A metal–organic framework with a 9-phenylcarbazole moiety as a fluorescent tag for picric acid explosive detection: collaboration of electron transfer, hydrogen bonding and size matching†
Xiaoli Jiang‡
,
Yanhong Liu‡,
Pengyan Wu*,
Lei Wang,
Qiyuan Wang,
Guangzhou Zhu,
Xiu-ling Li and
Jian Wang*
School of Chemistry and Chemical Engineering & Jiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials, Jiangsu Normal University, Xuzhou, Jiangsu 221116, P. R. China. E-mail: wpyan@jsnu.edu.cn; wjian@jsnu.edu.cn
First published on 17th September 2014
Abstract
We report here an electron-rich luminescent metal–organic framework (MOF), Zn–PDA, displaying high selectivity in the detection of PA (picric acid) explosive as a fluorescent sensor, which can serve as the first case of MOFs for detecting PA through synergistic effects including electron transfer, hydrogen-bonding and size matching.
Picric acid (PA) is well known for its wide use as an explosive until World War I. Detection of PA by simple and inexpensive methods is an issue of international concern, due to the increasing use of explosive materials in terrorism, military operation safety, industrial, and environmental safety control.1 A great variety of sophisticated instruments like ion mobility spectroscopy (IMS), X-ray dispersion, Raman spectroscopy, etc., as well as canines, are currently being employed to detect nitro explosives.2,3 The above techniques are limited portability, high cost, and these instruments need frequent careful calibrations, which restrict them from widespread use. Among these competing methods, fluorescence-based detection is gaining increasing attention owing to its high sensitivity, simplicity, easy visualization, and real-time monitoring with fast response time.4,5 And numerous fluorescent conjugated compounds have been synthesized, and are used in the detection of PA.6 Its main mechanism is the photo-induced donor–acceptor electron-transfer from the excited compound to PA.7,8 Since PA molecular not only has the electron-deficient nitro group, but also has hydroxyl group, ascertaining the value of hydroxyl group in PA structure is very important for PA related explosives detection.
As a new class of crystalline porous materials, metal–organic frameworks (MOFs), composed of metal ions (or cluster) as nodes and multi-topic organic ligands as linkers have received tremendous attention not only owing to their fascinating structures9 but also for their potential applications in gas storage,10 chemical separation,11 optical sensing/detection,12 catalysis13 and drug delivery,14 and as nonlinear optical,15 ferroelectric16 and magnetic materials.17 The rich delocalized π-electron framework, the functional surface, as well as the confinement of analytes inside the MOF pores allow MOFs to act as fast, reversible and highly sensitive chemosensors. 9-Phenylcarbazole is a typically versatile functional material that exhibit good hole-transport capabilities, efficient light emission, and excellent electron-donating properties.18 As such, it is incorporated as the bridging ligands to create conjugated MOFs with advanced architectures and excellent properties. Through incorporating 9-phenylcarbazole-3,6-dicarboxylic acid (H2PDA) as the linker and an efficient luminescent emission group, we created luminescent metal–organic framework Zn–PDA. It is reasoned that the 9-phenylcarbazole moiety located around the cavity of MOFs impart electron-rich and fluorescent properties to MOFs, the new MOFs can be utilized as molecular sensors for the detection of nitro explosives (Scheme 1).
 | ||
| Scheme 1 Potential luminescence detection process between designed metal–organic framework and picric acid (PA). | ||
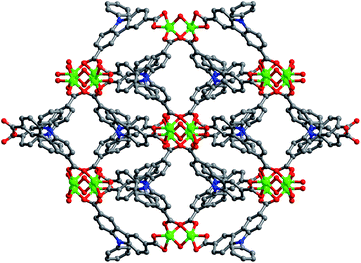
Solvothermal reaction of H2PDA and Zn(NO3)2·6H2O in mixed solvents of DMF and methanol gave compounds Zn–PDA in a high yield (75%). Elemental analyses and powder X-ray analysis indicated the pure phase of their bulky sample. Single crystal X-ray diffraction study revealed that Zn–PDA crystallizes in a monoclinic space group C2/c. The asymmetric unit contains one Zn2+ ion, one PDA2− ligand, one half coordinated and two and a half solvated water molecules and one methanol molecule. It was composed of paddle wheel dinuclear Zn2 units with a short Zn–Zn internuclear separation of 3.0028 (9) Å, bridged by four PDA2−. Each Zn ion is coordinated by five oxygen atoms: two oxygen atoms from one bidentate carboxylate group of one PDA2− anion, two oxygen atoms from one bimonodentate carboxylate group of two different PDA2− anions, and one oxygen atom from one bridge water, representing distorted trigonal-biyramidal geometry.19 Moreover, each PDA2− ligand connected two dinuclear Zn2 units to form a 1D chain, the adjacent chain is further associated together through π–π stacking (spacing 3.46 Å) to give a 2D layered structure. There exist one-dimensional rhombus channels of about 10.7 × 6.6 Å2 along the c axis (Fig. 1). The void space accounts approximately 43.9% of the whole crystal volume (2238.0 Å3 out of the 5101.1 Å3 per unit cell volume) by PLATON analysis. The thermogravimetric analysis (TGA) curve of Zn–PDA showed a weight loss of 14.3% between 23 and 380 °C, which corresponds to the loss of one methanol and two and a half guest H2O molecules (calculated 14.45%), indicating that the framework is robust, thus the highly open channels permits the accessibility of the small molecular.§
UV-vis absorption spectrum of Zn–PDA in the solid state exhibited an absorption band centered at 342 nm typically assignable to the π–π* transition of the 9-phenylcarbazole group.20 Upon excitation at this absorption band, Zn–PDA showed an intense luminescence band at about 366 nm. Fluorescence detection experiments were carried out with the ethanol suspension of compound Zn–PDA. As shown in Fig. 2b, different aromatic explosives have different effects on the fluorescence intensity of the dispersed solution of Zn–PDA in ethanol. The fluorescence intensity of Zn–PDA suspension was almost unchanged upon addition of analytes such as benzene (B), toluene (T) and aniline (A). But significant quenching of fluorescence intensity was observed upon addition of nitroaromatics such as nitrobenzene (NB), 2,4-dinitrotoluene (DNT), 2,4,6-trinitrotoluene (TNT) and picric acid (PA). The quenching of the emission intensity of Zn–PDA in one case was ascribed to a photo-induced electron transfer from the excited state of the complex to the nitroaromatic compound. Importantly, the most intense quenching was observed for PA. Fast and high fluorescence quenching was observed upon addition of PA solution into ethanol suspension of Zn–PDA (250 ppm) up to 0.35 mM. The Stern–Volmer constant Ksv was determined to be 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502 M−1 by fitting the linear plot to the Stern–Volmer equation I0/I = 1 + Ksv[PA], which is comparable to known organic polymers.21 EC50 (the concentration of guest added that half quenched the luminescence) is about 30 μM. The high quenching effect coefficient and the low detection concentration for PA allowed us to easily identify the existence of a small amount of explosive in solution. Another interesting point noticed is that a marked difference was observed in the quenching ability of PA and TNT, where the former displays an almost 1.5 times greater quenching efficiency compared to TNT, although PA and TNT have only one position difference in their structures, hydroxyl substituent for PA and methyl substituent for TNT, indicating that the hydroxyl group of PA has a great contribution for the good selectivity. This may be attributed to strong supramolecular interactions (e.g. hydrogen bonding) between the Zn–PDA framework and the PA molecules. This order would have been reversed if the analyte-receptor electron transfer interaction was the only mechanism.7
502 M−1 by fitting the linear plot to the Stern–Volmer equation I0/I = 1 + Ksv[PA], which is comparable to known organic polymers.21 EC50 (the concentration of guest added that half quenched the luminescence) is about 30 μM. The high quenching effect coefficient and the low detection concentration for PA allowed us to easily identify the existence of a small amount of explosive in solution. Another interesting point noticed is that a marked difference was observed in the quenching ability of PA and TNT, where the former displays an almost 1.5 times greater quenching efficiency compared to TNT, although PA and TNT have only one position difference in their structures, hydroxyl substituent for PA and methyl substituent for TNT, indicating that the hydroxyl group of PA has a great contribution for the good selectivity. This may be attributed to strong supramolecular interactions (e.g. hydrogen bonding) between the Zn–PDA framework and the PA molecules. This order would have been reversed if the analyte-receptor electron transfer interaction was the only mechanism.7
In order to verify our speculation for the mechanism of high selective PA sensing in the MOF Zn–PDA, fluorescence titrations were further performed with other hydroxyl-containing nitroaromatic moieties, such as p-nitrophenol (p-NP), m-nitrophenol (m-NP) and o-nitrophenol (o-NP). Interestingly, quenching efficiency of fluorescence intensity were different, p-NP shows the nearly same quenching responses as PA, while in the presence of m-NP and o-NP the fluorescence change was much smaller than that of p-NP. These results demonstrated that only nitrophenol with suitable configuration has the potential to make hydroxyl groups to interact with the framework through hydrogen bonding. Furthermore, we replace the –OH groups in nitroaromatics with –NH2 (the potential hydrogen-bond donor) and –CH3, the amino substituent molecular p-nitroaniline (p-NA), m-nitroaniline (m-NA), o-nitroaniline (o-NA) and the methyl substituent molecular p-nitrotoluene (p-NT), m-nitrotoluene (m-NT), o-nitrotoluene (o-NT) were measured as the analytes in the same condition. Importantly, a series of nitroaniline also display similar behavior like nitrophenol, otherwise, the quenching effect of the addition of nitrotoluene moiety was the similar to that of nitrobenzene, further demonstrating that existence of hydrogen-bonding type interaction in the p-site of nitro-group was important for the high sensitivity for explosives. However, in the presence of bulky nitrophenol HNBP, whose size is larger than the pore size of Zn–PDA as the analyte, the fluorescent quenching only gave 15% under the same detection conditions. The size selective sensing of the substrate suggested that the detection of PA occurred mostly in the channel of the MOF, not on the external surface. In addition, the infrared spectroscopy of Zn–PDA impregnated with an ethanol solution of p-NA exhibited red shift of the stretching vibrations and scissoring vibration of –NH2, not only suggesting the absorbance of p-NA in the channels of Zn–PDA, but also indicating the existence of hydrogen-bonding interaction between Zn–PDA and p-NA.22 Since the hydrogen-bonding interaction plays an important role in the PA detection, analyte with the richness in hydrogen-bonding sites, p-aminophenol (p-AP) was tested, the addition of p-AP hardly quenched the luminescence of the MOF significantly under the same experimental conditions. These results give a powerful proof for the selective detection of PA explosive was through synergistic effects including electron transfer, hydrogen-bonding interaction and size matching. To the best of our knowledge, Zn–PDA represents the first example of the MOF chemosensors for PA explosive in solution through multiple synergisms.
Conclusions
In conclusion, we successfully created a new luminescent MOFs Zn–PDA through incorporating 9-phenylcarbazole moiety as an efficient active site for the highly selective detection of the explosive PA. Comprehensive study makes the mechanism of their fluorescent quenching effect clearer, thus, synergistic effects including electron transfer, hydrogen-bonding and size matching, will supply the new strategy for designing and synthesizing new promising sensing materials for infield explosives monitoring. It is expected that this supramolecular synergistic effect coupled with appropriate site distribution enable detecting PA of MOFs with less interference and even industrial chemicals, improving application range in real application.Acknowledgements
This work was financial support from the NSFC (21401087, 21271091 and 21071121), the NSF of Jiangsu Province (BK20140234), and the open project of Jiangsu Key Laboratory of Green Synthetic for Functional Materials (K201305, K201306), the Major Basic Research Project of Natural Science Foundation of the Jiangsu Higher Education Institutions (11KJA430009), and Start-Up Fund of Jiangsu Normal University.Notes and references
- Y. Salinas, R. Martínez-Máñez, M. D. Marcos, F. Sancenón, A. M. Costero, M. Parra and S. Gil, Chem. Soc. Rev., 2012, 41, 1261 RSC; S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC.
- A. W. Czarnik, Nature, 1998, 394, 417 CrossRef CAS PubMed.
- D. S. Moore, Rev. Sci. Instrum., 2004, 75, 2499 CrossRef CAS PubMed.
- M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543 RSC; S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Angew. Chem., Int. Ed., 2013, 52, 2881 CrossRef CAS PubMed.
- Y. M. Yang, Q. Zhao, W. Feng and F. Y. Li, Chem. Rev., 2013, 113, 192 CrossRef CAS PubMed; D. Banerjee, Z. C. Hu and J. Li, Dalton Trans., 2014, 43, 10668 RSC.
- K. K. Kartha, S. S. Babu, S. Srinivasan and A. Ajayaghosh, J. Am. Chem. Soc., 2012, 134, 4834 CrossRef CAS PubMed; A. Lan, K. Li, H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. Hong and J. Li, Angew. Chem., Int. Ed., 2009, 48, 2334 CrossRef PubMed.
- B. Gole, S. Shanmugamraju, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 10046 RSC; S. R. Zhang, D. Y. Du, J. S. Qin, S. J. Bao, S. L. Li, W. W. He, Y. Q. Lan, P. Shen and Z. M. Su, Chem.–Eur. J., 2014, 20, 3589 CrossRef CAS PubMed.
- Y. Che, D. E. Gross, H. Huang, D. Yang, X. Yang, E. Discekici, Z. Xue, H. Zhao, J. S. Moore and L. Zang, J. Am. Chem. Soc., 2012, 134, 4978 CrossRef CAS PubMed.
- M. Eddaoudi, D. B. Molar, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319 CrossRef CAS PubMed; O. R. Evans and W. Lin, Acc. Chem. Res., 2002, 35, 511 CrossRef PubMed; S. Kitagawa, R. Kitaura and S. I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef PubMed; G. Férey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 217 CrossRef PubMed; O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166 CrossRef PubMed.
- S. Yang, X. Lin, A. J. Blake, G. S. Walker, P. Hubberstry, N. R. Champness and M. Schröder, Nat. Chem., 2009, 1, 487 CrossRef CAS PubMed; L. J. Murray, M. Dinča and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC.
- Y. Lan, H. Jiang, S. Li and Q. Xu, Adv. Mater., 2011, 23, 43 CrossRef PubMed; S. Xiang, Z. Zhang, C. Zhao, K. Hong, X. Zhao, D. Ding, M. Xie, C. Wu, M. C. Das, R. Gill, K. M. Thomas and B. Chen, Nat. Commun., 2011, 2, 204 CrossRef PubMed.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC; Y. Takashima, V. M. Martinez, S. Furukawa, M. Kondo, S. Shimomura, H. Uehara, M. Nakahama, K. Sugimoto and S. Kitagawa, Nat. Commun., 2011, 2, 168 CrossRef CAS PubMed; Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126 CrossRef PubMed.
- F. Song, C. Wang, J. M. Falkowski, L. Ma and W. Lin, J. Am. Chem. Soc., 2010, 132, 15390 CrossRef CAS PubMed; L. Ma, C. D. Wu, M. M. Wanderley and W. Lin, Nat. Chem., 2010, 2, 838 CrossRef PubMed.
- J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2009, 131, 8376 CrossRef CAS PubMed; K. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tranand and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261 CrossRef PubMed.
- C. Wang, T. Zhang and W. Lin, Chem. Rev., 2012, 112, 1084 CrossRef CAS PubMed.
- T. Hang, W. Zhang, H. Y. Ye and R. G. Xiong, Chem. Soc. Rev., 2011, 40, 3577 RSC; W. Zhang and R. G. Xiong, Chem. Rev., 2012, 112, 1163 CrossRef CAS PubMed.
- Y. Z. Zheng, W. Xue, S. L. Zheng, M. L. Tong and X. M. Chen, Adv. Mater., 2008, 20, 1534 CrossRef CAS.
- S. H. Kim, I. Cho, M. K. Sim, S. Park and S. Y. Park, J. Mater. Chem., 2011, 21, 9139 RSC; S. K. Chiu, Y. C. Chung, G. S. Liou and Y. O. Su, J. Chin. Chem. Soc., 2012, 59, 331 CrossRef CAS.
- S. L. Zheng, J. H. Yang, X. L. Yu, X. M. Chen and W. T. Wong, Inorg. Chem., 2004, 43, 830 CrossRef CAS PubMed; M. Eddaoudi, J. Kim, D. Vodak, A. Sudik, J. Wachter, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4900 CrossRef PubMed.
- Z. G. Zhu and J. S. Moore, J. Org. Chem., 2000, 65, 116 CrossRef CAS PubMed.
- N. Sabbatini, M. Guardigli and J.-M. Lehn, Coord. Chem. Rev., 1993, 123, 201 CrossRef CAS.
- U. Okwieka, K. H. Natkaniec, T. Misiaszek, W. Medycki, J. Baran and M. M. Szostak, J. Chem. Phys., 2009, 131, 144505 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and related spectra. CCDC 1009908. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra07067d |
| ‡ These authors contributed equally to this work. |
| § Crystal data of Zn–PDA: C21H11NO8Zn, M = 470.68, monoclinic, space group C2/c, a = 25.951(3), b = 11.8662(14), c = 20.436(4) Å, α = 90.00, β = 125.847(6), γ = 90.00, V = 5101.1(13) Å3, Z = 8, Dc = 1.226 g cm−3, μ(Mo-Kα) = 0.71073 mm−1, T = 296(2) K. 4481 unique reflections [Rint = 0.0773]. Final R1[with I > 2σ(I)] = 0.0758, wR2 (all data) = 0.2699, GOOF = 1.002. |
| This journal is © The Royal Society of Chemistry 2014 |