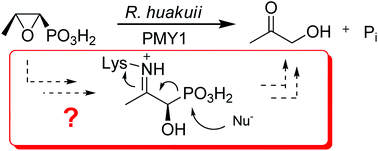
Three functionalised propylphosphonic acids were synthesised to study C–P bond cleavage in R. huakuii PMY1. (R)-1-Hydroxy-2-oxopropylphosphonic acid [(R)-5] was prepared by chiral resolution of (±)-dimethyl 1-hydroxy-2-methylallyllphosphonate [(±)-12], followed by ozonolysis and deprotection. The N-(L-alanyl)-substituted (1R,2R)-2-amino-1-hydroxypropylphosphonic acid 10, a potential precursor for 2-oxopropylphosphonic acid (5) in cells, was obtained by coupling the aminophosphonic acid with benzotriazole-activated Z-L-alanine and hydrogenolytic deprotection. (1R*,2R*)-1,2-Dihydroxy-3,3,3-trifluoropropylphosphonic acid, a potential inhibitor of C–P bond cleavage after conversion into its 2-oxo derivative in the cell, was accessed from trifluoroacetaldehyde hydrate via hydroxypropanenitrile 21, which was silylated and reduced to the aldehyde (±)-23. Diastereoselective addition of diethyl trimethylsilyl phosphite furnished diastereomeric α-siloxyphosphonates. The less polar one was converted to the desired racemic phosphonic acid (±)-(1R*,2R*)-9 as its ammonium salt.