Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers
Kamiya
Jain
a,
Raman
Vedarajan
a,
Masaki
Watanabe
b,
Mamoru
Ishikiriyama
b and
Noriyoshi
Matsumi
*a
aSchool of Materials Science, Japan Advanced Institute of Science and Technology, 1-1 Asahidai, Nomi, Ishikawa – 923 1292, Japan. E-mail: matsumi@jaist.ac.jp; Tel: +81-761-51-1600
bToyota Motors Corporation, Japan
First published on 18th August 2015
Abstract
Poly(N-isopropylacrylamide), PNIPAM, is a thermoresponsive polymer widely known for its lower critical solution temperature (LCST) phenomenon at 32 °C in aqueous solutions. Precise tuning of the LCST of PNIPAM to a broader temperature range offers a larger window of applications especially in the field of biotechnology and nanotechnology. A series of free radical random copolymerizations between N-isopropylacrylamide (NIPAM) and various imidazolium based ionic liquids (ILs) were conducted. IL structures were varied in terms of their alkyl chain length at the N-3 (or N-1) position of the imidazolium ring and counter anion. The LCST behavior of the aqueous solutions of the copolymers was investigated through UV-VIS transmission measurements. The results confirm that introduction of IL into the PNIPAM offers a wide range of LCST behaviors with a synergism between the hydrophobic part of the ionic liquid and the basic strength of the counter anion, probably by varying the hydrogen bonding abilities of the copolymer.
Introduction
Thermo-responsive or thermo-sensitive polymers are an important class of stimuli-responsive (stimuli such as temperature, pH, light and electric field) polymers1,2 which show discontinuous changes in their physical properties with changes in temperature. These polymers possess inherent ability to show abrupt transitions in their conformations with changes in temperature. These polymers can be classified on the basis of their solubility. The increase of polymer solubility with the rise in temperature is a characteristic of thermo-responsive polymers which are known to exhibit an upper critical solution temperature (UCST).3 On the contrary, polymers showing a decrease in solubility with rising temperature are known to exhibit a well-known phenomenon called the lower critical solution temperature (LCST).4 Generally, LCST is addressed in terms of its cloud point (Tcp) or phase separation temperature i.e. the temperature at which the solution suddenly becomes milky. Any reduction in temperature from Tcp will tend to make the solution clear owing to the formation of a single phase. These types of polymers have received great importance in recent years as they have a wide range of applications in drug-delivery,5 bio-engineering,6 sensors,7,8 thermal affinity separation9etc.One of the best known thermo-responsive polymers is poly(N-isopropylacrylamide), PNIPAM, having a structure as shown in Fig. 1. The repeating unit has both hydrophobic and hydrophilic moieties, and therefore, the aqueous polymer solution undergoes transitions between the water soluble state and the insoluble state depending on its degree of order at different temperatures. PNIPAM has been intensively investigated as it possesses a sharp cloud point ranging from 31 °C to 33 °C in water (irrespective of the polymer concentration).4,10 Fujishige et al. studied the conformational changes of an aqueous PNIPAM solution with changes in temperature.11 They reported that an aqueous solution of PNIPAM undergoes a coil to globule transition on heating. Below its LCST, PNIPAM chains exist as coils due to hydrogen bonding of amide groups with water. But above its LCST, it is considered plausible that hydrogen bonding is weakened as the kinetic energies of the molecules become larger than the energy of hydrogen bonding between water and the molecules. Thus, hydrophobic interactions between the hydrophobic backbone and isopropyl groups become dominant12 and cause a change in the polymer conformation from linear and flexible PNIPAM chains to the collapsed globules due to intra or intermolecular aggregation of hydrophobic moieties. Interactions that lead to attraction between polymer–water and polymer–polymer are the dominant factors for the determination of LCST behavior. Structural factors that enhance the polymer–water interactions result in an increase in LCST. On the contrary, increment in the polymer–polymer interactions decrease the value of LCST. Precise tuning of the LCST of PNIPAM to a broader temperature range offers a larger window of applications.
The LCST phenomenon of the polymers can be modulated by a variety of techniques. One of the most interesting methods is the preparation of thermo-responsive polymers by copolymerization with hydrophilic and hydrophobic comonomers.13–16 Addition of salts17 and/or surfactants18 to the polymer solution was also found to be an effective method to control LCST behavior. Maeda et al. reported the synthesis of random copolymers of N-isopropylacrylamide (NIPAM) and N,N-diethylacrylamide (DEA) and their thermo-responsive behavior.19 McCormick et al. showed that di- and tri-block copolymers of NIPAM and N,N-dimethylacrylamide (DMA) are capable of reversibly forming micelles with changes in temperature.20 More recently, Tenhu et al. have prepared di-block copolymers of polymerized ionic liquids (PIL) and PNIPAM.21 Further, they studied the effect of the PNIPAM block length on the properties of PIL and the effect of the cationic structure of the ionic liquid on the thermal properties of PNIPAM in aqueous dispersions. Many studies have been conducted for the tuning of the LCST pattern in PNIPAM-co-PIL systems but hardly any systematic investigations have been performed to understand the effect of IL on the LCST of PNIPAM by varying the degree of hydrophobicity of the cation or the polymerizing anion of the IL with NIPAM.
Hence, in our current study, the LCST behavior of PNIPAM has been systematically tailored to obtain copolymers showing Tcp below and above 32 °C in aqueous solutions. This has been demonstrated by the random copolymerizations of NIPAM with different N,N-di-substituted imidazolium based ionic liquids via free radical polymerization and their LCST behavior was confirmed by UV-Vis transmittance measurements.
Experimental
Materials and instruments
Acrylic acid (Tokyo Chemical Industry Co. Ltd), N-isopropylacrylamide (NIPAM, Tokyo Chemical Industry Co. Ltd), 1-bromotetradecane (Tokyo Chemical Industry Co. Ltd), vinyl phosphonic acid (Wako Co. Ltd), 1-methylimidazole (Wako Co. Ltd), 2,2′-azobis(2-methylpropionitrile) (AIBN, Wako Co. Ltd), dehydrated tetrahydrofuran (THF, Wako Co. Ltd), acetonitrile (Wako Co. Ltd) and diethyl ether (Wako Co. Ltd) were used as received without any further purification.A 400 MHz Nuclear Magnetic Resonance (NMR) spectrometer (Ultrashield™ Plus Bruker, Z101355) was employed to characterize ionic liquid and polymer structures. The molecular weight of the polymer was determined using Gel Permeation Chromatography (GPC, Shodex Asahipak GF-7M HQ) in methanol containing 0.05 M LiCl and polyethylene glycol was used for calibration as a standard. Cloud point measurements were performed using a UV-Vis spectrometer (JASCO V-630) equipped with a temperature controller system (Eyela NCB-1200).
Ionic liquid synthesis
A series of 1-alkyl-3-methylimidazolium bromide (as a precursor to acrylate (Ac) and vinylphosphonate (VP) salts) ILs (Scheme 1) were synthesized as reported in the literature.22TDMImAc. 1H NMR (400 MHz, DMSO-d6): δ = 9.35 (s, 1H, NC(H)N), 7.71–7.80 (d, 2H, NC(H)C(H)N), 5.85–5.97 (m, 1H, H–CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–COO−), 5.62–5.71 (d, 1H, H–CH
CH–COO−), 5.62–5.71 (d, 1H, H–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–COO−), 5.11–5.18 (d, 1H, H–CH
CH–COO−), 5.11–5.18 (d, 1H, H–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–COO−), 4.16 (t, 2H, N–CH2), 3.84 (s, 3H, N–CH3), 1.76 (m, 2H, CH2),1.22 (br. s, 22H, N–CH2–CH2–(–CH2–)11–), 0.87 (t, 3H, CH3).
CH–COO−), 4.16 (t, 2H, N–CH2), 3.84 (s, 3H, N–CH3), 1.76 (m, 2H, CH2),1.22 (br. s, 22H, N–CH2–CH2–(–CH2–)11–), 0.87 (t, 3H, CH3).
TDMImVP. 1H NMR (400 MHz, DMSO-d6): δ = 9.35 (s, 1H, NC(H)N), 7.71–7.80 (d, 2H, NC(H)C(H)N), 5.92–6.05 (m, 1H, H–CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–PO3−), 5.45–5.58 (m, 1H, H–CH
CH–PO3−), 5.45–5.58 (m, 1H, H–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–PO3−), 5.23–5.45 (m, 1H, H–CH
CH–PO3−), 5.23–5.45 (m, 1H, H–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–PO3−), 4.16 (t, 2H, N–CH2), 3.85 (s, 3H, N–CH3), 1.78 (m, 2H, CH2),1.24 (br. s, 22H, N–CH2–CH2–(–CH2–)11–), 0.87 (t, 3H, CH3).
CH–PO3−), 4.16 (t, 2H, N–CH2), 3.85 (s, 3H, N–CH3), 1.78 (m, 2H, CH2),1.24 (br. s, 22H, N–CH2–CH2–(–CH2–)11–), 0.87 (t, 3H, CH3).
Polymerization
NIPAM (13 mmol) was polymerized using AIBN as an initiator (1 mol% or 5 mol%) in THF for 12 h at 65 °C. After polymerization, the solvent was removed and the polymer was precipitated in cold diethyl ether. The polymers were dried under vacuum for 5 h at 50 °C.Random copolymerization of each ionic liquid with NIPAM was performed by free radical polymerization as shown in Scheme 2. In a typical procedure, NIPAM (13 mmol), ionic liquid (1.3 mmol) and AIBN (1 mol% or 5 mol%) were added under N2 in a 35 mL round neck test tube equipped with a magnetic stirrer. Thereafter, dehydrated THF (5 mL) was added into the reaction mixture and the test tube was placed in a preheated oil bath maintained at 65 °C for 12 h. Then the reaction mixture was quenched by cooling under liquid nitrogen. Copolymers were purified by reprecipitating in cold diethyl ether. Then, the solvent was removed by decantation and the polymers were dried under vacuum for 5 h at 50 °C. All polymerizations were carried out under a nitrogen atmosphere.
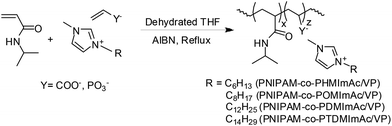 | ||
| Scheme 2 Synthesis of random copolymers of NIPAM and ionic liquids via free radical copolymerization. | ||
Polymer characterization
Polymer structures were characterized by analysing 1H NMR spectra (400 MHz) in CDCl3. The cloud point measurements were performed with an aqueous polymer solution (5 mg mL−1) using a UV-Vis spectrometer equipped with a temperature controller system. The UV-Vis transmission properties of the solutions were studied in the temperature range of 20 to 60 °C to determine the precise cloud point temperature.A series of copolymers were synthesized via free-radical copolymerization between NIPAM with a variety of ionic liquids. The amount of radical initiator (AIBN) varied between 1 mol% and 5 mol%. The polymerization results are summarized in Table 1. It was observed that the conversion rate for polymerization was predominantly around 90%. However, it was found that the conversion rate at 1 mol% of AIBN was invariably lower than 5 mol%. The copolymers were characterized by 1H NMR operated at 400 MHz. It was found that when the ionic liquid polymerized with NIPAM, vinyl protons of IL and NIPAM disappeared from the olefin region. The amount of NIPAM units incorporated in the copolymers was calculated from the 1H NMR spectra by considering per unit incorporation of IL as indicated by ‘a’ in Fig. 2(c).
| Chain length | AIBN | PNIPAM-co-PIL_Ac | PNIPAM-co-PIL_VP | ||||
|---|---|---|---|---|---|---|---|
| Yielda (%) | NIPAM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ILb ILb |
T cp | Yielda (%) | NIPAM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ILb ILb |
T cp | ||
| a (Experimental yield × 100)/theoretical yield. b Incorporation ratio of NIPAM and IL (calculated by 1H NMR spectroscopy). c After the addition of a chaotropic agent (0.5 M KBr salt). d Not determined. | |||||||
| 14 | 1 | 44.6 | — | — | 43.6 | — | — |
| 5 | 88.7 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
24.6 | 95.3 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
25.5 | |
| 12 | 1 | 87.8 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
28.0 | 98.0 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
33.0 |
| 5 | 92.6 | 09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
28.2 | 99.5 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
32.0 | |
| 8 | 1 | 97.8 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
50.0 | 94.7 | 07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
40.0 |
| 5 | 98.9 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
50.0 | n.d.d | 07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
38.5 | |
| 6 | 1 | 88.4 | 09![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
54.0c | 96.8 | 07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
44.0 |
| 5 | 93.9 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
56.0c | 98.9 | 08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 01 01 |
45.0 | |
| PNIPAM | |||||||
| 0 | 1 | 93.3 | — | 31.5 | |||
| 5 | 98.7 | — | 31.0 | ||||
The typical number average molecular weights (Mn) of the two polymers namely PNIPAM-co-DMImAc_5 mol% AIBN and PNIPAM-co-DMImVP_5 mol% AIBN were determined using GPC and were found to be 6200 g mol−1 and 2800 g mol−1 respectively. The difference between the Mn of the two polymers can be due to the different interactions of different anions (acrylate and vinylphosphonate) with propagating radicals.
Lower critical solution temperature measurements
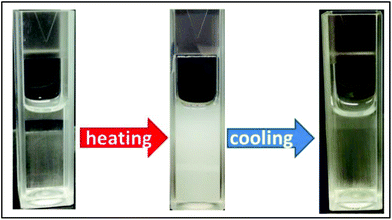
The cloud point temperature of PNIPAM depends on the degree of hydrogen bonding with water. Therefore, it was expected that the chemical incorporation of an ionic liquid in the PNIPAM chain could modify hydrogen bonding and consequently regulate the cloud point temperature. The lower critical solution temperature (LCST) transition behavior of polymer solutions was studied through UV-Vis measurements at various temperatures and is discussed in the subsequent section.The cloud point temperature of the solution was determined by studying the UV-Vis transmittance at 563 nm and a fall in transmittance to 50% of the initial value was noted as the Tcp of the polymer. Fig. 4 and 5 show the LCST behavior of random copolymers of NIPAM and IL and Fig. 3 shows their reversible nature.
Results and discussion
Several parameters can influence the LCST behavior of these copolymers: (1) the effect of hydrophobicity induced by the cation structure in IL and (2) the effect of the counter anion in IL. The influences of such parameters on LCST behavior are discussed in the subsequent sections.Effect of the concentration of the initiator (AIBN)
The LCST behaviors of random copolymers obtained with the varied AIBN amounts were found to be nearly similar (Fig. 4 and 5). This is evident from the almost similar incorporation ratios of IL and NIPAM in the random copolymer, as confirmed by NMR measurements. As no significant differences were observed with the change in the concentration of the initiator, subsequent studies will be carried out for copolymers prepared with 5 mol% AIBN as the initiator unless mentioned otherwise.Effect of cation (hydrophobicity)
Fig. 4(a) and (b) show the LCST behavior of random copolymers prepared by structurally varying the length of the alkyl chain in the cationic part of IL, while the counter anion (acrylate) was maintained the same. The hydrophobicity of the copolymer was varied with respect to the alkyl-chain attached to N-1 (or sometimes N-3) of the imidazolium ring of the IL. The hydrophobicity increased with the increasing alkyl chain length. The LCST behavior of all copolymers is summarized in Table 1. It was found that changing the kind of ionic liquid in the copolymer provides a broad range of LCST distributions both below and above the characteristic Tcp of PNIPAM (31 °C). For instance, it was observed that the LCST transitions of PNIPAM-co-PTDMImAc (25 °C) and PNIPAM-co-PDMImAc (28 °C) were lower than PNIPAM (31 °C). On the other hand, the LCST of PNIPAM-co-POMImAc (50 °C) and PNIPAM-co-PHMImAc (>90 °C) were higher than PNIPAM. Introducing hydrophobic molecules/moieties in water tends to minimize their surface energy by forming aggregates (hydrophobic effect24). Hence a larger hydrophobic moiety offers greater interference with the hydrogen bonding. Moreover, an increase in the surface tension between polymer/water interfaces leads to a lower LCST. LCST of the acrylate based copolymers was found to increase in the order of the alkyl chain length on the IL unit tetradecyl < dodecyl < octyl. The copolymer containing a hexyl chain had a high Tcp above 90 °C. Similar trends (Fig. 5(a) and (b)) were observed for other copolymers (having vinyl phosphonate as the anion) with their corresponding cloud point temperatures in the order of the alkyl chain length on the IL unit tetradecyl < dodecyl < octyl < hexyl.These observations (Fig. 4 and 5) clearly indicate the effect of hydrophobicity induced by the alkyl-chain of ILs. The results can be envisaged in terms of the interference of the hydrophobic interaction with the hydrogen bonding of the main polymer chain between two separate moieties: (1) hydrogen bonding between PNIPAM chains and water and (2) hydrogen bonding of the acrylate anion with water molecules.
Effect of chaotropic agent
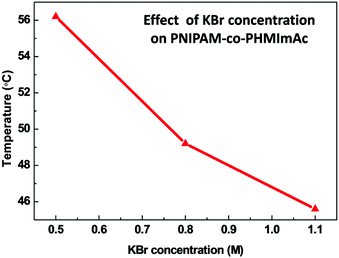
Chaotropic agents are salts, particularly anions, which lower the Tcp of a polymer solution when added in an excess amount.17 The hexyl group containing acrylate copolymer PNIPAM-co-PHMImAc showed specific LCST behavior. The Tcp of this copolymer was found to be higher than 90 °C (beyond the experimental range of measurement) which led us to investigate the effect of the excess of chaotropic agent. It is widely known that the excess of chaotropic agent assists in the disruption of macromolecular intra/intermolecular interaction networks.17 The LCST behavior of PNIPAM-co-PHMImAc was observed at 56 °C through the addition of a chaotropic agent solution (KBr, 0.5 M). The effect of chaotropic agent concentration on the LCST value is shown in Fig. 6. Hence, more precise modulation of LCST was achieved through the addition of chaotropic agents.Effect of counter anion
It was observed that the LCST behavior varied by changing the kind of anion, namely acrylate and vinyl phosphonate. Fig. 5(a) shows the results of LCST behavior of random copolymers having vinyl phosphonate as the anion at 1 mol% of AIBN. It was observed that all the vinyl phosphonate based copolymers show a higher cloud point temperature than PNIPAM except for PNIPAM-co-PTDMImVP. On the other hand, quite complex behavior was observed for the acrylate based copolymers (Fig. 4(a)). Tetradecyl- and dodecyl-containing acrylate based copolymers show a relatively lower Tcp than the corresponding vinyl phosphonate based copolymers. On the contrary, octyl- and hexyl-containing acrylate based copolymers show a higher Tcp than the corresponding vinyl phosphonate based copolymers as shown in Fig. 7. The obtained results can be understood on the basis of two competing effects i.e. primary and secondary effects. The primary effects originate from the widely known acid–base theory, i.e. a stronger base possesses a higher tendency to form hydrogen bonding and vice versa, while the secondary effects accompany hydrophobic interactions between the ions originating from the bulkier substituent which results in weaker hydrogen bonding. In the case of tetradecyl- and dodecyl-containing acrylate based copolymers, the secondary effects dominate owing to the presence of the bulky alkyl chain. Interestingly, the secondary effects were less effective in the case of the corresponding vinyl phosphonate based copolymers. These observations can be attributed to the better dimensional flexibility of hydrogen bonding due to the greater charge distribution sphere of vinyl phosphonates than acrylates. That is, more flexible hydrogen bonding is possible for vinyl phosphonates under different conformations of the cation. Beyond the C10-alkyl chain length the anionic effect becomes less dominant and exhibits converging behaviour irrespective of the anion type. | ||
Fig. 7 Synergistic correlation between hydrophobicity with the counter anion structure on the LCST behavior of acrylate ( , ,  ) and vinyl phosphonate based random copolymers ( ) and vinyl phosphonate based random copolymers ( , ,  ). ). | ||
On the other hand, octyl- and hexyl-containing acrylate based copolymers show higher Tcp than the corresponding vinyl phosphonate based copolymers. These results can be comprehended in terms of the dominant primary effects. Obviously, reduction in the length of the alkyl chain suppresses the secondary effects and offers a greater interaction of the chemical entities with the surroundings. Moreover, it is known that acrylic acid (pKa = 4.52) is a weaker acid compared to vinylphosphonic acid (pKa = 2.74)25 and consequently acrylates (strong conjugate base) offer stronger hydrogen bonding and higher Tcp. Furthermore, octyl- and hexyl-containing acrylate based copolymers offer lower steric shielding effects and facilitate stronger hydrogen bonding, respectively than the corresponding vinyl phosphonate based copolymers.
Conclusions
PNIPAM and its random copolymers with various kinds of ILs were synthesized and their LCST behavior was systematically elucidated. It was found that the LCST behavior of the synthesized random copolymers was greatly affected by varying the kind of IL in terms of their hydrophobicity and counter anion structure. It was found that both these factors synergistically affected the LCST behavior of these random copolymers. This study contributes toward the profound understanding of the LCST behavior of NIPAM-IL based random copolymers and will be useful for developing custom designed thermo-sensitive materials.References
- R. Liu, M. Fraylich and B. R. Saunders, Colloid Polym. Sci., 2009, 287, 627–643 CAS.
- A. S. Hoffman, Artif. Organs, 1995, 19, 458–467 CrossRef CAS PubMed.
- M. Koyama, T. Hirano, K. Ohno and Y. Katsumoto, J. Phys. Chem. B, 2008, 112, 10854–10860 CrossRef CAS PubMed.
- M. Heskins and J. E. Guillet, J. Macromol. Sci., Part A: Pure Appl. Chem., 1968, 2, 1441–1455 CrossRef CAS PubMed.
- G. Fundueanu, M. Constantin and P. Ascenzi, Int. J. Pharm., 2009, 379, 9–17 CrossRef CAS PubMed.
- P. S. Stayton, T. Shimoboji, C. Long, A. Chilkoti, G. H. Chen and J. M. Harris, Nature, 1995, 378, 472–474 CrossRef CAS PubMed.
- H. Akiyama and N. Tamaoki, Macromolecules, 2007, 40, 5129–5132 CrossRef CAS.
- T. Hoare and R. Pelton, Macromolecules, 2007, 40, 670–678 CrossRef CAS.
- A. Kondo, T. Kaneko and K. Higashitani, Biotechnol. Bioeng., 1994, 44, 1–6 CrossRef CAS PubMed.
- H. G. Schild and D. A. Tirrel, J. Phys. Chem., 1990, 94, 4352–4356 CrossRef CAS.
- S. Fujishige, K. Kubota and I. Ando, J. Phys. Chem., 1989, 93, 3311–3313 CrossRef CAS.
- C. Wu and S. Zhou, Macromolecules, 1995, 28, 8381–8387 CrossRef CAS.
- H. Feil, Y. H. Bae, J. Feijen and S. W. Kim, Macromolecules, 1992, 25, 5528–5530 CrossRef CAS.
- C. S. Brazel and N. A. Peppas, Macromolecules, 1995, 28, 8016–8020 CrossRef CAS.
- S. Zhou and B. Chu B, J. Phys. Chem. B, 1998, 102, 1364–1371 CrossRef CAS.
- M. D. Determan, J. P. Cox, S. Seifert, P. Thiyagarajan and S. K. Mallapragada, Polymer, 2005, 46, 6933–6946 CrossRef CAS PubMed.
- K. V. Durme, H. Rahier and B. V. Mele, Macromolecules, 2005, 38, 10155–10163 CrossRef.
- W. McPhee, K. C. Tam and R. Pelton, J. Colloid Interface Sci., 1993, 156, 24–30 CrossRef CAS.
- Y. Maeda and M. Yamabe, Polymer, 2009, 50, 519–523 CrossRef CAS PubMed.
- A. J. Convertine, B. S. Lokitz, Y. Vasileva, L. J. Myrick, C. W. Scales, A. B. Lowe and C. L. McCormick, Macromolecules, 2006, 39, 1724–1730 CrossRef CAS.
- E. Karjalainen, N. Chenna, P. Laurinmaki, S. J. Butcher and H. Tenhu, Polym. Chem., 2013, 4, 1014–1024 RSC.
- S. V. Dzyuba and R. A. Bartsch, J. Heterocycl. Chem., 2001, 38, 265–268 CrossRef CAS PubMed.
- F. Fukaya, A. Sugimot and H. Ohno, Biomacromolecules, 2006, 7, 3295–3297 CrossRef PubMed.
- N. T. Southall, K. A. Dill and A. D. J. Haymet, J. Phys. Chem. B, 2002, 106, 521–533 CrossRef CAS.
- B. Bingöl, W. H. Meyer and G. Wegner G, Polym. Prepr., 2007, 48, 144–145 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |