and M. Schäferling, Sens. Actuators, B, 2013, 177, 500–506 CrossRef CAS PubMed.
- K. Okabe, N. Inada, C. Gota, Y. Harada, T. Funatsu and S. Uchiyama, Nat. Commun., 2012, 3, 705 CrossRef PubMed.
- H. Ritter, J. Cheng and M. Tabatabai, Beilstein J. Org. Chem., 2012, 8, 1528–1535 CrossRef CAS PubMed.
- B. V. K. J. Schmidt, M. Hetzer, H. Ritter and C. Barner-Kowollik, Macromol. Rapid Commun., 2013, 34, 1306–1311 CrossRef CAS PubMed.
- J. Bigot, B. Charleux, G. Cooke, F. Delattre, D. Fournier, J. Lyskawa, L. Sambe, F. Stoffelbach and P. Woisel, J. Am. Chem. Soc., 2010, 132, 10796–10801 CrossRef CAS PubMed.
- L. n. Sambe, F. o. Stoffelbach, J. Lyskawa, F. o. Delattre, D. Fournier, L. Bouteiller, B. Charleux, G. Cooke and P. Woisel, Macromolecules, 2011, 44, 6532–6538 CrossRef CAS.
- S. Reinelt, D. Steinke and H. Ritter, Beilstein J. Org. Chem., 2014, 10, 680–691 CrossRef PubMed.
- A. Burkhart and H. Ritter, Beilstein J. Org. Chem., 2014, 10, 1951–1958 CrossRef CAS PubMed.
- L. Sambe, V. R. de La Rosa, K. Belal, F. Stoffelbach, J. Lyskawa, F. Delattre, M. Bria, G. Cooke, R. Hoogenboom and P. Woisel, Angew. Chem., Int. Ed., 2014, 53, 5044–5048 CAS.
- V. R. de la Rosa and R. Hoogenboom, Chem. – Eur. J., 2014, 21, 1302–1311 CrossRef PubMed.
- Y.-G. Jia and X. X. Zhu, Langmuir, 2014, 30, 11770–11775 CrossRef CAS PubMed.
- M. W. M. Fijten, J. M. Kranenburg, H. M. L. Thijs, R. M. Paulus, B. M. van Lankvelt, J. de Hullu, M. Springintveld, D. J. G. Thielen, C. A. Tweedie, R. Hoogenboom, K. J. Van Vliet and U. S. Schubert, Macromolecules, 2007, 40, 5879–5886 CrossRef CAS.
- P. Brocos, X. Banquy, N. Díaz-Vergara, S. Pérez-Casas, Á. Piñeiro and M. Costas, J. Phys. Chem. B, 2011, 115, 14381–14396 CrossRef CAS PubMed.
- M. V. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875–1918 CrossRef CAS PubMed.
- C. Dethlefs, J. Eckelmann, H. Kobarg, T. Weyrich, S. Brammer, C. Näther and U. Lüning, Eur. J. Org. Chem., 2011, 2066–2074 CrossRef CAS.
- L. Fielding, S. C. McKellar and A. J. Florence, Magn. Reson. Chem., 2011, 49, 405–412 CrossRef CAS PubMed.
- E. A. Lewis and L. D. Hansen, J. Chem. Soc., Perkin Trans. 2, 1973, 2081–2085 RSC.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844–4870 CrossRef CAS PubMed.
- K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394–418 RSC.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1754 CrossRef CAS PubMed.
- J. Mohanty and W. M. Nau, Angew. Chem., Int. Ed., 2005, 44, 3750–3754 CrossRef CAS PubMed.
- D. M. Bailey, A. Hennig, V. D. Uzunova and W. M. Nau, Chem. – Eur. J., 2008, 14, 6069–6077 CrossRef CAS PubMed.
- C. Yuan, Z. Jin and X. Li, Food Chem., 2008, 106, 50–55 CrossRef CAS PubMed.
- C. Trinadha Rao, J. Pitha, B. Lindberg and J. Lindberg, Carbohydr. Res., 1992, 223, 99–107 CrossRef.
- Á. Buvári-Barcza and L. Barcza, Talanta, 1999, 49, 577–585 CrossRef.
- C. Yong, C. Washington and W. Smith, Pharm. Res., 2008, 25, 1092–1099 CrossRef CAS PubMed.
- S. Concha-Santos, S. Pérez-Casas, P. Brocos and Á. Piñeiro, J. Chem. Thermodyn., 2013, 67, 112–119 CrossRef CAS PubMed.
- C. Schönbeck, P. Westh, J. C. Madsen, K. L. Larsen, L. W. Städe and R. Holm, Langmuir, 2011, 27, 5832–5841 CrossRef PubMed.
- C. Schönbeck, P. Westh, J. C. Madsen, K. L. Larsen, L. W. Städe and R. Holm, Langmuir, 2010, 26, 17949–17957 CrossRef PubMed.
- C. Tang, A. Inomata, Y. Sakai, H. Yokoyama, T. Miyoshi and K. Ito, Macromolecules, 2013, 46, 6898–6907 CrossRef CAS.
- H. Chen and H. Ji, Supramol. Chem., 2014, 1–9 Search PubMed.
- H.-J. Schneider and A. Yatsimirsky, Principles and Methods in Supramolecular Chemistry, John Wiley and Sons Ltd, Chichester, 1999 Search PubMed.
- H. Bakirci, X. Zhang and W. M. Nau, J. Org. Chem., 2004, 70, 39–46 CrossRef PubMed.
- W. M. Nau and X. Zhang, J. Am. Chem. Soc., 1999, 121, 8022–8032 CrossRef CAS.
- J. Kim, I.-S. Jung, S.-Y. Kim, E. Lee, J.-K. Kang, S. Sakamoto, K. Yamaguchi and K. Kim, J. Am. Chem. Soc., 2000, 122, 540–541 CrossRef CAS.
- A. Day, A. P. Arnold, R. J. Blanch and B. Snushall, J. Org. Chem., 2001, 66, 8094–8100 CrossRef CAS PubMed.
- M. M. Bloksma, C. Weber, I. Y. Perevyazko, A. Kuse, A. Baumgärtel, A. Vollrath, R. Hoogenboom and U. S. Schubert, Macromolecules, 2011, 44, 4057–4064 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR spectroscopy and size exclusion chromatography data of PEtOx90-ran-PNonOx12. See DOI: 10.1039/c4ob02654c |
| This journal is © The Royal Society of Chemistry 2015 |