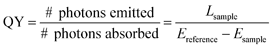Absolute quantum yield measurements of colloidal NaYF4: Er3+, Yb3+ upconverting nanoparticles†
John-Christopher
Boyer‡
and
Frank C. J. M.
van Veggel
*
University of Victoria, Department of Chemistry, P.O. Box 3065, Victoria, British Columbia, Canada V8W 3V6. E-mail: fvv@uvic.ca; Fax: +1 250-472-5193; Tel: +1 250-721-7184
First published on 29th May 2010
Abstract
In this communication we describe a technique for measuring the absolute quantum yields (QYs) of upconverting nanomaterials based on the use of a commercially available fluorimeter and an integrating sphere. Using this setup, we have successfully acquired luminescence efficiency data (pump laser, absorbed pump, and visible emitted intensities) for lanthanide-doped upconverting nanoparticles. QYs in the range of 0.005% to 0.3% were measured for several NaYF4: 2% Er3+, 20% Yb3+ nanoparticles with particle sizes ranging from 10 to 100 nm while a QY of 3% was measured for a bulk sample.
In this communication we describe a technique for the quantitative measurement of the absolute quantum yields (QYs) of several upconverting materials based on the use of a commercially available fluorimeter and an integrating sphere. Upconversion is the generation of higher energy light from lower energy radiation, usually near-infrared (NIR) or infrared (IR), typically through the use of lanthanide ions doped into a solid-state host. These materials have an assorted range of applications such as display phosphors,1–3 telecommunication,4 biolabels,5,6 lasers,7 and security labeling.8,9 Based on this intense interest, it is surprising to find so few publications in the literature dealing with the QYs of these materials where the intensities of the emitted and absorbed pump light are quantified.10–16 To our knowledge there are no reports on the measurement of absolute efficiencies of colloidal upconverting nanoparticles (NPs).
In response to this lack of data, we have successfully designed a setup (Fig. 1 and Fig. S1 of the ESI†) using a commercially available spectrophotometer (Edinburgh Instruments FLS 920), an integrating sphere, and a 980 nm laser diode to acquire luminescence efficiency data (pump laser, absorbed pump, and visible emitted intensities). This method makes use of a relatively basic spectrophotometer and an inexpensive diode laser. Using these data, we have successfully measured QYs in the range of 0.005% to 0.3% for the green emission of several hexagonal-phase NaYF4: 2% Er3+, 20% Yb3+ NPs with particle sizes ranging from 10 to 100 nm. A QY of 3% was measured for bulk samples with the same dopant levels. In order to obtain reliable measurements it was essential to calibrate the responses of the monochromators, detectors and integrating sphere before any other spectroscopic measurements were performed. The final result is a reliable and straightforward method to determining QYs of upconverting materials both in powder and colloid form.
 | ||
| Fig. 1 Diagram of the integrating sphere setup for luminescence measurements. Dashed line—excitation light. Solid line—sample emission. | ||
For the absolute QY measurements, we employed a barium sulfate coated integrating sphere (150 mm in diameter) from Edinburgh instruments. The integrating sphere was mounted on the fluorimeter with the entry and output ports of the sphere located in 90° geometry from each other in the plane of the spectrometer (Fig. S1, ESI†). All the powder or colloidal samples were held in a quartz cuvette located in the center of the integrating sphere. Samples were excited with a JDS uniphase 980 nm laser diode (device type 63-00342) coupled to a 105 µm (core) fibre. The emission from the fibre tip was collimated to a beam diameter of 1 mm and directed on the samples using a Newport F-91-C1-T Multimode Fiber Coupler. Baffles were employed on both sides of the sample holder to ensure that no scattered excitation light or emissions would be collected before scattering off the inside of the sphere. All the spectroscopic data collected were corrected for the spectral response of both the fluorimeter and the integrating sphere. The response of the detection systems in photon flux (sphere, monochromators, detectors) was determined using a calibrated tungsten lamp (Ocean Optics HL-2000-CAL). These normalization curves were then applied to all measured spectra.
The QY is defined as:
 | ||
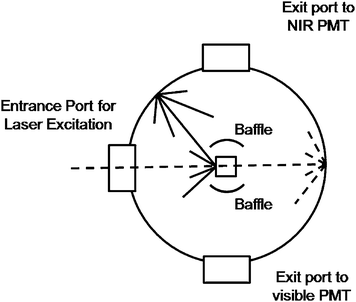
| Fig. 2 Upconversion photoluminescence data for ErYb3 nanoparticles (1 wt% colloidal solution in hexanes) excited with a 980 nm laser diode (power density = 150 W cm−2). Laser diode profile for reference (solid line) and sample (dotted line) is shown on the right. Inset: digital photograph of the ErYb3 sample under the same excitation conditions. | ||
The excitation light not absorbed by the sample and the reference was measured using a liquid-nitrogen cooled Hamamatsu R5509 NIR PMT. The difference in the integrated areas of the sample and reference samples gives the number of absorbed photons. For these measurements the collected signal was measured across the 976 nm excitation wavelength (±80 cm−1). The upconversion photoluminescence was measured using a red-sensitive Peltier-cooled Hamamatsu R955 PMT. The photons emitted were determined by integrating the area under the photoemission spectra. We neglected self-absorption of the emitted radiation due to the small absorption cross-sections of the lanthanide ions which is evident from the photo in Fig. 2. All spectra were recorded using a 1 nm resolution on the emission monochromators and were based on an average at least 10 scans. There is a considerable difference in intensity between the luminescence of the upconverting NPs and the excitation laser line due to its high power density. In order to obtain accurate data it is important to ensure that saturation of the photomultiplier tubes of the fluorimeter does not occur. To avoid saturating the NIR detector a neutral density filter was employed to attenuate the intensity of the scattered radiation.
We investigated several NaYF4: Er3+ 2%, Yb3+ 20% samples with different particle sizes as a demonstration of the validity of this technique. We choose only to examine samples with a hexagonal crystal phase as it is widely known that the hexagonal phase of NaYF4 is one of the best hosts known for the upconversion process.18 A series of micrometre and nanometre particle sized samples were synthesized using literature methods (see ESI† for synthetic details).19–22 These samples were given sample codes which are listed in Table 1. The average particle size of each sample determined by TEM measurements is also given in Table 1.
| Sample # | Average particle size/nm | Power density/W cm−2 | QY (%) |
|---|---|---|---|
| ErYb1 | ≫100 | 20 | 3.0 ± 0.3 |
| ErYb2 (1 wt%) | 100 | 150 | 0.30 ± 0.10 |
| ErYb3 (1 wt%) | 30 | 150 | 0.10 ± 0.05 |
| ErYb4 (1 wt%) | 8–10 | 150 | 0.005 ± 0.005 |
| ErYb5 core shell (1 wt%) | 30 | 150 | 0.30 ± 0.10 |
The method was first confirmed using a micrometre sized NaYF4 sample whose efficiency has been reported previously in the literature. A QY of 4% measured through the use of an integrating sphere and a custom built acquisition system was reported previously for the green emission in a bulk NaYF4: Er3+, Yb3+ sample.13 Both the emission of the sample and the laser profile with our doped and undoped sample were measured employing a power density of 20 W cm−2 (within saturation regime of power dependence curve). A QY of 3.0 ± 0.3% was determined which matches well with literature results thus validating the accuracy of our technique. The advantage of our technique is that it employs a commercially available spectrometer and an integrating sphere. A relatively inexpensive diode laser is also used for the measurements meaning this technique is now accessible for a large number of researchers.
The QYs of several NP samples were measured as well. Three samples ranging in particles size from 10 to 100 nm were employed to examine the effect of particles size on the efficiency of this material. The upconversion emission profile and the laser profile for the 30 nm sample (ErYb3) are show in Fig. 2. The QYs of the various materials and the raw data for the calculations are tabulated in Tables 1 and S1†, respectively. Upconversion is a nonlinear process thus the QYs of upconverting materials are highly dependent on the power density of the excitation laser.13 Therefore all measurements on the NPs samples were performed using a power density of 150 W cm−2 which was at the beginning of the saturation regime of the power dependence curve for all particles examined. Thus the QYs calculated for the colloidal samples represent the maximum possible QYs attainable for these materials. The QYs of the NP colloids were also found to be independent of concentration. Drastic drops in the QYs of the samples are observed with decreasing particle size as expected from previous observations on upconverting NPs. Previous studies have observed decreased upconversion luminescence in smaller NPs. The drop in quantum yield with decreasing particle size is attributed to the increase in surface area of the smaller NPs which places a higher percentage of the dopant lanthanide ions closer to the surface. This leads to an increase of non-radiative relaxations of the emitting and intermediate levels by solvent molecules and hence an overall decrease in the QYs. The higher surface areas of the smaller particles could also increase the amount of surface defects in close proximity to the lanthanide ions which can lead to additional luminescence quenching.
The unexpected result from our measurements is how sharp the decrease in QY is with decreasing particle size. When the particle size decreases from 30 to 10 nm there is a 95% decrease in quantum yield. This seems to indicate that there is a minimum particle size that is capable of supporting efficient upconversion luminescence. It should also be noted that all of the measurements reported here were performed in nonpolar organic solvents. For many of the application these NPs would have to be rendered dispersible in aqueous environments which would lead to even greater decreases in QY.22
Optimization of the synthetic procedures to obtain NPs with the highest possible QYs, such as core/shell structures, is an important step in realizing the potential of these upconverting NPs.23–25 To examine this point, we measured the QY of a 30 nm NaYF4: Er3+ 2%, Yb3+ 20%/NaYF4 core/shell upconverting NP sample (ErYb5). A 300% increase in the measured QY was observed after the growth of an undoped NaYF4 shell over the Er3+ doped core NPs. The 30 nm core/shell material has roughly the same QY as the 100 nm upconverting NP sample (ErYb2) demonstrating the effectiveness of shell growth at increasing the luminescence efficiency of these materials.
Conclusions
In this communication we have described a technique for measuring the absolute quantum yields of upconverting nanomaterials based on the use of a commercially available fluorimeter equipped with an integrating sphere. Using this setup, we have shown that it is possible to acquired QYs for lanthanide-doped upconverting nanomaterials. Quantum yields in the range of 0.005% to 3% were measured for the green emission of several NaYF4: 2% Er3+, 20% Yb3+ nanoparticles with different particle sizes and a bulk sample. The beneficial effects of a core/shell structure were also proven as a significant increase in QY was observed over a similarly sized uncoated NP sample. The final result is a reliable and straightforward method to determining quantum yields of upconverting materials that are now accessible to a wide range of researchers.Acknowledgements
Natural Science and Engineering Research Council (NSERC), the Canada Foundation for Innovation (CFI), and the British Columbia Knowledge Development Fund (BCKDF) of Canada are gratefully acknowledged for support.Notes and references
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061 CrossRef CAS.
- C. Li, Z. Quan, J. Yang, P. Yang and J. Lin, Inorg. Chem., 2007, 46, 6329 CrossRef CAS.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185 CrossRef CAS.
- R. Dekker, D. J. W. Klunder, A. Borreman, M. B. J. Diemeer, K. Worhoff, A. Driessen, J. W. Stouwdam and F. C. J. M. van Veggel, Appl. Phys. Lett., 2004, 85, 6104 CrossRef CAS.
- D. K. Chatterjee, A. J. Rufaihah and Y. Zhang, Biomaterials, 2008, 29, 937 CrossRef CAS.
- S. W. Wu, G. Han, D. J. Milliron, S. Aloni, V. Altoe, D. V. Talapin, B. E. Cohen and P. J. Schuck, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10917 CrossRef CAS.
- H. Scheife, G. Huber, E. Heumann, S. Bar and E. Osiac, Opt. Mater. (Amsterdam), 2004, 26, 365 Search PubMed.
- K. H. Cheng, J. Aijmo, L. Ma, M. Yao, X. Zhang, J. Como, L. J. Hope-Weeks, J. Huang and W. Chen, J. Phys. Chem. C, 2008, 112, 17931 CrossRef CAS.
- W. J. Kim, M. Nyk and P. N. Prasad, Nanotechnology, 2009, 20, 185301 CrossRef.
- F. Auzel and D. Pecile, J. Lumin., 1973, 8, 32 CrossRef CAS.
- F. Auzel and D. Pecile, J. Lumin., 1976, 11, 321 CrossRef CAS.
- A. Bril, J. L. Sommerdijk and A. W. De Jager, J. Electrochem. Soc., 1975, 122, 660 CrossRef CAS.
- R. H. Page, K. I. Schaffers, P. A. Waide, J. B. Tassano, S. A. Payne, W. F. Krupke and W. K. Bischel, J. Opt. Soc. Am. B, 1998, 15, 996 Search PubMed.
- A. Rapaport, J. Milliez, F. Szipocs, M. Bass, A. Cassanho and H. Jenssen, Appl. Opt., 2004, 43, 6477 CrossRef CAS.
- J. F. Suyver, J. Grimm, M. K. van Veen, D. Biner, K. W. Kraemer and H. U. Guedel, J. Lumin., 2006, 117, 1 CrossRef CAS.
- F. Vetrone, J.-C. Boyer, J. A. Capobianco, A. Speghini and M. Bettinelli, Appl. Phys. Lett., 2002, 80, 1752 CrossRef CAS.
- J. C. De Mello, F. H. Wittmann and R. H. Friend, Adv. Mater., 1997, 9, 230 CrossRef CAS.
- K. W. Krämer, D. Biner, G. Frei, H. U. Güdel, M. P. Hehlen and S. R. Lüthi, Chem. Mater., 2004, 16, 1244 CrossRef.
- Z. Li and Y. Zhang, Nanotechnology, 2008, 19, 345606 CrossRef.
- H. S. Qian and Y. Zhang, Langmuir, 2008, 24, 12123 CrossRef CAS.
- G. S. Yi and G. M. Chow, Adv. Funct. Mater., 2006, 16, 2324 CrossRef CAS.
- G.-S. Yi and G.-M. Chow, Chem. Mater., 2007, 19, 341 CrossRef CAS.
- J.-C. Boyer, J. Gagnon, L. A. Cuccia and J. A. Capobianco, Chem. Mater., 2007, 19, 3358 CrossRef CAS.
- J.-C. Boyer, M.-P. Manseau, J. I. Murray and F. C. J. M. van Veggel, Langmuir, 2010, 26, 1157 CrossRef CAS.
- Z. L. Wang, Z. W. Quan, P. Y. Jia, C. K. Lin, Y. Luo, Y. Chen, J. Fang, W. Zhou, C. J. O'Connor and J. Lin, Chem. Mater., 2006, 18, 2030 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, powder XRDs and TEM micrographs of the samples. See DOI: 10.1039/c0nr00253d |
| ‡ Current address: 4D Labs, Simon Fraser University, Department of Chemistry, 8888 University Drive, Burnaby, British Columbia, Canada, V5A 1S6. Fax: +1 778-782-3765, Tel: +1 778-782-8060. E-mail: jboyer@sfu.ca |
| This journal is © The Royal Society of Chemistry 2010 |