Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Compositional flexibility in irreducible antifluorite electrolytes for next-generation battery anodes†
Victor
Landgraf
 ,
Mengfu
Tu
,
Zhu
Cheng
,
Alexandros
Vasileiadis
,
Mengfu
Tu
,
Zhu
Cheng
,
Alexandros
Vasileiadis
 ,
Marnix
Wagemaker
,
Marnix
Wagemaker
 * and
Theodosios
Famprikis
* and
Theodosios
Famprikis
 *
*
Faculty of Applied Sciences, Delft University of Technology, 2629 JB Delft, The Netherlands. E-mail: t.famprikis@tudelft.nl; m.wagemaker@tudelft.nl
First published on 23rd December 2024
Abstract
Solid-state batteries currently receive ample attention due to their potential to outperform lithium-ion batteries in terms of energy density when featuring next-generation anodes such as lithium metal or silicon. One key remaining challenge is identifying solid electrolytes that combine high ionic conductivity with stability in contact with the highly reducing potentials of next-generation anodes. Fully reduced electrolytes, based on irreducible anions, offer a promising solution by avoiding electrolyte decomposition altogether. In this study, we demonstrate the compositional flexibility of the disordered antifluorite framework accessible by mechanochemical synthesis and leverage it to discover irreducible electrolytes with high ionic conductivities. We show that the recently investigated Li9N2Cl3 and Li5NCl2 phases are part of the same solid solution of Li-deficient antifluorite phases existing on the LiCl–Li3N tie line with a general chemical formula of Li1+2xCl1−xNx (0.33 < x < 0.5). Using density functional theory calculations, we identify the origin of the 5-order-of-magnitude conductivity increase of the Li1+2xCl1−xNx phases compared to the structurally related rock-salt LiCl phase. Finally, we demonstrate that SCl- and BrCl-substituted analogues of the Li1+2xCl1−xNx phases may be synthesized, enabling significant conductivity improvements by a factor of 10, reaching 0.2 mS cm−1 for Li2.31S0.41Br0.14N0.45. This investigation demonstrates for the first time that irreducible antifluorite-like phases are compositionally highly modifiable; this finding lays the ground for discovery of new compositions of irreducible antifluorite-like phases with even further increased conductivities, which could help eliminate solid-electrolyte decomposition and decomposition-induced Li losses on the anode side in high-performance next-generation batteries.
Introduction
Solid-state batteries have the potential to supersede conventional Li-ion batteries in terms of energy density and safety.1,2 Three main advantages of solid electrolytes are as follows. (1) The solid nature of solid electrolytes potentially enables bipolar stacking of individual cells, increasing the overall pack energy density. (2) Solid electrolytes are typically less flammable than liquid equivalents and thus safer. (3) Solid electrolytes are potentially better compatible with high-energy anodes such as silicon or metallic lithium.1,2 An initial challenge has been to develop solid electrolytes with sufficiently high ionic conductivities to compete with liquid electrolytes. In recent years, multiple derivatives of the argyrodite and Li10GeP2S12 solid electrolytes have been developed with Li-ion conductivities beyond 10 mS cm−1, thus exceeding the conductivities of their liquid equivalents.3–6 Nevertheless, the main issue with all known highly conducting (>1 mS cm−1) solid electrolytes is that they are generally not (electro-)chemically stable at the high potentials of typical Li-ion battery cathodes nor at low potentials of desirable high-capacity anodes such as silicon or lithium metal. The electrochemical instability of solid electrolytes with electrodes inevitably leads to electrolyte decomposition at the electrolyte–electrode interfaces.1,7 Electrolyte decomposition at the electrode interfaces causes lithium loss, formation of resistive interphases and contact loss between solid-electrolyte- and electrode particles, which are all directly linked to battery degradation and failure.8,9This study aims to design solid electrolytes that are highly conducting and thermodynamically stable against the low potentials of desirable high-capacity anodes such as lithium metal and silicon. Such electrolytes would eliminate (electro)chemical degradation on the anode side and thus eliminate complications associated with electrolyte degradation. With regards to thermodynamic stability at low potentials, fully reduced phases become immediately pertinent; i.e. phases in which the only cation present is Li and in which all anions are in their lowest permitted formal oxidation state and thus irreducible.10,11 Commonly known examples of such irreducible phases are the lithium binaries LiCl, LiBr, Li2S, LiI, LiF, Li3N and Li3P. While these phases all feature thermodynamic stability at the low potentials (<0 V vs. Li+/Li), they are fraught with low ionic conductivities (<10−6 mS cm−1), except for Li3N, which is reported to have a conductivity of 0.5 mS cm−1.10,12 New irreducible phases were recently discovered by exploring the tie lines between the above-listed binaries using mechanochemical synthesis. Examples include the Li2+xS1−xPx phases reported by Szczuka et al.11 and the Li2+xS1−xNx phases reported by Landgraf et al.;13 both systems reaching high conductivities of 0.2 mS cm−1. Additionally, the Li5NCl2 (ref. 10) and Li9N2Cl3 (ref. 14) phases existing on the Li3N–LiCl tie line were recently investigated. Li et al. demonstrated excellent stability against Li-metal, stability in dry air and high critical-current density for dendrite formation of 10 mA cm−2 for the Li9N2Cl3 phase.14 Additionally, excellent performance of Li9N2Cl3 in full cells was demonstrated where Li9N2Cl3 is used as an anolyte to protect the Li2.73Ho1.09Cl6 halide electrolyte against a Li metal anode.14 These results are promising, however an essential drawback remains the low room-temperature conductivity of Li9N2Cl3 which is reported to be 0.04 mS cm−1.14 Moreover, the mechanistic origin of the increased conductivity of Li9N2Cl3 phases compared to the structurally related rocksalt LiCl phase has not been established.
The present study develops compositional design strategies to improve the conductivity of Li9N2Cl3 through the following advances:
(1) Synthetically, we demonstrate that the antifluorite framework is compositionally flexible; Li-deficient and Li-excess antifluorite phases can be mechanochemically stabilized. We find that the previously reported Li5NCl2 and Li9N2Cl3 phases (ref. 15 and 16) are both members of the same solid solution of Li-deficient antifluorite phases on the Li3N–LiCl tie line with the general chemical formula of Li1+2xCl1−xNx with 0.33 < x < 0.5. Additionally, we show that Li1+2xCl1−xNx phases are compositionally highly modifiable; we synthesize SCl- and BrCl-substituted analogues boosting the ionic conductivities of Li1+2xCl1−xNx phases by an order of magnitude enabling conductivity enhancements up to 0.2 mS cm−1 for Li2.31S0.41Br0.14N0.45.
(2) Computationally, we explain how introducing nitrogen into the LiCl anionic-framework brings tetrahedral and octahedral lithium sites closer in energy so that vacant sites become energetically accessible for diffusion. Our analysis of diffusion bottlenecks resolving the effect of the local anion coordination shows that nitrogen widens diffusion bottlenecks further facilitating Li diffusion.
(3) Finally, we find through both experiments and computations that the oxidation limit of the irreducible antifluorite-like phases is compositionally tunable and generally higher compared to Li3N which may be a critical advantage to stabilize next-generation anodes.
Results and discussion
Our starting point for this work is our previous work on fully reduced electrolytes, showing the conductivity of Li2S could be highly improved by dissolving Li3N into the antifluorite Li2S phase.13 Dissolving Li3N in Li2S results in a series of phases where N and S share sites (Fig. S1†). A solid solution exists with the general chemical formula Li2+xS1−xNx (0 < x < ∼0.5), exhibiting an anion-disordered Li-rich antifluorite crystal structure (Fig. S1†) and showing much higher conductivity (>0.2 mS cm−1 for x = 0.5) than the Li2S host phase (10−6 mS cm−1).13 This motivated our investigation of whether a similar solid solution may be found between Li3N and LiCl (schematically illustrated in Fig. 1a), and examine their functional solid–electrolyte properties. To answer this question, Li3N and LiCl were mixed in different ratios, followed by a high-energy mechanochemical treatment (ball milling).A solid solution of Li-deficient antifluorite phases on the Li3N–LiCl tie line
Fig. 1 presents the results of the mechanochemical treatment of xLi3N + (1 − x)LiCl samples and their resulting structure and ionic conductivities. Fig. 1a presents schematically the crystal structures of the parent LiCl rocksalt phase and the target antifluorite phases, confirmed by the powder X-ray diffraction experiments shown in Fig. 1b. Attempting to dissolve small quantities of Li3N into LiCl did not result in a pure phase but a mixture of two cubic phases: LiCl and Li5NCl2. The stoichiometric formula of the latter may alternatively be written as Li1.66N0.33Cl0.66 (i.e. Li1+2xCl1−xNx with x = 0.33), and has been previously reported as a stable phase prepared by conventional solid-state synthesis.10,15 Only when the Li1.66N0.33Cl0.66 stoichiometry is reached, having a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 LiCl/Li3N ratio, a pure Li1.66N0.33Cl0.66 phase is observed (i.e. the LiCl phase is not observed in the X-ray diffraction pattern). The mixtures with a higher nitrogen composition than the Li1.66N0.33Cl0.66 phase (Li1+2xCl1−xNx with 0.33 < x < 0.5) show the same cubic Fm
1 LiCl/Li3N ratio, a pure Li1.66N0.33Cl0.66 phase is observed (i.e. the LiCl phase is not observed in the X-ray diffraction pattern). The mixtures with a higher nitrogen composition than the Li1.66N0.33Cl0.66 phase (Li1+2xCl1−xNx with 0.33 < x < 0.5) show the same cubic Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m diffraction pattern as the x = 0.33 phase but with an increasing shift towards larger scattering vector Q indicating a decreasing lattice parameter with increasing nitrogen content. When exceeding an overall stoichiometry of Li2N0.5Cl0.5 (i.e. x = 0.5) a second phase identified as β-Li3N is observed in the diffraction pattern while the lattice parameter of the first phase remains constant. As expected based on the smaller ionic radius of N3− compared to Cl− (1.46 vs. 1.81 Å),17 the lattice parameter of the Li1+2xCl1−xNx phases decreases with increasing nitrogen content, where the linear shift is in accordance with Vegard's law (Fig. 1c). A solid-solution region of cubic Li1+2xCl1−xNx phases is thus observed on the on the xLiCl–(1 − x)Li3N tie line for compositions between 0.33 < x < 0.5, i.e. between the end members Li1.66N0.33Cl0.66 and Li2N0.5Cl0.5.
m diffraction pattern as the x = 0.33 phase but with an increasing shift towards larger scattering vector Q indicating a decreasing lattice parameter with increasing nitrogen content. When exceeding an overall stoichiometry of Li2N0.5Cl0.5 (i.e. x = 0.5) a second phase identified as β-Li3N is observed in the diffraction pattern while the lattice parameter of the first phase remains constant. As expected based on the smaller ionic radius of N3− compared to Cl− (1.46 vs. 1.81 Å),17 the lattice parameter of the Li1+2xCl1−xNx phases decreases with increasing nitrogen content, where the linear shift is in accordance with Vegard's law (Fig. 1c). A solid-solution region of cubic Li1+2xCl1−xNx phases is thus observed on the on the xLiCl–(1 − x)Li3N tie line for compositions between 0.33 < x < 0.5, i.e. between the end members Li1.66N0.33Cl0.66 and Li2N0.5Cl0.5.
Rietveld refinements of the X-ray diffractograms (ESI Fig. S2 and Tables S1–4†) show that the crystal structure of the Li1+2xCl1−xNx (x < 0.33 < 0.5) phases consist in a face-centered-cubic arrangement of the anions where N3− and Cl− share the same site. At the nitrogen-poor boundary of the solid solution (i.e. Li1.66N0.33Cl0.66) the tetrahedral interstitials are partially occupied by Li ions (83%).‡ With increasing N content the Li content increases to balance the charge, and the tetrahedral sites become increasingly populated until reaching the N-rich solid-solution boundary (i.e. Li2N0.5Cl0.5). Consequently, where the Li2N0.5Cl0.5 phase can be described as a (stoichiometric) antifluorite phase (with fully occupied tetrahedral sites), the solid-solution members with (x < 0.5) may be described as Li-deficient antifluorite phases (having only partially occupied tetrahedral sites). Further lithium insertion (x > 0.5) seems to destabilize the antifluorite, leading to decomposition into a mixture of Li2Cl0.5N0.5 and Li3N (Fig. 1b). This is in contrast to the lithium-rich antifluorites Li2+xS1−xNx which exhibit partial occupation of their octahedral sites in addition to full occupation of their tetrahedral sites (vide infra). The structural shift that occurs when going from rock-salt LiCl to Li-deficient antifluorite Li1.66N0.33Cl0.66 entails a significant increase of the cubic lattice parameter from 5.17 Å to 5.39 Å (Fig. 1b), which is likely related to the rearrangement of lithium from the octahedral sites (in the former) to tetrahedral sites (in the latter).
Fig. 1c also shows the ambient-temperature ionic conductivity of the mechanochemically prepared samples as quantified by impedance spectroscopy experiments on pelletized samples which could be invariably fitted by a single bulk-diffusion process (ESI Fig. S3†). The rock-salt LiCl phase has a conductivity of the order of 10−7 mS cm−1 (Fig. S4†). The conductivity of Li-deficient antifluorite Li1.66N0.33Cl0.66 is significantly higher reaching 0.01 mS cm−1, and introducing more nitrogen into the solid solution further increases the conductivity up to 0.03 mS cm−1 for Li2N0.5Cl0.5 as shown in Fig. 1c.
We have thus discovered a new solid solution on the LiCl–Li3N tie line with the general formula Li1+2xCl1−xNx (x < 0.33 < 0.5) accessible by mechanochemistry (in contrast to conventional solid state synthesis previously explored in ref. 10 and 15). These phases crystallize in an anion disordered Li-deficient antifluorite-like crystal structure and their conductivity is orders of magnitude higher than the conductivity of rock-salt LiCl. In the next section, we will analyze the mechanistic origin of this 5-order-of-magnitude boost in ionic conductivity.
Rock-salt LiCl vs. Li-deficient antifluorite Li1+2xCl1−xNx – origin of the improved conductivity
Fast Li diffusion relies on two prerequisites: (1) Li sites connected by low Li-hop activation energies into a percolating network and (2) a sufficient fraction of vacancies among these Li sites. Vacant Li-sites may be introduced by defects (e.g. Frenkel defect pairs) and/or may be synthetically introduced via compositional tuning. To investigate Li diffusion in (defect-free) rock-salt LiCl and Li-deficient antifluorite Li1+2xCl1−xNx phases we performed ab initio molecular dynamics (AIMD) simulations of 2 × 2 × 2 supercells. As done in previous studies, we dissected our AIMD simulations into individual jump events.10,18–21 From the frequency of jumps between two sites A and B (vA→B), we calculate jump-activation energies (jump-Ea) by using eqn (1) and assuming a prefactor frequency (ν0) of 1013 Hz: § | (1) |
| ΔEsite(A, B) = jump-Ea,A→B − jump-Ea,B→A | (2) |
ΔEsite(tet, oct) may be approximated from eqn (2) as the average of ΔEsite(A, B) for all sites where A and B are tetrahedral and octahedral sites respectively.
Fig. 2 presents the result of our analysis of molecular dynamics of rock-salt LiCl and Li-deficient antifluorite Li1+2xCl1−xNx phases.
In LiCl, the octahedral interstitials are preferably occupied over the tetrahedral ones (Fig. 2a), indicating that the former are more stable than the latter. All tetrahedral interstitials in LiCl are vacant thus, in principle, LiCl features a high concentration of vacant interstitials enabling potential diffusion pathways via an octahedral–tetrahedral–octahedral jump sequence. |ΔEsite(tet, oct)| is 0.61 ± 0.02 eV in the case of LiCl indicating that tetrahedral sites are on average significantly destabilized by ca. 0.6 eV versus octahedral sites. The jump-activation energy for the tet–oct jumps in LiCl is low (ca. 0.03 eV) suggesting that the tetrahedral site is highly metastable and should arguably more generally be referred to as ‘position’ than ‘site’ as further detailed in ESI Note 2.† For the remaining discussion of this work however this distinction will not be made and the tetrahedral positions in LiCl will also be referred to as sites. One potential reason for the high metastability of tetrahedral Li sites may be the small void space at the tetrahedral site enabling occupation of an ion with a max radius of 0.38 Å (see ESI Note 3†) which is smaller than the Li-ion radius (0.59 Å).17 Additionally, oct–tet transitions are sterically hindered in LiCl due to a highly constrained bottleneck with a diameter of 0.8 Å, which requires energetically unfavorably close Li–Cl ion distances and/or lattice distortion to accommodate the passing of Li ions (Fig. 2e). To summarize, in rock-salt LiCl, Li is confined to the octahedral sites and low-activation-energy jumps to vacant sites are not available, rationalizing the absence of diffusion during simulations (localized density in Fig. 2g) and the low RT ion conductivity of LiCl.¶
We now turn our attention to Li-diffusion in the Li1.66N0.33Cl0.66 phase. We performed AIMD simulations on seven different disordered Li1+2xCl1−xNx (2 × 2 × 2) supercells (including Li1.66N0.33Cl0.66) to investigate the ion jumps and their jump activation energies present in these phases. Shared site occupations and partial occupancies in Li1+2xCl1−xNx phases were treated by random decoration of the Wyckoff 4a (0, 0, 0) position with nitrogen and chlorine and the 8c (0.25, 0.25, 0.25). Li-positions were randomly decorated with Li and vacancies in order to reach the targeted stoichiometry (see Methodology for full computational details). In contrast to LiCl, in the Li-deficient antifluorite Li1.66N0.33Cl0.66 structure the tetrahedral Li sites are occupied, indicating that they are stabilized with respect to the octahedral sites. Li1.66N0.33Cl0.66 features two types of intrinsic vacancies. (1) The tetrahedral sites are partially occupied and (2) the octahedral interstitials are essentially vacant (Fig. 2b).|| Compared to LiCl the difference in site energy between octahedral and tetrahedral is much smaller, with |ΔEsite(tet–oct)| = 0.07 ± 0.01 eV as compared to |ΔEsite(tet–oct)| = 0.61 ± 0.02 eV in LiCl (Fig. 2c and d). This flat(ter) energy landscape enables low-activation-energy oct–tet jumps and increased bulk diffusion reflected in the much more diffuse Li-density compared to the LiCl case (Fig. 2h) and higher experimentally-measured conductivities of Li1.66N0.33Cl0.66 (Fig. 1c).
Fig. 3 presents our analysis of ion hopping in disordered Li1+2xCl1−xNx as a function of local environment and jump geometry.
From our AIMD simulations we observe that diffusion occurs via Li jumps between oct and tet sites through triangular bottlenecks consisting of three anions as well as between tet sites through linear bottlenecks consisting of two anions (Fig. 3a). Li diffusion through triangular and linear bottlenecks is facilitated by the presence of N in the anionic lattice. Because N3− (1.46 Å) anions are considerably smaller than Cl− (1.81 Å) anions, the bottleneck becomes larger, enabling Li diffusion. To quantify this further, we calculated the bottleneck diameters for 5 Li1.66N0.33Cl0.66 DFT relaxed (2 × 2 × 2) supercells (featuring >500 bottlenecks) to account for local distortions that may not be evident in the average crystallographic unit cell. The bottleneck diameters for different bottlenecks as well as the average jump-Ea for jumps through the respective bottlenecks are shown in Fig. 3. Comparing the bottleneck diameter to the diameter of Li-ions (1.18 Å) is a good proxy for the constraints on Li diffusion; bottlenecks with significantly smaller diameters than 1.18 Å will constrain Li diffusion more than bottlenecks with diameters >1.18 Å. Fig. 3b for instance shows that triangular bottlenecks consisting of three chlorides are considerably smaller (∼0.8 Å) than bottlenecks containing one or more nitrogen (>1.25 Å). Fig. 3b and c show that jumps through nitrogen-containing bottlenecks for oct–tet and tet–tet jumps have lower activation energies indicating easier diffusion through N containing bottlenecks. Consequently, increasing the nitrogen content in the Li1+2xCl1−xNx phase, results in more nitrogen-rich, low-activation-energy bottlenecks, explaining the increase in conductivity upon increasing the nitrogen content in the Li1+2xCl1−xNx solid solution shown in Fig. 1c.
In conclusion, it is not possible to continuously dissolve Li3N into rock-salt LiCl to form Li-excess rock-salt phases as the excess Li ions would occupy tetrahedral sites which are sterically too constrained to accommodate Li ions. However, once a critical amount of Li3N (x ≥ 0.33) is mixed with LiCl sufficient Li ions are available to stabilize (Li-deficient) antifluorite phases where Li ions occupy tetrahedral sites. The occupation of tetrahedral sites indicates that the tetrahedral sites are more stable than octahedral sites in Li-deficient antifluorite phases. The vacant octahedral sites and the occupied tetrahedral sites are energetically in close proximity (|ΔEsite(tet–oct)| ∼ 0.07 eV) and oct–tet (and tet–tet) transitions are facilitated by the presence of N3− anions in the anionic lattice as the smaller ionic radius of N3− compared to Cl− increases the bottleneck size (Fig. 2f and 3). Consequently, the vacant octahedral sites which are innate to Li-deficient antifluorite phases are thermodynamically and kinetically accessible so that tet–oct jumps may be achieved with jump-Ea values of 0.37 ± 0.01 eV on average. In contrast, in LiCl the vacant tetrahedral sites are at much larger energies than the occupied octahedral sites (|ΔEsite(tet–oct)| ∼0.6 eV). Additionally, the oct–tet transitions are sterically hindered by small bottlenecks consisting of 3 Cl− ions so that an oct–tet transition has a jump-Ea value of 0.64 ± 0.01 eV on average. Consequently, the vacant tetrahedral sites which are innate to rock-salt LiCl phases are thermodynamically and kinetically inaccessible explaining the absence of diffusion in simulations and the experimentally obtained low ionic conductivity.
Comparison between Li-rich antifluorite Li2+xS1−xNx and Li-deficient antifluorite Li1+2xCl1−xNx
The presently discovered Li1+2xCl1−xNx (0.33 < x < 0.5) solid-solution phases are a structural analog of the Li2+xS1−xNx (0 < x < ∼0.5) system previously discovered.13 Comparing the Li1+2xCl1−xNx and Li2+xS1−xNx phases in the (0.33 < x < 0.5) range, where both exist as solid solutions, is insightful in understanding the relationship between structure and Li-ion conductivity. For this we consider three phases of each solid solution, x = 0.33, x = 0.4 and x = 0.45, —representing the lower, center and upper limit of the 0.33 < x < 0.5 range, respectively—and compare their ionic conductivity metrics as quantified by variable-temperature impedance spectroscopy. Fig. 4 presents the resulting dependence of the ambient-temperature conductivity, activation energy and conductivity prefactor as a function of composition in Li1+2xCl1−xNx and Li2+xS1−xNx. | ||
| Fig. 4 Comparison of the experimentally obtained conductivity, activation energy and Arrhenius prefactor of Li1+2xCl1−xNx and Li2+xS1−xNx phases. (a) Comparison of the conductivity at 22 °C determined experimentally via impedance spectroscopy. (b) Comparison of the Arrhenius plot for the activation energy. (c) Comparison of the Arrhenius prefactor. Data for Li2+xS1−xNx originally reported in ref. 13. The Arrhenius fits for (b) are shown in Fig. S6.† | ||
A notable difference is that the Li2+xS1−xNx phases are Li-excess antifluorite structures (more than 2 Li per anion), with partial Li occupancy of the octahedral sites (ref. 13) while the Li1+2xCl1−xNx phases are Li-deficient antifluorite phases (less than 2 Li per anion) where the tetrahedral sites are partially occupied by Li (Fig. S1 and 2†). Fig. 4a shows that the Li-excess Li2+xS1−xNx phases have much higher (∼10×) conductivity than the Li-deficient Li1+2xCl1−xNx phases, whereas the bulk activation energies, determined by EIS, for the Li2+xS1−xNx and the Li1+2xCl1−xNx phases differ by at most 0.03 eV (Fig. 4b) suggesting that the energy thresholds for diffusion in both systems are similar. However, the difference in the Arrhenius perfactors is large, on average a factor of 7 larger for the phases compared to of the Li1+2xCl1−xNx phases (Fig. 4c). This suggests that the origin of the higher conductivity of Li2+xS1−xNx phases is largely comprised in the Arrhenius prefactor. The larger charge carrier concentration in Li2+xS1−xNx (and potentially the inducing of concerted motion though we do not explicitly investigate this here) are likely the origin for the larger Arrhenius prefactor of Li-excess Li2+xS1−xNx antifluorite phases.
Oxidation limits of Li-rich antifluorite Li2+xS1−xNx and Li-deficient antifluorite Li1+2xCl1−xNx phases
We computationally investigated the metastability of Li2+xS1−xNx and Li1+2xCl1−xNx antifluorite-like phases. For compositions x = 0.11, 0.17, 0.36, 0.55, 0.72 we built 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 random 2 × 2 × 2 antifluorite-like Li2+xS1−xNx supercells and calculated their electrostatic energies with the formal oxidation states Li+, S2− and N3− assigned to the ions. We took the 30 supercells with the lowest electrostatic energies and relaxed the structures using density functional theory. With the energies obtained, the energy above the hull of these phases was calculated using entries of the materials project database (see also ESI Note 4†). Fig. 5 compares the calculated energies with the experimentally obtained stability limits of Li2+xS1−xNx and Li1+2xCl1−xNx as a function of composition.
000 random 2 × 2 × 2 antifluorite-like Li2+xS1−xNx supercells and calculated their electrostatic energies with the formal oxidation states Li+, S2− and N3− assigned to the ions. We took the 30 supercells with the lowest electrostatic energies and relaxed the structures using density functional theory. With the energies obtained, the energy above the hull of these phases was calculated using entries of the materials project database (see also ESI Note 4†). Fig. 5 compares the calculated energies with the experimentally obtained stability limits of Li2+xS1−xNx and Li1+2xCl1−xNx as a function of composition.
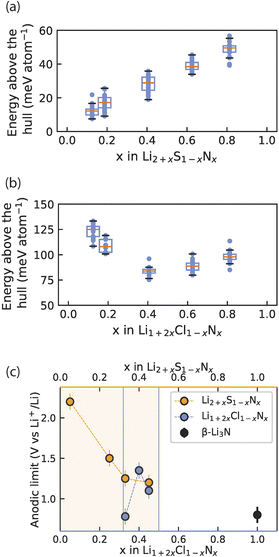 | ||
| Fig. 5 Interplay of phase (meta)stability and anodic limit of irreducible antifluorite phases. (a) and (b) Calculated energy above the hull for 30 Li2+xNxS1−x and Li1+2xCl1−xNx antifluorite supercells at each of several compositions x. At each composition above the distribution of energies above the hull is shown as a box plot where the orange line indicates the arithmetic mean and the box indicates the first standard deviation. (c) Experimental anodic limits obtained from LSV for Li2+xS1−xNx, Li1+2xCl1−xNx and β-Li3N for reference. Orange shading and blue lines denote the solid-solution range for Li2+xS1−xNx and Li1+2xCl1−xNx respectively. Data for Li2+xS1−xNx in (c) originally reported in ref. 13. | ||
Fig. 5a shows the average energy above the hull for the Li2+xS1−xNx phases and shows that increased nitrogen content and Li stuffing increase metastability (i.e. higher energy above the hull). Note the energy above the hull was calculated for antifluorite-like Li2+xS1−xNx phases even at nitrogen contents that cannot be stabilized experimentally (i.e. x > 0.55) to clearly demonstrate the effect of Li3N dissolution into Li2S on phase (meta)stability.
Turning to the Li1+2xCl1−xNx phases, the metastability of Li1+2xCl1−xNx phases was calculated in an analogous manner to the Li2+xS1−xNx phases and is shown in Fig. 5b. Note the energy above the hull is calculated for antifluorite-like Li1+2xCl1−xNx phases even at nitrogen contents that cannot be stabilized experimentally (i.e. x < 0.33 and x > 0.5) to clearly demonstrate the effect of nitrogen content on phase (meta)stability. For Li1+2xCl1−xNx phases the metastability is high at low and at high nitrogen content. The metastability of Li1+2xCl1−xNx phases is lowest around the center of the LiCl–Li3N tie line. This non-monotonic trend in the metastability is consistent with the experimentally observed low-N-content and high-N-content boundaries of the Li1+2xCl1−xNx (0.33 < x < 0.5) solid solution (Fig. 1b and c).
Fig. 5c presents the oxidative stability limits of the Li2+xS1−xNx and Li1+2xCl1−xNx antifluorite-like phases as determined experimentally by linear-sweep voltammetry (ESI Fig. S7 and S8†). The trends in the experimental oxidative limits mirror the trends observed for energy above the hull in Fig. 5a and b: the increased metastability of N-rich antifluorite-like Li2+xS1−xNx phases is reflected in the oxidation stability which monotonically decreases the higher the nitrogen content and a non-monotonic trend is observed in the oxidation stability of Li1+2xCl1−xNx phases with a maximum for x = 0.4.
SCl and BrCl substitutions on Li1+2xCl1−xNx phases to boost conductivity
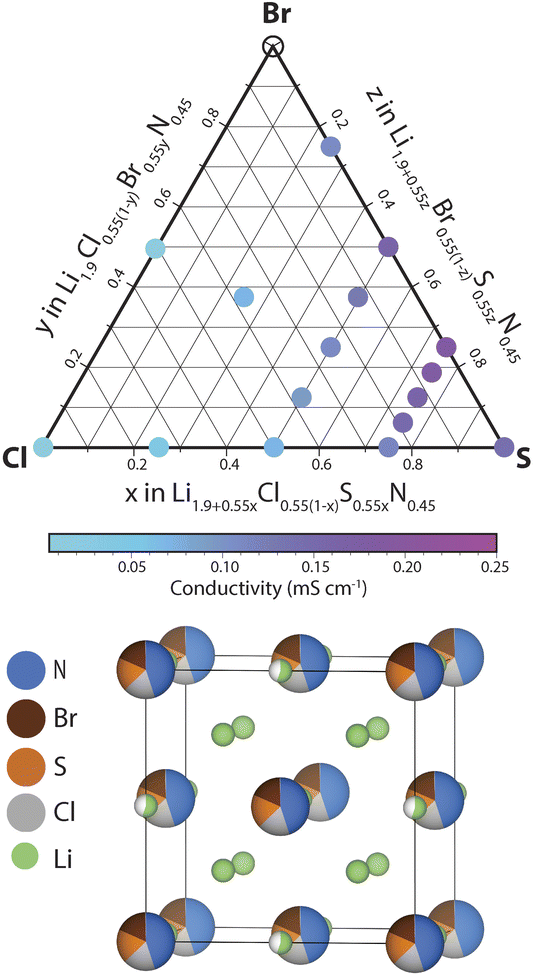
In this section we investigate the effect of SCl and BrCl substitutions in Li1+2xCl1−xNx phases. SCl substitutions in Li1+2xCl1−xNx may increase the number of charge carriers and BrCl substitutions could increase the lattice parameter facilitating Li diffusion. To narrow down this vast compositional space, the effect of the SCl, BrS and BrCl substitutions considered at a fixed nitrogen content x = 0.45. Thus the phase space we set out to investigate can be expressed by the following solid-solution formula: Li1.9+0.55yCl0.55(1−y−z)S0.55yBr0.55zN0.45 (0 < y,z < 1). We explored the ionic conductivity of this phase space by synthesizing various compositions in the ternary phase diagram and measuring their ionic conductivity using impedance spectroscopy (provided a single antifluorite-like phase was obtained). The results of this investigation are shown in ESI Fig. S9–S14.†Fig. 6 presents the ionic conductivity of ternary and quaternary Li1.9+0.55yCl0.55(1−y−z)S0.55yBr0.55zN0.45 antifluorite-like samples synthesized as a function of composition in a quasi-ternary phase diagram.We find that nearly all compositions in the phase space shown in Fig. 6 exist as Li-deficient or Li-excess antifluorite structures. Only highly Br-rich samples where the bromine content exceeds 40% of all anions (that is, values of z > 0.75 in Li1.9+0.55yCl0.55(1−y−z)S0.55yBr0.55zN0.45) did not result in phase-pure antifluorite-like phases.
Three main trends may be derived from Fig. 6. (1) SCl substitutions entail a continuous increase in ionic conductivity. For instance, when substituting Cl in Li1.9Cl0.55N0.45 by S to form Li2.45S0.55N0.45 the conductivity increases by an order of magnitude (from 0.02 to 0.15 mS cm−1). This trend can be rationalized based on the increased mobile charge-carrier concentration through charge compensation in replacing S2− by Cl− ions. (2) The second main trend we derive from Fig. 6 is that BrCl substitutions entail a continuous increase in ionic conductivity. For instance, substituting all Cl in Li2.175Cl0.275S0.275N0.45 by Br to form Li2.175Br0.275S0.275N0.45 leads to a conductivity increase by a factor of ca. 2. This trend may be rationalized by the larger ion radius of Br− compared to Cl−: the presence of Br− increases the lattice parameter, which facilitates diffusion through the sterically constrained diffusion bottlenecks. Additionally, the higher polarizability (softness) of Br compared to Cl (ref. 23) may ease diffusion through Br-containing bottlenecks compared to Cl-containing bottlenecks. (3) The third trend we derive from Fig. 6 is that partial BrS substitutions increase ionic conductivities. Unlike the two previous trends, this trend is not monotonic. While partial BrS substitution leads to higher conductivities, carrying the substitution too far leads to decreasing conductivities. This discontinuous trend may be explained as follows. While BrS substitutions increase the lattice parameter, BrS substitutions concurrently reduce the Li concentration to compensate for the lower valence of Br− compared to S2− ions.
In agreement with the trends described above we find that the phase with the highest conductivity is a moderately Br-substituted Li2.45S0.55N0.45 phase – that is a phase with a stoichiometry of Li2.31S0.41Br0.14N0.45 reaching a conductivity of ca. 0.2 mS cm−1 at 22 °C. Overall this investigation demonstrates the high structural and compositional flexibility of the antifluorite framework; Li-deficient and Li-rich antifluorite phases may be stabilized and may feature numerous elements on the anion site.
Perspectives of irreducible antifluorite-like electrolytes for batteries
Solid-state batteries necessitate high-energy, low-voltage anodes such as Li metal or LixSi to supersede conventional Li-ion batteries.8,24 However, the best ion conductors known to date—reaching >0.1 mScm−1 in conductivity and comprising oxide, sulfide and halide chemistries suffer from electrochemical decomposition <1 V vs. Li/Li+7,25–27—with the exception of garnet oxides which are compatible with Li metal.28** Electrochemical decomposition on the anode side is associated with increased cell resistance, capacity loss, dendrite formation and short-circuiting.29,30 A promising strategy to avoid reductive decomposition are bilayer separators comprising a catholyte facing the cathode and an anolyte facing the anode.1 Anolytes should be highly-conducting and electrochemically stable at the low potentials of Li metal anodes. Highly conducting fully-reduced phases are thus inherently promising anolyte candidates which has also been demonstrated experimentally.13,14,31High conductivity and reductive stability are not the only criteria suitability criteria of anolytes. The suitability of anolyte layers is system-dependent and multiple factors need to be considered. Mechanical and microstructural properties of solid electrolytes play a key role, for instance in dendrite formation and mechanical degradation in solid-state batteries.32,33 Chemical compatibilities of anolytes with the paired catholytes also need to be considered.10 Additionally, the oxidation limit of anolytes needs to be considered; for applications e.g. with LixSi anodes whose operation window ranges from 0.01 V to 1.1 V.8 Due to the low oxidation limit of Li3N (0.8 V vs. Li/Li+), Li3N would be inert to reduction but not inert to oxidation when in contact with LixSi anodes. In contrast, some compositions of the antifluorite-like phases have oxidation limits exceeding 1.1 V (see Fig. 5c) and would be suitable anolytes for LixSi anodes as they would be inert to reduction and oxidation. In summary, irreducible electrolytes are promising anolyte candidates. The high compositional and structural flexibility of fully-reduced antifluorite-like phases we demonstrate in this study will enable the further tunability of ionic conductivity, electrochemical stability, mechanical and microstructural properties – essential for functional electrode/electrolyte interfaces in batteries.32
Conclusion
Fully reduced electrolytes based on the antifluorite framework recently received ample attention as their stability against low potentials eliminates performance degradation due to reductive decomposition.14 In this study we elucidated the mechanism underlying the increased conductivity in herein discovered irreducible antifluorite-like Li1+2xCl1−xNx phases (which includes Li9N2Cl3 ref. 14 and Li5NCl2 ref. 10 and 15) compared to the structurally similar LiCl phase. Computationally, we find that introducing nitrogen into the LiCl anionic framework brings tetrahedral and octahedral Li sites closer in energy so that vacant sites become energetically accessible for diffusion. Analyzing local diffusion bottlenecks we further showed that nitrogen widens diffusion bottlenecks further facilitating Li diffusion. Experimentally we demonstrated that the antifluorite framework is stoichiometrically flexible; Li-deficient and Li-excess antifluorite phases can be stabilized. We further found that fully reduced antifluorite-like phases have an increased oxidation limit compared to Li3N (0.8 V vs. Li) which may be critical advantage over Li3N (the archetypical fully reduced electrolyte) to stabilize next-generation anodes. Additionally, we showed that Li1+2xCl1−xNx phases are compositionally highly modifiable: SCl- and BrCl-substituted analogues were synthesized, boosting the ionic conductivities of Li1+2xCl1−xNx phases by an order of magnitude. We demonstrate that ternary and quaternary solid solutions can be synthesized mechanochemically in the LiCl–LiBr–Li2S–Li3N phase space, unlocking an expansive compositional domain for future materials exploration.Methodology
Synthesis
All preparation steps were performed in an argon atmosphere (H2O < 1 ppm, O2 < 1 ppm). Li1+2xCl1−xNx phases: the synthesis precursors were LiCl (Sigma-Aldrich, 99%) and Li3N (Sigma-Aldrich, >99.5%). Stoichiometric amounts of the precursors were milled in a planetary ball mill (Jar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZrO2, 45 mL) with 10 mm ZrO2 balls and a ball
ZrO2, 45 mL) with 10 mm ZrO2 balls and a ball![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) powder mass ratio of 30 at 550 rpm for 99 (5 min milling–5 min-pause) cycles. Li2+xS1−xNx phases (originally reported in ref. 13): the synthesis precursors were Li2S (Sigma-Aldrich, 99%) and Li3N (Sigma-Aldrich, >99.5%). Stoichiometric amounts of the precursors were milled in a planetary ball mill (Jar
powder mass ratio of 30 at 550 rpm for 99 (5 min milling–5 min-pause) cycles. Li2+xS1−xNx phases (originally reported in ref. 13): the synthesis precursors were Li2S (Sigma-Aldrich, 99%) and Li3N (Sigma-Aldrich, >99.5%). Stoichiometric amounts of the precursors were milled in a planetary ball mill (Jar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZrO2, 45 mL) with 10 mm ZrO2 balls and a ball
ZrO2, 45 mL) with 10 mm ZrO2 balls and a ball![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) powder mass ratio of 30 at 550 rpm for 99 (5 min milling–5 min-pause) cycles.
powder mass ratio of 30 at 550 rpm for 99 (5 min milling–5 min-pause) cycles.
Electrochemical characterization
The same procedure was applied to all solid electrolytes (SE) investigated in this work: Li1+2xCl1−xNx and Li2+xS1−xNx and Li1.9+0.55yCl0.55(1−y−z)S0.55yBr0.55zN0.45. Electrochemical Impedance Spectroscopy (EIS): pellets (diameter = 10 mm) of the SE powder samples were pressed (3.2 tons) in custom-made cells. These lab cells consist of an alumina tube and two stainless steel (SS) plungers and an airtight seal. The stainless steel plungers act as current collectors. Solid electrolyte powder is filled in the alumina tube and compressed on both sides with the stainless steel plungers. The cell configuration used was SS|SE|SS. AC impedance was performed with a Metrohm Autolab (AUT86298) in the frequency range 10 MHz to 0.1 Hz with a voltage amplitude of 10 mV. EIS spectra were fitted with a resistor in parallel with a constant phase element (CPE) representing the solid electrolyte and a CPE representing the solid electrolyte–SS interface. RT conductivities were measured in ambient conditions (22 °C in our labs). Linear sweep voltammetry (LSV): LSV measurements were also performed with an Metrohm Autolab (AUT86298). To measure the anodic limit of SE phases, Li|SE|SE–C cells were used. To make the SE–C composite cathode a mixture of SE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Super P with a weight ratio of 0.7
Super P with a weight ratio of 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 was milled in a planetary ball mill (Jar
0.3 was milled in a planetary ball mill (Jar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZrO2, 45 mL) with 10 mm ZrO2 balls and a ball/powder ratio of 30 at 400 rpm for 2 h (5 min milling; 5 min pause). Li|SE|SE-C cells were assembled by pressing a SE pellet (130 mg, 3.2 tons) and subsequently the SE–C composite (15 mg, 3.2 tons) on top. Finally, a Li disk was placed on the opposite side of the SE pellet. The LSV scanning rate was 0.01 mV s−1.
ZrO2, 45 mL) with 10 mm ZrO2 balls and a ball/powder ratio of 30 at 400 rpm for 2 h (5 min milling; 5 min pause). Li|SE|SE-C cells were assembled by pressing a SE pellet (130 mg, 3.2 tons) and subsequently the SE–C composite (15 mg, 3.2 tons) on top. Finally, a Li disk was placed on the opposite side of the SE pellet. The LSV scanning rate was 0.01 mV s−1.
X-ray diffraction
Powder diffraction patterns were collected using Cu Kα X-rays (1.54 Å) on a PANalytical X'Pert Pro X-ray diffractometer. The air sensitive SE probes were loaded into air-tight holders in an Ar-filled glovebox prior to the measurements. GSAS-II34 and FullProf35 used for LeBail and Rietveld refinements. As a starting point the structure solution of Li5NCl2 (i.e. Li1.66Cl0.66N0.33) was taken. 15For Li1+2xCl1−xNx phases with higher nitrogen content the nitrogen amount was increased, the chlorine amount reduced and the Li fraction of occupied tetrahedral sites increased according to the synthesized stoichiometry. Based on the initial structure solution of Li1.66Cl0.66N0.33 [ref. 15] all Li ions were assumed in tetrahedral sites though we note that generally a small occupation of the octahedral sites (≤5%) may potentially exist (see ESI Note 1†).Computational details
All DFT calculations were performed with the Vienna ab initio simulation package VASP with computational settings consistent with those used in the Materials Project database.36 Obtaining jump-activation energies for Li1+2xCl1−xNx and LiCl. For the generation and analysis of supercells the calculations were done on 7 different 2 × 2 × 2 Li1+2xCl1−xNx and one 2 × 2 × 2 LiCl supercells. Because of the shared site occupations and partial occupancies in Li1+2xCl1−xNx phases, different atomic arrangements were generated by random decoration of the Wyckoff 4a (0, 0, 0) position with nitrogen and chlorine and the 8c (0.25, 0.25, 0.25) positions were randomly decorated with Li and vacancies. For the generation and analysis of supercells the pymatgen package was used.37 For the AIMD simulations the Li pseudopotential was changed from one considering the semicore s electrons as valence (i.e. 1s22s1 “Li_sv”, which was used for relaxations) to on considering on the 2s electrons (i.e. 2s1 “Li”) as this enables the use of a lower energy cutoff and vastly improves computational speed. The simulation time was 200 ps for every AIMD simulation. The AIMD simulations were executed at 1000 K. The dissection of AIMD simulations into individual jump events and subsequent analysis of jump frequencies and individual Ea,jump values was done as first described by de Klerk and Wagemaker18 and currently developed as a python package in our group 39A comprehensive account can be found in ref. 18 but crucial aspects for the understanding of the reported data is presented here. Calculation of Ea, jump values between two sites: the sites are defined around the 0 K equilibrium positions of the Li ions. At every simulation step it is recorded in which site each Li ion is located or whether it is currently between two sites. From this information the jump frequency between two sites vA→B can be calculated according to eqn (3): | (3) |
Stability calculations for Li1+2xCl1−xNx and for Li2+xS1−xNx. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 2 × 2 × 2 supercells were generated by randomly decorating the Wyckoff 4a (0, 0, 0) position with nitrogen and chlorine. The 8c (0.25, 0.25, 0.25) positions were also randomly decorated with Li and vacancies according to the targeted stoichiometry. Then formal charges of −3, −1, and +1 were assigned to nitrogen, chlorine and Li, respectively and the electrostatic energy (‘Ewald energy’) was calculated via pymatgen. Subsequently, out of the Li1+2xCl1−xNx structures, 30 with the lowest Ewald energy were taken and relaxed by DFT. The energies obtained from DFT were used to calculate the energy of the hull of the Li1+2xCl1−xNx phases, corrections from the materials project data base were applied and the energies for the end-member phases (Li3N and Li2S) were also obtained from the materials project data base (see also ESI Note 4†).36
000 2 × 2 × 2 supercells were generated by randomly decorating the Wyckoff 4a (0, 0, 0) position with nitrogen and chlorine. The 8c (0.25, 0.25, 0.25) positions were also randomly decorated with Li and vacancies according to the targeted stoichiometry. Then formal charges of −3, −1, and +1 were assigned to nitrogen, chlorine and Li, respectively and the electrostatic energy (‘Ewald energy’) was calculated via pymatgen. Subsequently, out of the Li1+2xCl1−xNx structures, 30 with the lowest Ewald energy were taken and relaxed by DFT. The energies obtained from DFT were used to calculate the energy of the hull of the Li1+2xCl1−xNx phases, corrections from the materials project data base were applied and the energies for the end-member phases (Li3N and Li2S) were also obtained from the materials project data base (see also ESI Note 4†).36
Data availability
The data that support the findings of this study and the code to reproduce the results shown in the paper are openly available in 4TU. ResearchData at https://doi.org/10.4121/fcb46e92-06cd-4241-a97b-3390d6dc1f70. We used python version 3.10 and the following python packages: numpy39, gemdat38, matplotlib40, pymatgen37.Author contributions
The study was conceptualized by V. L. Simulation data were acquired by V. L. Experimental data were acquired by V. L., M. T. and Z. C. Data analysis and interpretation were done by V. L., T. F., M. T. and M. W. Writing and editing of the draft were done by V. L., T. F., M. W. The funding for this study was acquired by M. W. and T. F. The project was supervised by T. F. and M. W. All authors have approved the submitted version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. W. and V. L. acknowledge the funding received from the Netherlands Organization for Scientific Research (NWO) under the VICI grant (no. 16122). T. F. acknowledges the funding provided by the European Union's HORIZON EUROPE programme in the form of a Marie Skłodowska-Curie individual postdoctoral fellowship (project no. 101066486). A. V. acknowledges financial support from the Netherlands Organization for Scientific Research (NWO) under the VENI grant number 18123 and the eScience Centre under the NLESC.OEC.2022.013 grant. T. F., A. V. and V. L. acknowledge financial support by the NWO in the form of an open-competition XS grant (OCENW.XS22.4.210). All authors acknowledge the use of computational resources of the DelftBlue supercomputer, provided by Delft High Performance Computing Centre (https://www.tudelft.nl/dhpc).References
- T. Famprikis, P. Canepa, J. A. Dawson, M. S. Islam and C. Masquelier, Fundamentals of Inorganic Solid-State Electrolytes for Batteries, Nat. Mater., 2019, 18, 1278–1291 CrossRef CAS PubMed.
- J. Janek and W. G. Zeier, Challenges in Speeding up Solid-State Battery Development, Nat. Energy, 2023, 8(3), 230–240 CrossRef.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsui, M. Yonemura, H. Iba and R. Kanno, High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors, Nat. Energy, 2016, 1(4), 1–7 Search PubMed.
- L. Zhou, A. Assoud, Q. Zhang, X. Wu and L. F. Nazar, New Family of Argyrodite Thioantimonate Lithium Superionic Conductors, J. Am. Chem. Soc., 2019, 141(48), 19002–19013 CrossRef CAS PubMed.
- M. A. Kraft, S. Ohno, T. Zinkevich, R. Koerver, S. P. Culver, T. Fuchs, A. Senyshyn, S. Indris, B. J. Morgan and W. G. Zeier, Inducing High Ionic Conductivity in the Lithium Superionic Argyrodites Li6+xP1–xGexS5I for All-Solid-State Batteries, J. Am. Chem. Soc., 2018, 140(47), 16330–16339 CrossRef CAS PubMed.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama and K. A. Kawamoto, Lithium Superionic Conductor, Nat. Mater., 2011, 10(9), 682–686 CrossRef CAS PubMed.
- Y. Zhu, X. He and Y. Mo, First Principles Study on Electrochemical and Chemical Stability of Solid Electrolyte-Electrode Interfaces in All-Solid-State Li-Ion Batteries, J. Mater. Chem. A, 2016, 4(9), 3253–3266 RSC.
- H. Huo and J. Janek, Silicon as Emerging Anode in Solid-State Batteries, ACS Energy Lett., 2022, 7(11), 4005–4016 CrossRef CAS.
- W. Zaman and K. B. Hatzell, Processing and Manufacturing of next Generation Lithium-Based All Solid-State Batteries, Curr. Opin. Solid State Mater. Sci., 2022, 26(4), 101003 CrossRef CAS.
- V. Landgraf, T. Famprikis, J. de Leeuw, L. J. Bannenberg, S. Ganapathy and M. Wagemaker, Li5NCl2 : A Fully-Reduced, Highly-Disordered Nitride-Halide Electrolyte for Solid-State Batteries with Lithium-Metal Anodes, ACS Appl. Energy Mater., 2023, 6(3), 1661–1672 CrossRef CAS PubMed.
- C. Szczuka, B. Karasulu, M. F. Groh, F. N. Sayed, T. J. Sherman, J. D. Bocarsly, S. Vema, S. Menkin, S. P. Emge, A. J. Morris and C. P. Grey, Forced Disorder in the Solid Solution Li3P–Li2S: A New Class of Fully Reduced Solid Electrolytes for Lithium Metal Anodes, J. Am. Chem. Soc., 2022, 144(36), 16350–16365 CrossRef CAS PubMed.
- X. Xu, G. Du, C. Cui, J. Liang, C. Zeng, S. Wang, Y. Ma and H. Li, Stabilizing the Halide Solid Electrolyte to Lithium by a β-Li3N Interfacial Layer, ACS Appl. Mater. Interfaces, 2022, 14(35), 39951–39958 CrossRef CAS.
- V. Landgraf, M. Tu, Z. Cheng, J. de Leeuw, S. Ganapathy, M. Wagemaker and T. Famprikis, Entropy-Induced High Conductivity in Fully-Reduced Electrolytes for Solid-State Batteries with Lithium Metal Anodes, ChemRxiv, 2023, preprint, DOI:10.26434/chemrxiv-2023-33r87.
- W. Li, M. Li, P.-H. Chien, S. Wang, C. Yu, G. King, Y. Hu, Q. Xiao, M. Shakouri and R. Feng, Lithium-Compatible and Air-Stable Vacancy-Rich Li9N2Cl3 for High–Areal Capacity, Long-Cycling All–Solid-State Lithium Metal Batteries, Sci. Adv., 2023, 9(42), eadh4626 CrossRef CAS.
- R. Marx and H. M. Mayer, Preparation and Crystal Structure of Ordered and Disordered Lithium Nitride Dichloride, Li5NCl2, J. Solid State Chem., 1997, 130(1), 90–96 CrossRef CAS.
- W. Weppner, P. Hartwig and A. Rabenau, Consideration of Lithium Nitride Halides as Solid Electrolytes in Practical Galvanic Cell Applications, J. Power Sources, 1981, 6(3), 251–259 CrossRef CAS.
- R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides, Acta Crystallogr., Sect. A, 1976, 32(5), 751–767 CrossRef.
- N. J. J. De Klerk, E. Van Der Maas and M. Wagemaker, Analysis of Diffusion in Solid-State Electrolytes through MD Simulations, Improvement of the Li-Ion Conductivity in β-Li3PS4 as an Example, ACS Appl. Energy Mater., 2018, 1(7), 3230–3242 CrossRef CAS PubMed.
- C. Yu, S. Ganapathy, N. J. J. De Klerk, I. Roslon, E. R. H. Van Eck, A. P. M. Kentgens and M. Wagemaker, Unravelling Li-Ion Transport from Picoseconds to Seconds: Bulk versus Interfaces in an Argyrodite Li6PS5Cl-Li2S All-Solid-State Li-Ion Battery, J. Am. Chem. Soc., 2016, 138(35), 11192–11201 CrossRef CAS.
- N. J. J. De Klerk, I. Rosłoń and M. Wagemaker, Diffusion Mechanism of Li Argyrodite Solid Electrolytes for Li-Ion Batteries and Prediction of Optimized Halogen Doping: The Effect of Li Vacancies, Halogens, and Halogen Disorder, Chem. Mater., 2016, 28(21), 7955–7963 CrossRef CAS.
- N. J. J. De Klerk and M. Wagemaker, Diffusion Mechanism of the Sodium-Ion Solid Electrolyte Na3PS4 and Potential Improvements of Halogen Doping, Chem. Mater., 2016, 28(9), 3122–3130 CrossRef CAS.
- X. Li, H. Liu, C. Zhao, J. T. Kim, J. Fu, X. Hao, W. Li, R. Li, N. Chen, D. Cao, Z. Wu, Y. Su, J. Liang and X. Sun, Hopping Rate and Migration Entropy as the Origin of Superionic Conduction within Solid-State Electrolytes, J. Am. Chem. Soc., 2023, 145(21), 11701–11709 CrossRef CAS PubMed.
- N. Flores-González, N. Minafra, G. Dewald, H. Reardon, R. I. Smith, S. Adams, W. G. Zeier and D. H. Gregory, Mechanochemical Synthesis and Structure of Lithium Tetrahaloaluminates, LiAlX4(X = Cl, Br, I): A Family of Li-Ion Conducting Ternary Halides, ACS Mater. Lett., 2021, 3(5), 652–657 CrossRef.
- J. Janek and W. G. Zeier, A Solid Future for Battery Development, Nat. Energy, 2016, 1(9), 1–4 Search PubMed.
- Y. Zhu, X. He and Y. Mo, Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations, ACS Appl. Mater. Interfaces, 2015, 7(42), 23685–23693 CrossRef CAS PubMed.
- T. K. Schwietert, V. A. Arszelewska, C. Wang, C. Yu, A. Vasileiadis, N. J. J. de Klerk, J. Hageman, T. Hupfer, I. Kerkamm and Y. Xu, Clarifying the Relationship between Redox Activity and Electrochemical Stability in Solid Electrolytes, Nat. Mater., 2020, 19(4), 428–435 CrossRef CAS.
- S. Wang, Q. Bai, A. M. Nolan, Y. Liu, S. Gong, Q. Sun and Y. Mo, Lithium Chlorides and Bromides as Promising Solid-State Chemistries for Fast Ion Conductors with Good Electrochemical Stability, Angew. Chem., Int. Ed., 2019, 58(24), 8039–8043 CrossRef CAS PubMed.
- K. Subramanian, G. V. Alexander, K. Karthik, S. Patra, M. S. Indu, O. V. Sreejith, R. Viswanathan, J. Narayanasamy and R. Murugan, A Brief Review of Recent Advances in Garnet Structured Solid Electrolyte Based Lithium Metal Batteries, J. Energy Storage, 2021, 33, 102157 CrossRef.
- L. M. Riegger, R. Schlem, J. Sann, W. G. Zeier and J. Janek, Lithium-Metal Anode Instability of the Superionic Halide Solid Electrolytes and the Implications for Solid-State Batteries, Angew. Chem., 2021, 133(12), 6792–6797 CrossRef.
- M. Du, K. Liao, Q. Lu and Z. Shao, Recent Advances in the Interface Engineering of Solid-State Li-Ion Batteries with Artificial Buffer Layers: Challenges, Materials, Construction, and Characterization, Energy Environ. Sci., 2019, 12(6), 1780–1804 RSC.
- P. Yu, H. Zhang, F. Hussain, J. Luo, W. Tang, J. Lei, L. Gao, D. Butenko, C. Wang, J. Zhu, W. Yin, H. Zhang, S. Han, R. Zou, W. Chen, Y. Zhao, W. Xia and X. Sun, Lithium Metal-Compatible Antifluorite Electrolytes for Solid-State Batteries, J. Am. Chem. Soc., 2024, 146(18), 12681–12690 CrossRef CAS.
- T. Krauskopf, F. H. Richter, W. G. Zeier and J. Janek, Physicochemical Concepts of the Lithium Metal Anode in Solid-State Batteries, Chem. Rev., 2020, 120(15), 7745–7794 CrossRef CAS.
- K. B. Hatzell, X. C. Chen, C. L. Cobb, N. P. Dasgupta, M. B. Dixit, L. E. Marbella, M. T. Mcdowell, P. P. Mukherjee, A. Verma, V. Viswanathan, A. S. Westover and W. G. Zeier, Challenges in Lithium Metal Anodes for Solid-State Batteries, ACS Energy Lett., 2020, 5(3), 922–934 CrossRef CAS.
- B. H. Toby and R. B. Von Dreele, GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package, J. Appl. Crystallogr., 2013, 46(2), 544–549 CrossRef CAS.
- J. Rodriguez-Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction, Phys. B, 1993, 192, 55, DOI:10.1016/0921-4526(93)90108-I.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation, APL Mater., 2013, 1(1), 001002 Search PubMed.
- S. P. Ong, W. D. Richards, A. Jain, G. Hautier, M. Kocher, S. Cholia, D. Gunter, V. L. Chevrier, K. A. Persson and G. Ceder, Python Materials Genomics (Pymatgen): A Robust, Open-Source Python Library for Materials Analysis, Comput. Mater. Sci., 2013, 68, 314–319 CrossRef CAS.
- V. Azizi; S. Smeets; A. K. Lavrinenko; S. Ciarella and T. Famprikis, GEMDAT, Zenodo, July 2024 Search PubMed.
- C. R. Harris, K. J. Millman, S. J. Van Der Walt, R. Gommers, P. Virtanen, D. Cournapeau, E. Wieser, J. Taylor, S. Berg and N. J. Smith, Array Programming with NumPy, Nature, 2020, 585(7825), 357–362 CrossRef CAS PubMed.
- J. D. Hunter, Matplotlib: A 2D Graphics Environment, Comput. Sci. Eng., 2007, 9(3), 90–95 Search PubMed.
- C. R. A. Catlow, Static Lattice Simulation of Structure and Transport in Superionic Conductors, Solid State Ionics, 1983, 8(SUPPL. 3), 89–107 CrossRef CAS.
- R. Marx, F. Lissner and T. Schleid, Li9N2S3: Das Erste Nitridsulfid Der Alkalimetalle in Einer Li2O-Typ-Variante, Z. Anorg. Allg. Chem., 2006, 632(12–13), 2151 CrossRef.
- Z. Deng, T. P. Mishra, E. Mahayoni, Q. Ma, A. J. K. Tieu, O. Guillon, J.-N. Chotard, V. Seznec, A. K. Cheetham and C. Masquelier, Fundamental Investigations on the Sodium-Ion Transport Properties of Mixed Polyanion Solid-State Battery Electrolytes, Nat. Commun., 2022, 13(1), 4470 CrossRef CAS.
- M. Wilkening, D. Gebauer and P. Heitjans, Diffusion Parameters in Single-Crystalline Li3N as Probed by 6Li and 7Li Spin-Alignment Echo NMR Spectroscopy in Comparison with Results from 8Li β-Radiation Detected NMR, J. Phys. Condens. Matter, 2007, 20(2), 22201 CrossRef.
- H. Xu, Y. Li, A. Zhou, N. Wu, S. Xin, Z. Li and J. B. Goodenough, Li3N-Modified Garnet Electrolyte for All-Solid-State Lithium Metal Batteries Operated at 40 °C, Nano Lett., 2018, 18(11), 7414–7418 CrossRef CAS.
- C. Zhu, T. Fuchs, S. A. L. Weber, F. H. Richter, G. Glasser, F. Weber, H. J. Butt, J. Janek and R. Berger, Understanding the Evolution of Lithium Dendrites at Li6.25Al0.25La3Zr2O12 Grain Boundaries via Operando Microscopy Techniques, Nat. Commun., 2023, 14(1), 1300 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The following references used in the ESI (ref. 13, 15, 36, 41 and 42). See DOI: https://doi.org/10.1039/d4ta07521h |
| ‡ A small fraction of the Li ions ≤5% may potentially occupy the octahedral sites as detailed in ESI Note 1. |
| § Assuming v0 = 1013 s−1 is commonly adopted in the solid electrolyte field.19,43,44 Additionally we justify this choice by calculating the average vibration frequency around the equilibrium Li-sites in our AIMD simulations and find it to be 1.0 ± 0.2 1013 Hz for 5 different Li1+2xCl1−xNx supercells (Table S5). |
| ¶ Due to the high activation energy required for oct–tet jumps in LiCl and the high metastability of tet sites/positions diffusion in LiCl is likely mediated by Schottky defects just as in NaCl. Accordingly, the charge carrier concentration in LiCl at room temperature is extremely low (∼1 × 10−9cLi where cLi is the Li concentration in LiCl) as the formation energy for Schottky defects is typically ∼1 eV. This low charge carrier concentration additionally contributes to the low ionic conductivity in LiCl. |
| || Potentially a small fraction of Li ions ≤5% on average may occupy the octahedral sites in which case the octahedral sites as detailed in ESI Note 1. |
| ** Even oxide garnet electrolytes may potentially benefit from protection layers against Li metal.45,46 |
| This journal is © The Royal Society of Chemistry 2025 |