 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transforming the chemical industry: a BASF perspective on net-zero and circularity
Bernhard von Vacano *a,
Wolfgang Hübingerb,
Willis Mugandaa,
Grigorios Koliosa,
Talke Schaffrannekc,
Alois Kindlera,
Kai Ehrhard
*a,
Wolfgang Hübingerb,
Willis Mugandaa,
Grigorios Koliosa,
Talke Schaffrannekc,
Alois Kindlera,
Kai Ehrhard a and
Markus Weber*a
a and
Markus Weber*a
aBASF SE, Group Research, Carl-Bosch-Str. 38, 67056 Ludwigshafen, Germany. E-mail: bernhard.von-vacano@basf.com; markus.weber@basf.com
bBASF SE, Petrochemicals Division, Carl-Bosch-Str. 38, 67056 Ludwigshafen, Germany
cBASF SE, Corporate Sustainability, Carl-Bosch-Str. 38, 67056 Ludwigshafen, Germany
First published on 5th February 2026
Abstract
This contribution to the collection of “industry perspectives” discusses pathways for a transformation of the chemical industry to achieve net-zero greenhouse gas emissions and switch to a circular feedstock base. It is based on the specific perspective of BASF as a multinational, deeply integrated chemical company. At the source of its CO2 emissions is the energy-intensive nature of basic chemical production, and the triple role of petrochemical feedstocks to provide carbon, hydrogen and energy. Along these themes, the importance of circularity is discussed and the shift to non-fossil carbon feedstocks, including bio-based, recycled, and potentially CO2-derived sources. It is shown how they are inextricably linked to the energy perspective, emphasizing the transition to renewable energy sources and exploring alternatives to conventionally thermally driven processes. As an industry currently facing unprecedented economic challenges, any transformation must be coupled to economically viable pathways, enabled by the right tools, business models and products, to leverage the potential of chemistry to drive the sustainability transformation of the whole economy.
Sustainability spotlightThe chemical industry's transformation toward net-zero emissions and circularity is critical for global climate action and resource protection. This work presents BASF's strategic approach to decoupling fossil feedstocks and energy, advancing renewable and recycled raw materials, and electrifying core processes. These innovations directly address the urgent need for sustainable industrial infrastructure (SDG 9), responsible consumption and production (SDG 12), and climate action (SDG 13). By integrating digital transparency tools and mass balance methodologies, the contribution explores scalable, economically viable pathways that catalyze sustainability across value chains and industries. |
1. Introduction
1.1 Chemical industry's role in sustainability
Addressing the pressing challenges of our globe – protecting the climate and biodiversity, keeping resource use and environmental impacts within the planetary boundaries – requires implementing sustainability in all human activities. Material consumption is visible in products used and discarded, and therefore the responsibility of the manufacturing sector seems obvious. In manufacturing, resources are extracted and refined, waste is generated and energy consumed in conversion steps, assembly and shipping. For consumers, companies making products they use seem to be the ones to hold accountable. However, those brands themselves almost never operate all steps in the production chain: they buy components from suppliers, which themselves source materials and parts from other economic actors earlier in the chain. Finished goods inherit all the environmental impacts caused by every step prior. The chemical industry is at the root of uncountable value chains.Consequently, with its products used in almost every aspect of modern life, the chemical industry contributes more than 1 trillion Euro to world GDP and employs around 15 million people worldwide,1 making it the fifth-largest global manufacturing sector. Its enabling character is reflected in the estimate that every Euro value generated directly leads to more than quadruple of this amount generated additionally elsewhere in the global economy.1 Today, though, the industry is almost exclusively based on fossil-derived raw materials such as naphtha and natural gas.
Being energy intensive, the chemical industry has a significant environmental footprint and is responsible for 3 billion tons of global CO2 emissions per year, which amounts to 6% of all CO2 released in the atmosphere by human activity in 2025.2 On a global, systemic perspective, the impact of the industry,3 or specifically the plastics value chain4 have been discussed in the framework of planetary boundaries:5 aiming at a comprehensive view on the global impacts, different categories are analyzed and reflected against a “safe operating space” that, under the specific assumptions made, would correspond to sustainable operations. Typical patterns indicate the predominant transgression of CO2-based thresholds (atmospheric CO2 concentration, energy imbalance and ocean acidification). Few high-volume basic chemicals such as ammonia, methanol, (poly)olefins, or benzene account for most impacts.3 In addition to the greenhouse gas emission dimension, the industry, routinely producing and using hazardous materials for example as intermediates to make polymers or pharmaceutical actives, also needs to ensure environmental protection, health and chemical safety along all production, use, and end of life of its products.6 The global chemicals industry, while heavily impacted by global realignment of production and consumption patterns, economic uncertainty and high energy prices, is undergoing a transformative phase driven by sustainability, regulatory compliance, and advancements in digital technology. With the sector's environmental impact, this implies mitigating CO2 emissions as energy intensive industry and shifting from conventional fossil-based feedstocks to more sustainable recycled or bio-based alternatives.
It is important to note that energy use and feedstock source are not decoupled in today's petrochemical processes: unlike in other raw materials industries, where raw material (e.g. iron ore for steel or bauxite for aluminium) is different from the needed energy carriers (e.g. coal for steel or electricity for aluminium), in petrochemistry mostly naphtha and fossil gases (methane, ethane, propane or LPG) are both raw materials and source of fuel, as these common hydrocarbon feedstocks themselves and side products of petrochemical transformations carry intrinsic energy which is used to drive the processes (Fig. 1).
The Resource Efficient and Circular Economy Industry Coalition (RECEIC) posits that a raw material shift will be essential for achieving net-zero emissions within the chemical sector.7 The green transition therefore encompasses not only the adoption of secondary, recycled or bio-based feedstocks and renewable energy, but also the integration of sustainable practices throughout the entire supply chain—from the procurement of raw materials to manufacturing processes and distribution.
1.2 BASF's strategic commitment to climate and circularity
Within the chemical industry, BASF SE is a leading global player integrated along several major value chains (Fig. 2). With its breadth and depth of operations, in many aspects it reflects the general connectedness and complexity of chemical industrial value chains.BASF has been integrating sustainability into its business strategy in 1994 (ref. 8) and setting targets since the early 2000s. Initiatives such as the “Eco-Efficiency Analysis” (1996)9 and the “Sustainable Solution Steering” method (2012) were implemented to assess the environmental impact of products and steer them towards sustainability. The first corporate report to include quantitative environmental objectives was already published in 2003. BASFs reduced greenhouse gas (GHG) emissions by 10% five years ahead of schedule in 2007, setting more ambitious targets. For 2020, BASF originally aimed for a 25% reduction in GHG emissions and a 25% increase in energy efficiency, however both goals were again revised after being achieved earlier than planned. BASF commits to achieving net-zero greenhouse gas emissions in 2050 for own production emissions (Scope 1), energy purchases (Scope 2) and purchase of raw materials (Scope 3 upstream, also termed Scope 3.1). The different frames of reference for CO2 accounting, referred to as the respective scopes, are depicted in Fig. 3.
As an intermediate target, by 2030, Scope 1 and Scope 2 emissions are to be reduced by 25% compared with 2018, while reducing the specific upstream Scope 3 (Scope 3.1) by 15% by 2030 compared with the 2022 baseline for BASF group.
While these CO2 targets mostly affect all operations and the production up to product delivery, the products themselves are key to the transformation of all industries and applications following “downstream” in the value chain. In target setting, BASF classifies respective products as “Sustainable-Future Solutions” if they offer significant sustainability benefits in terms of resource efficiency, climate change and energy, circularity or other dimensions. and are measured with the “Sustainable Solutions Steering” approach (see Section 5). Until 2030, the proportion of sales from “Sustainable-Future Solutions” is targeted to increase from 41% in 2024 to above 50%.
BASF embraces its pivotal role and set out the aspiration in the 2024 strategy update, to be the preferred chemical company to enable its customers' green transformation, while growing profitably and creating value for its shareholders with the broad portfolio of chemicals businesses as well as product and process innovations.10
What can you expect in this contribution? Written for the “industrial perspective” themed collection of this journal, the views presented here reflect those of BASF, in the authors' company-context only. Other companies and stakeholders may have different priorities and concerns.
- We will start with a comprehensive view of the status quo in Section 2, analyse the sources of CO2 emissions in all connected value chains. We will consider separately the two-faced nature of the industry's petrochemical feedstock today for carbon and hydrogen, clearly lining out material use and use as energy carrier.
- For the raw material perspective, circularity is the key to a green transformation: Section 3 discusses non-fossil carbon feedstocks: bio-based, recycled, and – under specific boundary conditions – via CO2 capture and utilization (CCU).
- For the energy perspective, the following Section 4 explores the change to renewable energy sources, rethinking historically thermally driven processes, and prioritizing less energy consuming synthesis sequences.
- Finally, in Section 5, the green transformation of production and raw materials in an economic context needs to be combined with the right tools, business models and product portfolio, that is not only carbon neutral but also fit for purpose to catalyze sustainable solutions in the customers' value chains that follow.
2. Current carbon flows and emissions in BASF's production network
In general, our industry today consumes these four resources: electricity, steam, fuel and feedstocks. BASF operates in a pronounced, highly connected, deeply staggered value chain logic: the output of an initial production is used as the input in a subsequent step, increasing chemical complexity and value (Fig. 4). From a dozen basic raw materials and energy sources, more than fifty thousand different products are produced.For overall ∼50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 “C”-containing products of BASF, one can calculate a mass-weighted average compositional chemical formula. As such, the BASF average product would have a composition of CH2.5O0.6N0.2 (Fig. 5), which in its C to H molar ratio sits between methane (CH4) and naphtha (CH2.3), the two main raw materials consumed today.
000 “C”-containing products of BASF, one can calculate a mass-weighted average compositional chemical formula. As such, the BASF average product would have a composition of CH2.5O0.6N0.2 (Fig. 5), which in its C to H molar ratio sits between methane (CH4) and naphtha (CH2.3), the two main raw materials consumed today.
More than 90% of all the carbon today comes from those fossil hydrocarbon feedstocks. For the hydrogen similarly: either because it remains in the molecules during chemical conversions, or it is produced via steam methane reforming (SMR). SMR uses methane as feedstock, effectively stripping off the hydrogen and releasing the carbon as CO2:
Steam Methane Reforming (SMR):
| 3CH4 + 6H2O → 3CO2 + 12H2 |
Nitrogen is taken from the air as N2, but needs to be converted into a reactive chemical species. This is done via ammonia (NH3), in the Haber–Bosch process, as originally invented by BASF. The process consumes significant amounts of hydrogen, leading to a direct coupling of hydrogen demand and nitrogen input into the chemical industry:
Haber–Bosch process:
| 4N2 + 12H2 → 8NH3 |
Oxygen is introduced by oxidation reactions on hydrocarbons (e.g., ethylene to ethylene oxide propylene to acrylic acid, or cyclohexane to cyclohexanone as a precursor for caprolactam), subsequently via CO from synthesis gas generated from hydrocarbons by partial oxidation (then used, e.g., in hydroformylation reactions), or by oxidizing ammonia to nitric acid.
BASF has provided a detailed and transparent annual reporting of CO2 (equivalent) emissions since 2008.11 >70% of its own total emissions (Scope 1 and 2, see Fig. 3) stem from just ten key upstream industrial process steps, directly related to carbon, hydrogen, nitrogen and oxygen introduction into the downstream products (Fig. 6).
 | ||
| Fig. 6 GHG emissions of the ten major chemical processes foundational in the BASF production Verbund, and an indication of the key elements introduced. (Based on 2021 nameplate capacities, excluding at-equity consolidated companies)12 TDI: toluidene diisocyanate, MDI: methylene diphenyl diisocyanate. | ||
The total emissions from own operations (Scope 1) and purchased energy (Scope 2) summed up to 18 megatons (Mt) in 2024 (ref. 11) (Fig. 7). However, the materials fed into BASF's production assets also come with their upstream emissions (Scope 3.1), and can create further emissions due to transport (Scope 3.9), use (Scope 3.11), and end of life (Scope 3.12).
 | ||
Fig. 7 BASF's GHG emissions along the value chain and product life cycle in 2024, in megatons (Mt) of CO2 equivalents. While significant, Scope 1 and 2 emissions from own operations and energy purchases add to sizable upstream emissions “imported” with raw materials, and downstream emissions, mainly from disposal (incineration or landfilling). For a net zero transformation, the pictograms symbolize the possible levers: using bio-based renewable feedstock ( ) at different entry points, using carbon capture and utilization (CCU) by converting CO2 into feedstock with green energy ( ) at different entry points, using carbon capture and utilization (CCU) by converting CO2 into feedstock with green energy (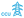 ), electrification of processes ( ), electrification of processes ( ), and recycling products back into the value chain, short-cutting both upstream emission burdens, as well as end-of-life emissions ( ), and recycling products back into the value chain, short-cutting both upstream emission burdens, as well as end-of-life emissions ( ). (*as reported in BASF's 2024 annual report,11 available online with more details). ). (*as reported in BASF's 2024 annual report,11 available online with more details). | ||
Like in financial reporting, different indicators can be calculated for CO2 emissions. Depending on the perspective and the means of steering, either all emissions are considered (life cycle perspective), or only the ones up to leaving BASF's gate (product carbon footprint perspective), Scope 1 and 2 (BASF primary target view), or only own emissions (Scope 1) for CO2 taxes or levies (Fig. 8).
 | ||
| Fig. 8 Different “accounting views” on CO2 emissions, depending on regulatory, customer or societal perspective. | ||
Within a linear economy, chemical products typically follow the end-of-life pathway of the applications in which they are utilized. If they are recoverable as waste, they are subject to disposal processes that differ by region. In areas with established waste management infrastructure, disposal often involves incineration with energy recovery—contributing to lifecycle CO2 emissions—or landfilling. In contrast, regions where infrastructure development has lagged behind economic growth and material consumption continue to experience high rates of mismanaged waste. This includes littering and accumulation in informal dumps, where uncontrolled burning poses environmental and health risks. These challenges are particularly evident in the case of plastic waste. A considerable proportion of chemical products is dispersed during their use phase, such as ingredients in personal care items like detergents and shampoos, or as nutritional additives in food and animal feed. Regulatory frameworks and product development efforts increasingly emphasize biodegradability and the minimization of adverse environmental effects in these applications. Nonetheless, the environmental fate and persistence of such substances across diverse usage scenarios remain enduring subjects of scientific investigation, regulatory attention, and public concern.
A recent publication by CEFIC (the European Chemical Industry Association), performed by and based on data from Accenture (Fig. 9), shows the different applications and their end-of-life scenarios.
3. The feedstock transformation – defossilizing carbon
For the green transformation, the chemical industry and its downstream customers face one of their greatest challenges with the increasing conversion of the raw material base to non-fossil sources, as well as changing over the energy sources to CO2 neutral options. For many aspects, technology building blocks exist or are being scaled. Others still need research and development. This transformation path must be aligned with the investment cycles of the industry, available capital and business cases, and the economic availability of the raw materials.Carbon in different (molecular) form is the main raw material of the chemical industry today. It cannot be stated enough, that a defossilization of the chemical industry's raw materials is targeted, and a decarbonization of its energy use. The raw materials and products will always contain carbon. Non-fossil carbon based raw materials can be based on renewable biomass, on recycling carbon that already circulates as material in the technosphere and becomes waste, or would be based on CO2.
Of course, chemical products do not only contain carbon: the stoichiometry matters. A key aspect to be discussed throughout the different non-fossil C-based options, is their fit to the “average” chemistry output CH2.5O0.6N0.2 (Fig. 5): many non-fossil options have a relative deficiency in hydrogen content. For the net-zero transformation, this H-gap (Fig. 10) needs to be filled by low-emission hydrogen and is therefore directly linked to external energy demands, which we will discuss in the next section.
3.1 Harnessing biomass for sustainable chemistry
Biomass as potential raw material is classified in three “generations”, alluding to the sequence in time they have been or could be introduced in the chemical industry. 1st generation biomass is used today: it uses the same crops that have been scaled in agricultural production for food and feed, are globally traded as commodities and hence in principle similarly available as fossil raw materials. The type of carbon-bearing molecules differentiates the sources between starchy crops (corn, rice, wheat), sugary crops (cane, beet) and oil crops (soybean, rapeseed, palm kernels). Those sources are already used today besides food and feed, mainly for energy uses bioethanol production from corn or cane as fuel, or biogas from corn. The use of food and feed crops directly puts up the challenge of competition with nutrition.The use of 2nd generation biomass for chemistry avoids this challenge: these are agro and forestry residues, not needed for soil fertilization or animal nutrition. They can include cover crops (used intermittently to fix nitrogen in the soil), and wood-based residues from the wood, pulp and paper industries. In contrast to first generation biomass, those feedstocks are mainly cellulose based.
Third-generation biomass is waste based: biogenic material today ending up in landfills or incineration. Seaweed and macro algae often are also counted under this category.
Within that scope of potential biomass sources such as feedstock, BASF emphasizes responsible sourcing: biodiversity protection, sustainable agricultural practices, and lifecycle assessments are evaluated in sourcing of bio-based feedstocks. Collaboration with suppliers who adhere to sustainable farming practices mitigates land use change and deforestation, thereby safeguarding ecological integrity. In addition, fair labor practices and community engagement are integrated within bio-based feedstock sourcing initiatives. Establishing long-term relationships with suppliers committed to sustainable practices is a cornerstone of the sourcing strategy.
As a principle for sustainability, the cascading use of biomass has been defined by the EU. Food and feed should in principle have priority over material use (including as chemical raw material), which itself precedes energy use (Fig. 11). Nevertheless, biomass follows the rules of the market economy: today, the fuel sector already generates demand due to legal requirements, which is commercially underpinned and directly competes with potential material use in terms of prices and quantities.
The integration of bio-based feedstocks into BASF's value chains can happen at multiple points. For each raw material, a technology needs to be matched that converts it to valuable molecules that can be used in the chemical industry. These can be key feedstocks that directly substitute a fossil equivalent at some entry point in the value chains: replacing natural gas by biomethane (entry point 1 in Fig. 12), fossil naphtha by bio-naphtha (entry point 2 in Fig. 12), or fossil ethanol by bioethanol. Renewable biomass can also be the starting point of their own value chain, or used as is (e.g. oils and fats in oleochemistry for surfactants, or as monomers for polyurethanes as in entry point 6 in Fig. 12).
Key transformation technologies include gasification of residual biomass to synthesis gas (entry point 3 in Fig. 12) and/or subsequent methanol synthesis as building blocks for a C1 value chain, but also hydrocarbons:13 naphtha replacement via Fischer–Tropsch syncrude (entry point 2 in Fig. 12), olefins via methanol-to-olefins (MTO, entry point 4 in Fig. 12) or methanol to aromatics MTA (entry point 5 in Fig. 12), to feed into BTX (benzene, toluene, xylene as key molecules).
However, starting with biomass also opens technology options (Fig. 13) that are markedly different from petrochemical routes: many structures of high value specialty chemicals are directly found in nature (like vitamins, salicylic acid, indigo…). While total synthesis of such molecules from fossil C1–C4 basic building blocks has often become cheaper than a complex extraction of small concentrations out of complex biologic matrices, natural building blocks for specific chemistry can avoid energy-intensive basic petrochemistry, save investment into long synthesis chains and become economically feasible. This is the case if the target molecule is markedly different from pure hydrocarbons, and an elegant synthesis route is available. Fermentation in industrial biotechnology is such an opportunity to convert bio-based feedstocks directly into intermediate chemicals. For instance, microorganisms ferment sugars derived from plant materials to yield organic acids, higher alcohols, and other valuable chemical entities. Also, enzymatic processes can be used to transform bio-based materials. Specific process development is time-consuming and expensive but will be an important driver for future sustainable chemical production. It will, however, very likely focus on “nature”-like oxygen-containing chemical products, which make up roughly 20% of the chemical industry output.
This shift towards renewable materials cannot only be environmentally beneficial but also unlock competitive advantages. Increasing consumer awareness regarding sustainability issues has already resulted in heightened demand for products formulated from renewable resources in some markets. This presents both challenges and opportunities for chemical manufacturers, and BASF is actively investing in research and development to effectively harness bio-based feedstocks. Efforts include collaborations with diverse stakeholders, academic institutions, research organizations, and industry partners, to expedite the development of bio-based processes and products. Today, BASF has first examples of renewable bio-based feedstocks used in product lines:
- Bio-based polyurethanes incorporating bio-based polyols derived from sugars, vegetable oils or other renewable sources (entry point 6 in Fig. 12).
- Bio-based surfactants derived from natural sources, characterized by biodegradability and a diminished environmental footprint compared to other options.
- Functional polymeric additives from Polysaccharide sources.
- Cosmetic bio-actives as example of high-value, functional materials from organically farmed rambutan fruit, with full-plant utilization and supporting local Vietnamese communities with certified organic farming, fair wages, and ecological practices.14
3.2 Recycled feedstocks via different technologies
A large share of carbon-based chemical products is used as materials in structural applications: plastics constitute around half of the chemical industry's carbon-based products (Fig. 9). As such, they are in principle collectible at their end of life and can be fed back as feedstock,15 reducing the need for new primary carbon inputs (from fossil, bio-based or CO2-derived sources) through recycling. Plastics recycling can be achieved through different routes, including mechanical, solvent-based, depolymerization and thermochemical processes, with each technology operating on different “loop” sizes in the value chain.Mechanical recycling is the most straightforward and established technology.16 It is based on sorting, cleaning, and re-melting plastics, physically reprocessing it without deliberate chemical reactions in a “polymer-to-polymer” loop. The advantage of mechanical recycling is its low energy demand and comparatively simple process. Melting and remolding plastic consumes less energy than synthesizing new polymers from scratch. However, the quality of the recycled output directly depends on the quality of the input. Contamination or foreign polymer types lead to a loss of quality and properties. Furthermore, the polymer chains will accumulate damage from their previous use phase and due to mechanical forces, heat and oxidation during processing. For engineering plastics, applications are limited so far to cases where pure, high quality scrap of the same materials can be collected. BASF, for example, produces a range of polyamide engineering plastics with mechanical recycled content (Nypel, entry point 5 in Fig. 14) and is exploring further options. The quality issue in mechanical recycling can be mitigated by using other chemical products in recycling: washing formulations for initial cleaning (including solutions by BASF subsidiary Chemetall), plastics additives that stabilize against oxidation and molecular weight degradation17 (e.g., the Irgacycle™ products offered under the VALERAS® brand in BASF's Performance Chemicals division), and compatibilizers that help intimately mix in foreign polymer components. Still, mechanical recycling needs clean single-type polymer waste streams.
Solvent-based recycling (sometimes also called dissolution recycling) is a variant of the small-loop approach where waste plastics are dissolved in selective solvents to separate polymers from additives and impurities without depolymerizing the chains.16 The polymer can then be recovered (e.g. by precipitation) in a purified form. This technique achieves decontamination and can also separate different polymer types: it can remove dyes, fillers, or legacy additives or delaminate multi-layer plastic composites by selective dissolution. The outcome is a recycled polymer with properties much closer to virgin material, suitable for high-quality uses. Extra steps and more energy are needed: solvents must be managed and recycled, and the process is more costly.18
Plastics such as thermosets (e.g. cured rubbers, crosslinked polyurethanes or epoxy resins), however, generally cannot be remelted or dissolved and thus are incompatible with purely physical recycling methods. Furthermore, damaged polymer chains and chemical degradation will not be restored.
Hence, further loops are needed.16 For polymers with a suitable structure (typically condensation-type polymers like polyesters or polyamides, but also polyurethanes) the polymers can be chemically cleaved into their constituent monomers or comparable chemical intermediates, which can then be re-polymerized in one subsequent step. This is referred to as depolymerization in the family of chemical recycling technologies. In addition to dealing with degraded polymer chains, depolymerization recycling can also be designed to deal with a larger share of contamination or mixed materials, and via purification of the monomer always go back to the identical quality as fossil production (entry point 3 in Fig. 14). BASF, on this basis, has launched loopamid® as a polyamide 6 recycled via depolymerization from mixed, post-consumer textile waste back into textile fibers. Depolymerization “resets” the material to its original quality, solving the degradation problem of physical recycling. It also cuts short the chemical value chain with its respective steps to synthesise the monomer: an advantage more pronounced for more complex monomer syntheses in the fossil value chain. Still, depolymerization is polymer-specific, each plastic type requires a different chemical process, and not all polymers have efficient depolymerization pathways. For economic operation,19 the plastics waste must be highly enriched in the target polymer.
Pyrolysis is one of the thermochemical recycling methods:16 mixed plastic waste is thermally decomposed in the absence of oxygen. Once the activation energy for C–C – bond cleavage is reached, long polymer chains break via a multitude of mechanistic pathways into smaller molecules. The result is a mixture of hydrocarbon liquids (oil), gases, and some solid residue (char). If the input is primarily polyolefin plastics (polyethylene, polypropylene) or other C–H rich materials like rubber, the pyrolysis oil can resemble a petroleum product. This pyrolysis oil can then be used as a secondary raw material instead of e.g., naphtha in steam cracking (entry point 1 in Fig. 14) or other hydrocarbon entry points. BASF's ChemCycling® project partners with pyrolysis technology firms to convert end-of-life tires or mixed plastic waste into oil, to replace fossil raw material. Due to the necessary mixing with fossil input into existing assets, a mass-balance approach is needed to allow output chemicals (like ethylene, propylene, etc.) to be certified as derived from recycled plastic. In quality, they are identical to conventionally produced chemicals. Raw pyrolysis oil is usually a complex mixture that can also fluctuate with the nature of the waste processed, and most often cannot be fed directly into existing petrochemical units without treatment. High amounts of unsaturated hydrocarbons can lead to unwanted formation of high molecular components, while the impurities cause corrosion, fouling, or catalyst poisoning in downstream units. An essential step therefore is upgrading the crude pyrolysis oil, e.g., by hydrotreatment – reacting the oil with hydrogen over catalysts to remove heteroatoms and stabilize the hydrocarbons. This necessary upgrading comes at an additional cost in energy and hydrogen demand. Pyrolysis itself requires heat, often provided by combustion of the gas fraction produced in the process.18 New designs are exploring electrically heated furnaces to use renewable electricity and avoid direct emissions. The advantage of pyrolysis, given suitable oil quality, is an immediate fit to the existing infrastructure of the fossil based chemical industry. Using mass balance allocation (see Section 5), a gradual feedstock transformation can be started without further burdensome investment demands downstream of the pyrolysis itself.
Another major feedstock recycling route is gasification,16 which takes the breakdown a step further: plastic waste (often along with other carbon-containing waste like biomass or refuse-derived fuel) is reacted at high temperature with a controlled amount of oxygen or steam, generating a synthesis gas (“syngas”) primarily composed of carbon monoxide (CO) and hydrogen (H2). Syngas can then be used (entry point 2 in Fig. 14) as a platform for C1 chemistry. It can be fed into existing production networks where fossil-generated syngas is used today, but also catalytically be transformed into liquid naphtha-like hydrocarbons (via Fischer–Tropsch synthesis), or methanol. In these cases, however, large transformative investments in the chemical industry are necessary for these conversions. Gasification itself is already a well-established process in other contexts (coal gasification, biomass gasification). Its major strength is its robustness to feedstock heterogeneity. A gasifier can process a diverse mix of waste – including plastics that are heavily soiled, composite materials, or even non-plastic organic waste alongside plastics. It requires, however, high process temperatures and is more energy intensive.18 Raw syngas will also contain CO2, tar vapors and reactive gases (HCl, H2S, NH3), stemming from respective heteroatoms in the feed. These gases need to be scrubbed and cleaned using industrial cleanup units. The syngas composition will mostly not match the needs of downstream synthesis; for example, the H2/CO ratio is always lower than required for hydrocarbon-based base chemicals. Hence, either water gas shifting must be used to adjust the ratio, leading to CO2 emissions, or extra H2 is added. One likely implementation of gasification, due to the large scales needed for efficient integrated plants, is co-gasification of plastic waste together with biomass.20 Co-feeding can even help mitigate practical process issues such as the “sticky” nature of a heated, pure plastic feed.
A third variant of feedstock recycling worth noting is the direct catalytic hydrogenation of waste plastics. Historically, such technology had been explored already for the conversion of coal into hydrocarbons. Instead of first pyrolyzing to an oil and then hydrotreating, hydrogen and special catalysts are used in a pressurized reactor for a hydrolytic breakdown of plastic. The idea is to directly transform plastics into saturated hydrocarbon liquids (as feedstock), while simultaneously stripping out heteroatoms by reaction with hydrogen. Heteroatoms will end up as H2O, HCl, NH3, etc., which are removed, but increase the hydrogen consumption. Hydrogenation is also highly exothermic, providing some self-heating. The challenges for scaling include high-pressure reactors and a low-carbon hydrogen supply for an environmentally sustainable solution. Compared to pyrolysis, the direct “compatibility” to the existing hydrocarbon-based chemical value chains is also given. Compared to gasification, for the same chemical products with their H/C rations, less supplemental hydrogen is needed, because the partial oxidation in gasification does not need to be reversed (see Fig. 10).
All the discussed technology options for circulating carbon in the technical circle need to be combined efficiently. They need to be matched to the waste stream (Fig. 15), to (i) minimize systemic energy (and hydrogen) demand, but also (ii) valorize a majority of collectible carbon to scale these non-fossil feedstock sources. Key waste streams today considered non-recyclable and lost towards incineration or use as refuse derived fuels, include automotive mixed shredded plastics waste. As a key material supplier to this industry, BASF is advancing and piloting projects in the recycling of automotive plastics via mechanical recycling, solvent based recycling, depolymerization and high temperature recycling via gasification.
 | ||
| Fig. 15 Matching of waste streams to different recycling technologies. In addition to the nature of the waste, the chemical composition of the polymers also determines which processes are applicable. Only a combination of technologies allows optimizing maximum amount of recycle-based circular carbon, at lowest energy intensity. Image adapted from ref. 16 under CC-BY license (Mangold and von Vacano, Macromolecular Chemistry and Physics 2022, DOI: https://doi.org/10.1002/macp.202100488). | ||
3.3 CO2 as a carbon source: opportunities and challenges
Many studies with decade-long net zero and renewable carbon perspectives tout CO2 as major carbon source for the chemical industry. CO2 is an abundant molecule and can be captured directly at the source of emitting operations. A leading technology for CO2 capture today is amine-based absorption and is offered by BASF under the brand OASE® blue from sources such as fossil power generation plants, steam reformers, waste incinerators or the cement industry.21 Extraction from the atmosphere in “direct air capture” (DAC)22 is also possible but increases effort and cost further. DAC, or “direct ocean capture” (DOC) of dissolved CO2 in marine waters,23 followed by conversion of the carbon into long-lived material applications, could even establish a carbon dioxide sink and mitigate already emitted CO2.After capturing and purification, the reduction of CO2 to, e.g., methanol is known in principle, and several technical implementations have been described.24 From methanol, all important value chains could be accessed, as discussed before for syngas. However, CO2 famously is a thermodynamic sink: converting it into usable chemical intermediates always involves putting in significant amounts of energy, by direct electrochemical reductive conversion or reduction via hydrogen equivalents.24 Of all possible feedstocks, the hydrogen gap (Fig. 10) is as large as it can get. Therefore, the viability of CCU is directly linked to the electric energy system and the availability of large amounts of emission-free electricity at very low prices.
The implementation of almost all these non-fossil C-feedstock routes, exacerbated for CCU, faces the economic challenge of higher costs today compared to fossil primary production, which has been optimized to the last cent in megaton-scales globally, and does not factor in economically CO2 emission or end of life dimensions today. Scaling and triggering the necessary investment will require clear regulatory pathways that influence these boundary conditions, and tools like mass balance allocation to capture the value of non-fossil content in chemical products. These tools and building blocks for transformation pathways will be described in Section 5.
For both bio-based and recycled-based C-feedstocks, and clearly most pronounced CCU, we have repeatedly highlighted the tight connection of technological pathways to the energy dimension. In the green transformation, a decoupling of the carbon flows (as resource) and energy flows is necessary. Let's therefore now dive into the energy dimension, directly linked to the H and N in our “average product formula” (Fig. 5), and the identified hydrogen gap of alternative C-feedstocks (Fig. 10).
4. Electrifying chemistry: decarbonizing the process energy
Energy sources for industrial chemistry changed over time and will change in the future with changing circumstances: in the 19th century with the development of the tar colors not only the feedstocks relied on coal tar, also the energy came from firing the coal. This was true for nearly a hundred years but changed in the middle of the 20th century first to oil (naphtha) and later to gas – a long-time to change due to long life cycles of chemical production plants. In some countries, especially in China, a significant share of production is still coal-based. With the development of electric power production, electricity became a rising share of energy use in chemical production – and also a source as driving force in reactions (electrochemistry). Conventional power generation from fossil energy carriers always produces heat, hence co-generation of electricity and, typically, steam for process energy is performed. With a rising share of renewable power production based on sun, water and wind, electrical power is considered the key to the decarbonization of the energy dimension – not only to replace today's sourcing of electricity as energy and to replace the fossil production of steam (Scope 2), but also for direct heating and as driving force for cleaner chemical reactions (Scope 1). Specific cases of use are described in the next section. Electrical power as a kind of feedstock (Scope 3.1), via carbon capture and utilization (CCU) or under the term “Power-to-X”, consumes so much energy that renewable power must become much cheaper and available in large amounts before CCU can take place on a commercial scale. These conditions will vary by geographical location: in some favored regions, economic viability will be reached first. Such favored locations for renewable power differ depending on technologies:- Waterpower follows precipitation and the big rivers and is therefore very locally concentrated, e.g. in northern Sweden or behind big dams in big rivers.
- Wind power is strongest mainly by west wind (northern hemisphere) on the eastern coastlines of the oceans.
- Sun is available everywhere – but still in different intensity. Most solar power can be harvested in regions with nearly no precipitation (arid zones) between latitudes of 40° north and 40° south.
For the intensity of solar and wind, publicly available data in the Global Solar and the Global Wind Atlas can be used.24 The electrification of chemical processes encompasses a range of technological strategies aimed at replacing fossil fuels with electrical energy. Effectively, it decouples the use of hydrocarbons as energy input from their essential use as “raw material”. The overarching objective of this transition is the elimination of CO2 emissions, inevitably linked to energy generation from fossil fuels. A central pillar of the Net-Zero strategy is the belief that electricity from renewable sources will become the preferred primary energy carrier—both economically and ecologically.
The DECHEMA Roadmap on Chemical Reaction Engineering25 provides a comprehensive overview of the current state of the art and outlines potential development trajectories. In essence, electrical energy is used either to overcome activation barriers or to supply the enthalpy required for chemical reactions.
Fig. 16 gives an overview of the reactor concepts that are considered for the electrification of chemical production. One technological approach involves electrochemical reactors, where reactions occur directly at the electrodes. Applications include water electrolysis, electrochemical CO2 reduction, and organic synthesis. This method offers advantages such as high selectivity, mild operating conditions, and direct coupling to electrical energy sources.
 | ||
| Fig. 16 Potential reactor concepts for the electrification of chemical production (Drawings newly created following concept illustrations or photographic examples in literature: Microwave Reactor,26 Plasma Reactor,27 Inductively Heated Reactor, Photoreactor,28 Ohmic Reactor29 and Electrochemical reactor30). | ||
A second approach focuses on (high)-temperature generation. The most straightforward implementation is resistive heating, where the reaction zone is heated either indirectly via heating elements or directly if the reactor internals are electrically conductive. Inductive and microwave heating are gaining traction due to their ability to heat reactor zones without physical contact. Another promising method is plasma technology, where gas discharges are used to activate molecules.
In photoelectrochemical systems, chemical conversion is driven either by direct photoactivation of reactants or through the use of photocatalysts.
Despite their promise, integrating these technologies into existing industrial systems presents several challenges. Electrified processes must be capable of dynamic operation to accommodate fluctuations in power availability. Moreover, many of these technologies remain at the laboratory or pilot scale, complicating scale-up. Functional materials such as electrodes and catalysts must be tailored to the specific process conditions. Additionally, appropriate infrastructure is required, including adapted power grids, energy storage systems, and market mechanisms.
Electrification is a key enabler for the defossilization of the chemical industry. High-temperature processes, which are foundational to the chemical value chain, play a particularly critical role.
BASF's development efforts began with a fundamental question: In which reactors should we apply electrical energy? It quickly became clear that radical innovation is needed to enable efficient electric heating of these processes. The goal is to scale these innovations from fundamental research to industrial applications. Within BASF, this development is pursued across a broad range of applications, including a new type of electrically heated steam cracker, as well as novel processes for hydrogen production via methane pyrolysis and NO synthesis via thermal nitrogen activation.
4.1 Electrifying the heart of petrochemistry
Among the various electrified reactor concepts, the electrically heated steam cracker (e-furnace) has made the most progress. In steam cracking, naphtha is converted into olefins and other base chemicals. This endothermic process requires temperatures around 850 °C.31In April 2024, BASF, in collaboration with SABIC and LINDE, commissioned a demonstration plant in Ludwigshafen.32 This facility serves as a prototype for a full-scale production furnace that could replace conventionally fired furnaces. Fig. 17 shows a schematic comparison of the conventional steam cracker and the eFurnace.
Traditionally, steam cracking is performed in fossil-fuel-fired furnaces.33 Natural gas or methane—often a by-product of olefin production—is used as fuel. This combustion process is the primary source of CO2 emissions in steam cracking. Electrifying the heating process can eliminate up to 90% of these emissions. However, this also means that methane can no longer be used as a fuel and must be repurposed. Two main approaches to electric heating are under consideration:
- Using the cracker coils themselves as resistive heating elements.
- Replacing conventional wall burners with electric heating elements.
Electrification compels a fundamental rethinking of furnace design. Material properties and limitations are critical: for example, electrical short-circuits must be avoided, and even advanced alloys lose mechanical strength at high temperatures. Additionally, alloy components can catalyze undesirable coke formation.
One promising solution is the use of ceramic materials. Alumina is ideal in terms of temperature resistance, corrosion resistance, chemical inertness, erosion resistance, and electrical insulation. However, its brittleness and sensitivity to thermal shock have historically limited its use.
We have overcome these limitations by reinforcing monolithic ceramics with oxide fiber composite ceramics. The resulting composite tube is suitable for operation at temperatures up to 1200 °C.34 The manufacturing process allows for targeted functionalization of the tube wall, enabling the integration of embedded heating resistors and precise distribution of heating power along the tube35,36 (Fig. 18).
The tube also acts as an electrical insulator, decoupling the heating circuit from the reactor periphery. This design enables the elegant integration of additional functional elements, such as cold lead ends for electrical connections.
4.2 Low-carbon hydrogen via electrified methane pyrolysis
The practical objective of methane pyrolysis is the production of hydrogen from methane without the obligatory formation of CO2, which is a major drawback of steam reforming—the current industrial standard. In methane pyrolysis, solid carbon is generated as a co-product in a gravimetric ratio of approximately 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 relative to the hydrogen produced. The economic and ecological potential of this process has been analyzed in a comparative study.37
1 relative to the hydrogen produced. The economic and ecological potential of this process has been analyzed in a comparative study.37
This section outlines the technical process developed by BASF for industrial implementation. At the heart of the system is a novel, directly electrically heated moving-bed reactor.38
In this reactor, natural gas and coke are fed in counter-current flow. An electric current is passed through the solid packing, which functions as a resistive heating element. Electrodes embedded in the central section of the reactor deliver the current. When powered by renewable electricity, the process becomes nearly carbon neutral.
The localized heating of the solid-state-packing, combined with counter-current heat exchange between the gas and the coke, eliminates the need for external heat exchangers and minimizes energy consumption. A beneficial side effect is that the reactor ends remain relatively cool, simplifying the handling of feed and product streams and enabling stable, long-term reactor operation.
A key design feature is the method of current delivery to the reaction zone. The vertically arranged, cell-shaped electrodes ensure a uniform current distribution parallel to the material flow, resulting in homogeneous heating across the reactor cross-section. The electrodes are made from highly conductive, high-temperature-resistant materials and are contacted via the upper and lower reactor hoods. This design provides a large cross-sectional area for current supply, reducing power losses to just a few per thousand of the effective thermal power.
The electrodes and hoods are electrically insulated from the surrounding reactor shell. The reaction section is free of internal structures and can be dimensioned according to specific process requirements. Thanks to its simple and robust design, the reactor can safely operate at temperatures of up to 1700 °C. Moreover, the design is modular and scalable, making it suitable for both standalone applications and integration into large-scale chemical production sites. This technology is currently moving from pilot scale39 to several kiloton demonstration scale in collaboration with ExxonMobil.40
The application of the electrically heated moving-bed reactor is not limited to methane pyrolysis. We view this reactor design as a technology platform for the emission-free, thermally integrated operation of other endothermic high-temperature processes, such as:
- Olefin production via hydrocarbon cracking.
- Synthesis gas generation from natural gas or biogas reforming.
A major advantage of the moving-bed concept over conventional reactor designs is its ability to continuously remove carbon deposits from the reaction zone along with the carrier material. This enables continuous operation without the need for frequent shutdowns for regeneration.
4.3 Electrified nitrogen activation: a new path to NO
Via the Haber–Bosch process, today all Nitrogen introduced into chemical value chains is linked to ammonia, and hence hydrogen and its implications for energy demand and CO2 emissions of in today's dominating fossil production (Section 2). Even for use as oxidized species, the conventional route for nitric oxide (NO) production relies on ammonia as an intermediate and is based on hydrocarbon feedstocks. It consists of three major process steps:Steam Methane Reforming (SMR):
| 3CH4 + 6H2O → 3CO2 + 12H2 |
Haber–Bosch process:
| 4N2 + 12H2 → 8NH3 |
Ostwald process:
| 8NH3 + 10O2 → 8NO + 12H2O |
As a result, nitric acid (HNO3) production is also one of the major sources of CO2 emissions in BASF's value chain (Fig. 6).
An alternative pathway is the direct synthesis of NO from air, known as the Birkeland–Eyde (B–E) process.1 This method uses an electric arc (plasma) to initiate thermal NO formation via the Zel'dovich mechanism:
| N2 + O2 → 2NO, ΔH = 90 kJ mol−1 |
Under non-equilibrium plasma conditions, NO concentrations exceeding 6.5 vol% can be achieved at temperatures well above 3500 K. The main advantage of the B–E process is its independence from hydrocarbons, offering the potential for a zero-carbon product footprint. However, its major drawback is extremely poor energy efficiency—only about 3% of the supplied energy is used for the reaction, rendering the process economically unviable.
To address this, we have developed a reactor and process concept for the direct synthesis of NO from air or oxygen-containing gas mixtures via high-temperature reaction of nitrogen and oxygen.41 The objective is to provide an energy-efficient, scalable alternative to the Ostwald process, while eliminating the need for ammonia as an intermediate.
The core innovation lies in the use of regenerative heat exchange between the product and feed streams to bridge the large temperature gap between ambient feed conditions and the reaction temperature (∼2500 K). The reactor consists of:
- A central reaction zone with direct electrical heating (e.g., plasma torch, induction heater).
- Two regenerator zones on either side of the reaction zone.
The system operates in periodic reverse-flow mode, with a switching interval of approximately 60 seconds. The regenerators serve two critical functions:
- Preheating the feed gas.
- Rapidly quenching the product gas to suppress the back-reaction of NO to N2 and O2.
An effective quenching requires temperature gradients on the order of −105 K s−1. This is enabled by structured ceramic packings made from refractory oxides (e.g., MgO, Y2O3, ZrO2) with narrow channels (∼1 mm). These materials provide both high thermal stability and excellent heat exchange efficiency.
As a result, the utilization efficiency of the applied heat can be increased to 30–50%, a significant improvement over the B–E process. Moreover, the specific energy consumption of the electrically heated reverse-flow reactor (e-RFR) process is estimated at ≈ 4 kWh per kg NO, outperforming even the highly optimized Haber–Bosch–Ostwald route (≈5.2 kWh per kg NO), while completely avoiding CO2 emissions.
Beyond NO synthesis, the e-RFR offers a versatile platform for a wide range of high-temperature endothermic reactions. Current development efforts include:
- Ammonia cracking for hydrogen and nitrogen production, as part of the TransHyDE flagship project under Germany's national hydrogen strategy, funded by the federal ministry for Research, Technology and Space BMFTR.42
- Catalytic processes to produce key intermediates such as syngas, propylene, and styrene.
The e-RFR's combination of thermal integration, electrification, and modular scalability makes it a promising candidate for future low-emission chemical manufacturing. The concept has been demonstrated, but more work is needed for scaling towards industrial production maturity.
Overall, electrification holds great potential for a net-zero transformation of the chemical industry: by driving reactions electrically, by providing heat with renewable electric heating, and finally, by using renewable electricity as “feedstock” for CO2 conversion in CCU. The necessary technologies are developed to different levels of maturity. Implementing a large systematic make-over of the industry will require enormous amounts of capital and time, given the typical timescales of plant commissioning and the lifetime of many decades of existing assets. Furthermore, it needs to be embedded in the general transformation of the surrounding systems. Refineries consider their transformation pathways, including electrification, as mapped out in detail by Weckhuysen et al.43 A recent paper by the Bardow group started to explore the interdependencies with energy system outside the chemical industry, and identified44 a suggested macroeconomic optimal pathway, that starts with electrification of low-temperature heat and the switch to battery electric vehicles for mobility outside the industry, before transitioning high temperature heat applications and the chemical industry itself, suggesting it towards the 2040s. While highlighting many crucial influences, navigating the transition will, as recognized in this study, require consideration of even more factors: the economic perspective of the chemical company needing to invest, the role of imports of “green” base chemicals (like methanol or ammonia), and finally the feedstock mix between residual fossil, bio-based, recycled and – prospectively – CCU, that will modulate the total electricity demands as discussed.
5. How to get the transition done: tools, economics, and systems integration
To convert the chemical industry to climate neutrality, chemical raw materials and process energy must come from renewable sources or CO2 must be collected as waste (carbon capture and storage, CCS). In addition to the technological feasibility and market availability of the processes, the minimization of energy use is an essential selection criterion. The energy input per ton of products dominates long-term profitability, especially in the case of large-volume base chemicals.The German chemical industry association VCI identifies the options for meeting the carbon demand for a climate-neutral, defossilized future (in addition to “competitive energy costs”) as follows:31
(1) Utilizing the full spectrum of recycling.
(2) Long-term and sustainable availability of biogenic raw materials.
(3) Carbon capture and utilization (CCU), direct air capture and utilization (DACU) and biotechnological carbon recycling (CCUBIO).
This list is, from our view, clearly a prioritization,45 because it optimizes both: the energy- und the investment-demand (Fig. 19). Such different dimensions will, in a greater context, steer the carbon feedstock transformation.46 Mechanical recycling is preferred, wherever possible – it is the only way which is less energy- and investment-consuming than the production of virgin polymers from fossil fuels. In many cases, for products similar to the current portfolio of the industry, chemical recycling, under these assumptions, is preferred over the use of biomass, because it avoids the energy-consuming removal of physical and chemical water and/or adjusting the hydrogen gap (Fig. 10). Still, the cost of recycled feedstock is already significantly higher than today's fossil products. Similarly, a hurdle to use more biogenic carbon, aside from sustainable sourcing considerations, is the market price: currently (as of August 2025), “carbon” equivalents from crude oil (“West Texas Intermediate” grade: about 60–65 USD per barrel, or about 40 USD per MWh) cost half or less than half of the carbon equivalents from sugar (0.17 $ per lb or 86 $ per MWh) in Brazil or ethanol (90 $ per bbl or 88 $ per MWh) in the US.31 If oxygen-free base products are the target, this hurdle often is too high. CCU is the by far most expensive way to get carbon-based products, mainly due to the need to lift the “C” from CO2 from the absolute energy minimum and add the lacking hydrogen (Fig. 10).
 | ||
Fig. 19 Case study for different production routes to low density polyethylene (LDPE), a typical large-volume petrochemical product with a C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H ratio of ∼1 H ratio of ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The indicated energy input and invest volumes have been calculated for different raw-material routes: conventional fossil production, mechanical recycling (assuming equal quality can be achieved), chemical recycling via pyrolysis and naphtha replacement in the steam cracker (entry point 1 in Fig. 14), production via bio-ethanol from sugar fermentation to ethylene, waste biomass gasification, methanol synthesis and subsequent methanol-to-olefin (MTO) conversion (entry point 2 in Fig. 14), and finally CCU, namely captured CO2 to methanol, and MTO. (Data is based on detailed calculations with non-public sources, and therefore shown on a relative scale also indicating major uncertainties due to different possible assumptions). 2. The indicated energy input and invest volumes have been calculated for different raw-material routes: conventional fossil production, mechanical recycling (assuming equal quality can be achieved), chemical recycling via pyrolysis and naphtha replacement in the steam cracker (entry point 1 in Fig. 14), production via bio-ethanol from sugar fermentation to ethylene, waste biomass gasification, methanol synthesis and subsequent methanol-to-olefin (MTO) conversion (entry point 2 in Fig. 14), and finally CCU, namely captured CO2 to methanol, and MTO. (Data is based on detailed calculations with non-public sources, and therefore shown on a relative scale also indicating major uncertainties due to different possible assumptions). | ||
The electrical power demand in the chemical industry is expected to rise over-proportionally due to the technological developments in electrification such as those described above.39 Renewable energy today, especially in a cash-constrained economic environment of the industry, is a significant investment hurdle: after all success in reducing specific investment for wind and especially solar energy, the upfront invest needed for a MWh generated is today still fourfold compared to base load power plants fueled by natural gas (own calculations for 2025). Power distribution and uninterrupted supply are further sources for significant transformation investments. The fossil base chemicals are currently produced in continuously running big production plants, as economies of scale are crucial to reduce cost for competitiveness, and need a huge amount of energy (today mainly fossil fuels). Power as electrical energy today costs a factor of 2 to 5 more compared to natural gas. With rising shares of renewable electricity, safeguarding uninterrupted supply 24/7 will become an increasing challenge. In times of dark doldrums and scarce renewable electricity supply, continuous chemical production needs back-up solutions. This can be in the short term a battery with round-trip efficiency of about 90% – but to compensate seasonality, stored volumes are much higher and self-discharge make Li-ion batteries unsuitable. Redox-flow batteries47 overcome the self-discharge, but specific storage capability production experience is still lower than for Li-ion batteries. Renewable electricity at locations without significant seasonality – especially solar in arid zones between ±40° latitude around the equator – can become a decision factor for new chemical plants in the near future: in such locations (>2000 h per a full load; and with low installation cost), a yield of 1 MWh per a per panel at 140 $ invest (500 W panel installed) can be reached. That results in a power price of $14 per MWh (at 10% depreciation + return on investment) – or even lower. This is much lower than from a fossil-fired thermal power plant. In North-Western Europe (NWE), the same photovoltaics (PV) system produces less than half the power and costs more to install (due to higher personnel cost) – and needs additional battery/backup power for the dark doldrums, which needs about the same investment as the PV-plant itself: power price forecast for grids in NWE are often higher than 100 $ per MWh. This is defined by the merit order price curve: PV and wind are on the far left side of the curve and undercut fossil generation, but the marginal producer, either a fossil based thermal (gas) power plant or a bio-based thermal power plant (woody biomass) will have costs between 100 and 200 $ per MWh, depending on plant size and effective production hours per annum (usually around 1000 h per a), and will define the market price for uninterrupted power supply via the grid. Hydrogen as storage option has a round-trip efficiency of only 33%. This, and the high investment into electrolysis and a power plant which is running only for 1000 h per a makes it currently much more costly (∼4 fold) than burning wood to get uninterrupted power.48–51
An alternative to mitigate fluctuating power supply could be chemical assets that break with the continuous production paradigm: those, however, would require new technologies in many cases and vastly increase the specific cost of, on a yearly average, poorly utilized assets. From today's industry perspective, this seems economically impossible at scale; while studies indicate that such an approach with overcapacities in the chemical industry to leverage fluctuating electricity could be beneficial from a macroeconomic point of view.44
Renewable energy could in principle also be imported like oil and gas today from preferred locations. To that end, electrical power needs transmission lines, which as standard high voltage transmission lines transport ∼1–2 GW, and in their most advanced form (high voltage long distance direct current) ∼8 GW.52 These don't reach the energy throughput (∼45 GW) of a dual-pipe natural gas pipeline,53 and are dwarfed by the energy throughput of a single crude oil pipeline with its liquid energy carrier (∼130 GW).54 Transporting electricity equivalents by ships or pipelines needs further transformations into hydrogen first (which, as a gas, is also expensive to transport) and to ammonia or methanol second as liquid energy carriers. These processes are energy consuming and have a limited efficiency: PEM-electrolysis loses a third of the input-energy (due to ohmic resistance in membrane and electrodes, oxygen-production and degradations, which produces waste heat at about 55 °C). From an economic point of view, all these factors will lead to higher costs, where today the market in its entirety does not yet pay accordingly for the green benefits such as lower PCF – and current CO2-prices do not compensate the disadvantage.
Therefore, the industry transformation will only be achieved via pathways that are economically feasible. We need the respective technologies, but crucially also tools in place that provide the necessary transparency to steer the complexity of the transformation, and finally enable the economics: transparently allocating benefits, connecting them to pricing that captures the value of more sustainable chemical products, while ensuring compliance with regulatory requirements. Some of those tools, which are also intricately linked with a digital transformation of business processes, we will present in the following.
5.1 Digital carbon transparency: SCOTT and product carbon footprints
One such tool to reach Net Zero is BASF's Strategic CO2 Transparency Tool (SCOTT) – an in-house digital platform designed to calculate and manage Product Carbon Footprints (PCFs) for BASF's product portfolio (∼40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 sales products) on a cradle-to-gate basis (Fig. 8).55 SCOTT integrates detailed primary data from BASF's production (e.g. plant-level emission measurements, energy use, bills of materials) and supply chain (e.g. raw material carbon footprints) by linking into existing enterprise digital systems, then consolidates these inputs using ISO-compliant allocation algorithms to apportion CO(eq)2 emissions across the highly integrated manufacturing network. The result is a fully automated PCF calculation for each product, computed with consistency and speed (the entire portfolio can be processed within hours). SCOTT's methodology is aligned with established life-cycle accounting standards – notably ISO 14067:2018 (carbon footprint of products), ISO 14040/44 and the GHG Protocol Product Standard – ensuring credibility and comparability of the results. In fact, because general PCF standards still allow methodological choices, BASF has introduced additional prescriptive criteria in its PCF calculations to enhance uniformity in emissions allocation, which is crucial for cross-industry comparability. The robustness of this approach is evidenced by independent certification: in 2023, TÜV Rheinland confirmed that BASF's PCF calculation system (SCOTT) fully complies with the new Together for Sustainability (TfS) PCF Guideline, a uniform industry standard agreed upon by 47 major chemical companies in 2022. The tool thus provides transparency into the carbon emission drivers of each product, allowing emission hotspots in manufacturing or supply chains, and supports business discussions to help customers with their transformation using reduced or low-PCF offerings, thereby linking environmental benefits with economic feasibility in the industry's green transformation.
000 sales products) on a cradle-to-gate basis (Fig. 8).55 SCOTT integrates detailed primary data from BASF's production (e.g. plant-level emission measurements, energy use, bills of materials) and supply chain (e.g. raw material carbon footprints) by linking into existing enterprise digital systems, then consolidates these inputs using ISO-compliant allocation algorithms to apportion CO(eq)2 emissions across the highly integrated manufacturing network. The result is a fully automated PCF calculation for each product, computed with consistency and speed (the entire portfolio can be processed within hours). SCOTT's methodology is aligned with established life-cycle accounting standards – notably ISO 14067:2018 (carbon footprint of products), ISO 14040/44 and the GHG Protocol Product Standard – ensuring credibility and comparability of the results. In fact, because general PCF standards still allow methodological choices, BASF has introduced additional prescriptive criteria in its PCF calculations to enhance uniformity in emissions allocation, which is crucial for cross-industry comparability. The robustness of this approach is evidenced by independent certification: in 2023, TÜV Rheinland confirmed that BASF's PCF calculation system (SCOTT) fully complies with the new Together for Sustainability (TfS) PCF Guideline, a uniform industry standard agreed upon by 47 major chemical companies in 2022. The tool thus provides transparency into the carbon emission drivers of each product, allowing emission hotspots in manufacturing or supply chains, and supports business discussions to help customers with their transformation using reduced or low-PCF offerings, thereby linking environmental benefits with economic feasibility in the industry's green transformation.
5.2 Mass balance: transparent allocation of recycled and renewable inputs
A key enabler for the feedstock transformation from BASF's position the mass balance allocation approach (Fig. 20), a chain-of-custody model that allows to attribute a share of renewable or recycled feedstock to specific products even when these sustainable inputs are co-processed with fossil resources in the same facilities. This is crucial for raw material entry early into chemical value chains, while on the level of intermediates or products, segregated production can become feasible. In practice, a rigorous accounting system has to ensure that an equivalent quantity of fossil raw material is replaced by sustainable alternatives at the start of production, enabling transparent and traceable claims for the final outputs. Mass balance is formalized international standards (e.g., ISO 22095:2020 for chain-of-custody) and implemented via third-party certification schemes like ISCC PLUS and REDcert.2Equally important, as mass balance enables value-based pricing: non-fossil raw materials today are clearly more expensive than fossil alternatives. Allocation allows a producer to market certified low-carbon or circular versions of existing chemicals at the premium needed to offset the higher costs of renewable or recycled raw materials, while transparently allocating the environmental benefits for customers. In (petro-)chemical processing, one added complexity are the different “routes” an input carbon atom can take in the vast production network (Fig. 4). Additional requirements can be layered on top of basic mass balance principles, in terms of the physical or chemical connectivity of value chains, and deductions for specific uses: a “fuel-exempt rule”, for example, deducts the share that ends up in energetic uses. A “polymer only” rule, discussed in the recycling context, proposes to only count carbon ending up in plastics. Such stricter rules lead to lower apparent “yields” of non-fossil feedstock in the allocation, and hence proportionally increase the substitution cost. Mass balance as a concept requires trust in the rules by all stakeholders. Critics point out potential dangers of misleading claims or unfair competition with other sustainable practices, demanding robust guardrails. Clarity in the definition of rules in regulatory contexts is crucial for any investment. From our industry perspective, rules have to balance trust requirements with realistic economic enablement, to bootstrap the feedstock transition also commercially.
5.3 Sustainable solution steering: shaping the product portfolio
Finally, on the product side, BASF has established a clear categorization of all products with regards to all dimensions of their sustainability, to allow active shaping of the portfolio across more than 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 products and approximately 70 business units and aiming at increasing the share of sales from Sustainable-Future Solutions from 41% (2023) to more than 50% by 2030. To achieve this, BASF employs the TripleS (Sustainable Solution Steering) methodology. The methodology involves a two-step analysis. A first step ensures compliance with BASF's Code of Conduct, relevant regulatory frameworks such as REACH and TSCA, customer needs, and emerging regulations. Products that pass this check proceed to the second step, the check for sustainability value contribution: this step evaluates how a product compares to standard market solutions in terms of sustainability performance. Based on this analysis, products are segmented into five categories: pioneer, contributor, standard, monitored, and challenged (Fig. 21). To manage the extensive data required for these assessments, BASF relies on semi-automated digital in-house workflows, integrating data from other internal BASF systems. The TripleS methodology is dynamic and iterative: products initially classified as innovative may become market standards and eventually be reclassified due to new research findings or regulatory changes. BASF decided to phase out products classified as Challenged within five years of their initial classification, replacing them with new formulations. The categories of the framework furthermore support early-stage decisions in the R&D phase,15 implementing a lean and coherent “Safe and Sustainable by Design” (SSbD) approach.
000 products and approximately 70 business units and aiming at increasing the share of sales from Sustainable-Future Solutions from 41% (2023) to more than 50% by 2030. To achieve this, BASF employs the TripleS (Sustainable Solution Steering) methodology. The methodology involves a two-step analysis. A first step ensures compliance with BASF's Code of Conduct, relevant regulatory frameworks such as REACH and TSCA, customer needs, and emerging regulations. Products that pass this check proceed to the second step, the check for sustainability value contribution: this step evaluates how a product compares to standard market solutions in terms of sustainability performance. Based on this analysis, products are segmented into five categories: pioneer, contributor, standard, monitored, and challenged (Fig. 21). To manage the extensive data required for these assessments, BASF relies on semi-automated digital in-house workflows, integrating data from other internal BASF systems. The TripleS methodology is dynamic and iterative: products initially classified as innovative may become market standards and eventually be reclassified due to new research findings or regulatory changes. BASF decided to phase out products classified as Challenged within five years of their initial classification, replacing them with new formulations. The categories of the framework furthermore support early-stage decisions in the R&D phase,15 implementing a lean and coherent “Safe and Sustainable by Design” (SSbD) approach.
6. Outlook: catalyzing the green transformation
Shaping the green transformation is at the center of “Transform”, one of the four pillars of BASF's strategy.10 Customer industries are facing tremendous challenges in achieving their own transformation targets: It will be sustainable chemical products that support them on this journey and leverage the fundamental position of BASF in the value chains, leading the way for a transformative role of the chemical industry.BASF is taking a phased approach to the green transformation (Fig. 22) based on and catering to increasing customer demand. In a first phase, BASF has accessed increasing amounts of renewable electricity, piloted new technologies and launched sustainable products. Today, BASF offers already thousands of products with reduced or even net-zero product carbon footprints (PCF) across its broad portfolio.
In a second phase, BASF is moving to secure increasing volumes of renewable feedstocks and is ramping up volumes of products with sustainable attributes according to customer needs. The mass balance approach offers a pragmatic pathway for integrating recycled and renewable feedstocks into existing chemical production systems, yet its effectiveness is constrained by regulatory and methodological inconsistencies. To unlock its full potential in the transition to net zero, policy frameworks20 must establish clear, enforceable rules for mass balance accounting, supported by robust verification mechanisms. Coordinated international efforts are essential to ensure credibility, market acceptance, and scalability of this approach within the chemical industry.
Recent steps on the transformation pathway have been reported: while still a minority share of total feedstock, in 2023, BASF purchased around 1 million metric tons of renewable raw materials. The complete European amine portfolio,56 as well as performance materials production sites in Europe, switched to 100% renewable electricity.57 In 2025, BASF has become the first producer of renewable ammonia in Central Europe.58 This has been achieved by integrating renewable energy-derived hydrogen into its existing ammonia plant.
This is only the beginning of a long journey that involves a phased re-wiring of chemical value chains that touch every industry. Many of the necessary steps and possible building blocks are sketched out. Their technical maturity and the concomitant timeline of implementation varies. The necessary ambition to change within just over two decades until 2050, in an industry that has long investment cycles, can only be reconciled with high uncertainties in today's economic climate, if well strategized steps are taken consistently in the right direction. There is still further research needed for:
- Enhancing availability of feedstocks from end of life products and (waste) biomass by improved pre-processing.
- Economic approaches to directly obtain functional building blocks from waste plastics by non-destructive methods.
- Making use of the functionality of biomass for more direct synthesis of final sales products, avoid energy-intensive transformations for better economics.
- Novel electrified routes incl. usage of plasma with optimized energy efficiency and minimized carbon footprint, together with the required reactor concepts and enabling materials.
- Embracing the parallel digital transformation and the emergence of “artificial intelligence” in development, engineering, operations and business as potential enabler to cope with the complexity, urgency and magnitude of the task at hand.
- Finally, engaging with downstream customers to jointly leverage chemistry solutions for material reduction, product redesign, lifetime extension, and substitution, to help keep the flow of C, H and N through the economy compatible with sustainability.
Only by fostering and creating new partnerships between research disciplines, between academia, industry, customers and regulators, will we be able to leverage the power of innovation for the green transformation, to create chemistry for a sustainable future.
Conflicts of interest
The authors are all employed by BASF. There are no conflicts of interest to be declared.Data availability
In general, for this perspective no major primary research results, software or code have been included and no new data were generated or analysed as part of this review. Some rationalization is based on internal evaluations and company data, which cannot further be disclosed.Acknowledgements
Many thanks to L.-M. Rehn, U. Hees, J. Lubbock, H. Mangold, C. Jaeckel, J. Hamprecht, J. C. Olucha Alcalde, H. Winterling and M. Liedemit for their valuable input, feedback and suggestions. We explicitly want to acknowledge the very valuable feedback from our anonymous reviewers, including sharing their “wrestling” with the nature of this contribution as single-company industry perspective, with its concomitant boundary conditions on data disclosure and scarcity of citable sources. We thank the editors for their guidance, and the opportunity to share industry thinking in this context, that hopefully contributes to continued exchange with different further perspectives.References
- O. Economics, The Global Chemical Industry: Catalyzing Growth and Addressing Our World's Sustainability Challenges, https://icca-chem.org/wp-content/uploads/2020/10/Catalyzing-Growth-and-Addressing-Our-Worlds-Sustainability-Challenges-Report.pdf, (accessed 08.08.2025, 2025).
- C. Early and T. Slavin, CO2 and Greenhouse Gas Emissions, https://www.reuters.com/sustainability/decarbonizing-industries/chemicals-industry-struggles-kick-its-fossil-fuel-habit-2025-07-28/, (accessed 08.08.2025, 2025).
- I. Barnosell and C. Pozo, Sustain. Prod. Consum., 2024, 44, 188–207 Search PubMed.
- M. Bachmann, C. Zibunas, J. Hartmann, V. Tulus, S. Suh, G. Guillén-Gosálbez and A. Bardow, Nat Sustainability, 2023, 6, 599–610 CrossRef.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. Chapin, E. F. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. de Wit, T. Hughes, S. van der Leeuw, H. Rodhe, S. Sörlin, P. K. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. W. Corell, V. J. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. A. Foley, Nature, 2009, 461, 472–475 CrossRef PubMed.
- CEFIC, Responsible Care®: An ethical framework towards safe chemicals management and performance excellence, https://cefic.org/guidance-and-management-frameworks/responsible-care/, (accessed 08.08.2025, 2025).
- V. R, P. L. Dhepe, R. K. Sukumaran, R. Verma, S. Goyal, A. Desai and S. Lahiri, presented in part at the Global Symposium of Resource Efficiency & Circular Economy, Scaling Resource Efficiency & Circular Economy: Pathway for Global Sustainability, New Delhi, 2025, https://receic.com/wp-content/uploads/2025/04/Material-Transition-in-Chemical.pdf Search PubMed.
- BASF, 25 Years of Sustainability at BASF, https://www.basf.com/global/en/who-we-are/history/25-years-sustainability, (accessed 08.08.2025, 2025).
- BASF, Eco-Efficiency Analysis, https://www.basf.com/global/en/who-we-are/sustainability/our-contributions-to-enabling-the-green-transformation/eco-efficiency-analysis, (accessed 08.08.2025, 2025).
- BASF, Our Strategy, https://www.basf.com/global/en/who-we-are/strategy, (accessed 30.06.2025, 2025).
- BASF, BASF Annual Report 2024: E1 Climate Change, https://report.basf.com/2024/en/combined-managements-report/consolidated-sustainability-statement/environment/e1-climate-change.html, (accessed 19.09.2025, 2025).
- BASF, BASF ESG Investment Story - August 2025, https://www.basf.com/dam/jcr:f9323816-2619-4d1b-9fef-be775dc72765/basf/www/global/documents/en/investor-relations/sustainable-investments/BASF_ESG-Investment-Story.pdf, (accessed 08.08.2025, 2025).
- I. Yarulina, A. D. Chowdhury, F. Meirer, B. M. Weckhuysen and J. Gascon, Nat. Catal., 2018, 1, 398–411 CrossRef CAS.
- BASF, Rambutan: A sustainable sourcing project, https://care360.basf.com/global/en/industries/personal-care/sustainability-personal-care/the-rambutan-program, (accessed 09.08.2025, 2025).
- B. von Vacano, H. Mangold, G. W. M. Vandermeulen, G. Battagliarin, M. Hofmann, J. Bean and A. Künkel, Angew. Chem., Int. Ed., 2023, 62, e202210823 CrossRef CAS PubMed.
- H. Mangold and B. von Vacano, Macromol. Chem. Phys., 2022, 223, 2100488 CrossRef CAS.
- B. von Vacano, O. Reich, G. Huber and G. Türkoglu, J. Polym. Sci., 2023, 61, 1849–1858 CrossRef CAS.
- M. Klotz, C. Oberschelp, C. Salah, L. Subal and S. Hellweg, Sci. Total Environ., 2024, 906, 167586 CrossRef CAS PubMed.
- T. Uekert, A. Singh, J. S. DesVeaux, T. Ghosh, A. Bhatt, G. Yadav, S. Afzal, J. Walzberg, K. M. Knauer, S. R. Nicholson, G. T. Beckham and A. C. Carpenter, ACS Sustain. Chem. Eng., 2023, 11, 965–978 CrossRef CAS.
- Circular Business Review, ETH Zurich and BASF Push Policy Debate on Co-Gasi
![[*]](https://www.rsc.org/images/entities/char_e103.gif) cation of End-of-Life Vehicle Plastics, https://www.circularbusinessreview.com/eth-zurich-and-basf-push-policy-debate-on-co-gasification-of-end-of-life-vehicle-plastics/, accessed 06.02.2026 Search PubMed.
cation of End-of-Life Vehicle Plastics, https://www.circularbusinessreview.com/eth-zurich-and-basf-push-policy-debate-on-co-gasification-of-end-of-life-vehicle-plastics/, accessed 06.02.2026 Search PubMed. - BASF, OASE® blue for Flue Gas and Post Combustion CO2 Capture, https://energy-resources.basf.com/global/en/gas-treatment/gas-treatment-oase/OASE_blue_for_Flue_gas_industrial_CO2, (accessed 08.08.2025, 2025).
- E. S. Sanz-Pérez, C. R. Murdock, S. A. Didas and C. W. Jones, Chem. Rev., 2016, 116, 11840–11876 CrossRef PubMed.
- P. Aleta, A. Refaie, M. Afshari, A. Hassan and M. Rahimi, Energy Environ. Sci., 2023, 16, 4944–4967 Search PubMed.
- S. Mbatha, R. C. Everson, N. M. Musyoka, H. W. Langmi, A. Lanzini and W. Brilman, Sustain. Energy Fuels, 2021, 5, 3490–3569 RSC.
- DECHEMA, Roadmap “Chemical Reaction Engineering”, https://dechema.de/dechema_media/Downloads/Positionspapiere/2023+Roadmap+%E2%80%9EChemical+Reaction+Engineering_+engl_-p-20009528.pdf, (accessed 09.08.2025, 2025).
- K. Rana and S. Rana, OALib, 2014, 01, 1–20 Search PubMed.
- P. Mehta, P. Barboun, D. B. Go, J. C. Hicks and W. F. Schneider, ACS Energy Lett., 2019, 4, 1115–1133 CrossRef CAS.
- D. Cambié, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noël, Chem. Rev., 2016, 116, 10276–10341 CrossRef PubMed.
- V. L. M. Silva, L. M. N. B. F. Santos and A. M. S. Silva, Chem.–Eur. J., 2017, 23, 7853–7865 CrossRef CAS PubMed.
- H. Jaroszek and P. Dydo, Open Chem., 2016, 14, 1–19 Search PubMed.
- BASF, The Heart of the Verbund, https://www.basf.com/global/en/who-we-are/organization/locations/europe/german-sites/ludwigshafen/production/the-production-verbund/Steamcracker, (accessed 19.09.2025, 2025).
- BASF, BASF, SABIC, and Linde celebrate the start-up of the world's first large-scale electrically heated steam cracking furnace, https://www.basf.com/global/en/media/news-releases/2024/04/p-24-177, (accessed 19.09.2025, 2025).
- H. Zimmermann and R. Walzl, Ullmann’s Encycl. Ind. Chem., 2012, 13, 465–529 Search PubMed.
- Walter-E.-C.-Pritzkow-Spezialkeramik, Material Basics - What does “Keramikblech”/“sheet ceramic” mean?, https://www.keramikblech.com/en/material/, (accessed 19.09.2025, 2025).
- J. Matthies, T. Schall, W. Pritzkow, U. Tuttlies and U. Nieken, Chem. Ing. Tech., 2023, 95, 701–707 CrossRef CAS.
- T. Roedelmeier and G. Kolios (BASF SE), PCT/EP2023/083814, 2023.
- O. Machhammer, A. Bode and W. Hormuth, Chem. Eng. Technol., 2016, 39, 1185–1193 CrossRef CAS.
- H. Appel, J. Bernnat, F. Glenk, G. Kolios, G. Olbert, F. Scheiff, B. Zoels, M. Kern, D. Flick, C. A. Anderlohr, D. Klinger and A. Wechsung (BASF SE), PCT/EP2019/051466, 2018.
- BASF, Innovative Processes for Climate-Smart Chemistry, https://report.basf.com/2021/en/shareholders/basf-on-the-capital-market/methane-pyrolysis.html, (accessed 19.09.2025, 2025).
- BASF, BASF and ExxonMobil Join Forces to Advance Low - Emission Hydrogen Through Methane Pyrolysis Technology, https://www.basf.com/global/en/media/news-releases/2025/11/p-25-233, (accessed 22.01.2026, 2026).
- K. R. Ehrhardt, G. Kolios, S. Titlbach and M. Sentko (BASF SE), WO2023174935A1, 2022.
- BMFTR, How TransHyDE aims to develop a hydrogen infrastructure, https://www.wasserstoff-leitprojekte.de/projects/transhyde, (accessed 19.09.2025, 2025).
- E. T. C. Vogt and B. M. Weckhuysen, Nature, 2024, 629, 295–306 CrossRef CAS PubMed.
- P. Mayer, F. J. Baader, D. Y. Shu, L. Leenders, C. Zibunas, S. Moret and A. Bardow, Energy Environ. Sci., 2025, 18, 7967–7979 RSC.
- W. Huebinger, T. Osterland and R. Albach, Nachr. Chem., 2025, 73, 42–47 CrossRef.
- J.-P. Lange, Green Chem., 2026, 28, 762–782 RSC.
- W. Sharmoukh, RSC Adv., 2025, 15, 10106–10143 RSC.
- J. von Heimburg, E. Roduner, K.-D. Franz, W. Huebinger and T. Osterland, Nachr. Chem., 2023, 71, 32–35 CrossRef CAS.
- E. Roduner, K.-D. Franz, T. Osterland, W. Hübinger and P. Staniek, Nachr. Chem., 2024, 72, 28–30 CrossRef.
- T. Osterland, W. Hübinger and E. Roduner, Nachr. Chem., 2025, 73, 30–33 CrossRef.
- E. Roduner, T. Osterland and W. Hübinger, Nachr. Chem., 2025, 73, 36–38 CrossRef CAS.
- Wikipedia, List of HVDC projects, https://en.wikipedia.org/wiki/List_of_HVDC_projects, (accessed 15.09.2025, 2025).
- Authors' calculation: major gas pipelines, like former Northstream, had a transport capacity of 55 billion m3 per a – corresponding to 1231 m3 s−1. With a heating value of 10.5 kWh m−3 this are 12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926 kWh s−1 or ∼46.5 GW. Note that hydrogen is less efficient to transport, due to the lower density (0.0899 kg m−3 vs. 0.717 kg m−3; heating value 3 kWh m−3).
926 kWh s−1 or ∼46.5 GW. Note that hydrogen is less efficient to transport, due to the lower density (0.0899 kg m−3 vs. 0.717 kg m−3; heating value 3 kWh m−3). - Authors' calculation, with 2 million barrels per day, 1 barrel (bbl) of crude oil = 0.159 m3 and consequently 3.68 m3 s−1. Crude oil density is about 850 kg m−3, hence the mass flow ∼3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 kg s−1 and energy flow rate (in GJ s−1 = GW) with 42 MJ kg−1 heating value ∼131 GJ s−1.
130 kg s−1 and energy flow rate (in GJ s−1 = GW) with 42 MJ kg−1 heating value ∼131 GJ s−1. - BASF, BASF calculates CO2 footprint of all sales products, https://www.basf.com/global/en/media/news-releases/2020/07/p-20-260, (accessed 15.09.2025, 2025).
- BASF Intermediates Division, BASF's Intermediates division converts its entire European amines portfolio to 100 percent renewable electricity, https://www.basf.com/global/en/media/news-releases/2025/05/p-25-086.
- BASF Performance Materials Division, BASF's Performance Materials division plants run entirely on renewable electricity in Europe as of 2025, https://www.basf.com/global/en/media/news-releases/2025/01/p-25-011, (accessed 08.10.2025, 2025).
- BASF Monomers Division, BASF becomes first producer of renewable ammonia in Central Europe, https://www.basf.com/global/en/media/news-releases/2025/05/p-25-099, (accessed 08.10.2025, 2025).
| This journal is © The Royal Society of Chemistry 2026 |
















