Open Access Article
Open Access ArticleMultifunctional halloysite nanotube–polydopamine agro-carriers for controlling bacterial soft rot disease†
Sandeep
Sharma‡
 ,
Ofer
Prinz Setter‡
,
Hanan
Abu Hamad
,
Ofer
Prinz Setter‡
,
Hanan
Abu Hamad
 and
Ester
Segal
and
Ester
Segal
 *
*
Faculty of Biotechnology and Food Engineering, Technion – Israel Institute of Technology, Haifa – 3200003, Israel. E-mail: esegal@technion.ac.il
First published on 11th January 2024
Abstract
Current pesticide formulations suffer from instability and susceptibility to drift, which diminish their effectiveness and harm the environment. To circumvent these challenges, we have developed a core–shell carrier by employing in situ polymerization of dopamine on halloysite nanotubes (HNTs), loaded with the antibacterial essential oil thymol. This hybrid system demonstrates a 2.5-fold higher content of loaded thymol and a 3-fold slower release rate compared to a control lacking the 10 nm thick polydopamine layer. Additionally, the polydopamine coating confers dual-stimuli responsiveness to both acidic pH (mimicking plant infection sites) and near-infrared (NIR) irradiation (mimicking natural sunlight). Consequently, the functionalized clay shows a 2.5-fold increase in inhibiting the major plant pathogen, Erwinia carotovora, at pH 5 compared to a neutral pH. A similar trend is observed under NIR irradiation, attributed to the photothermal properties of polydopamine in combination with thymol release. In terms of crop safety, our polydopamine-coated HNT-based nanocarriers are found to be non-phytotoxic to tomato plants and show no evidence of foliar uptake as confirmed by confocal microscopy. The mussel-inspired polydopamine shell also enhances thymol stability under UV irradiation, and improves the wettability and leaf adhesion of the formulation, thereby reducing drift under simulated rainy conditions. Ultimately, the designed hybrid material exhibits superior in planta efficacy in controlling soft rot disease in tomato crops. We believe our clay-based core–shell agro-nanocarrier loaded with thymol offers a sustainable alternative to existing pesticide formulations while providing enhanced efficacy, stability, and crop safety.
Environmental significanceEscalating global food demand necessitates extensive use of pesticides, which may contaminate soil and crops, exposing the consumer to toxic biocides. Our sustainable nanoformulation, shown to be highly effective in controlling soft rot disease in planta, is composed of natural and biodegradable components: a natural clay carrier, an antibacterial essential oil, that is generally recognized as safe, and the biocompatible polymer polydopamine. Not only does the latter enable the sustained and triggered release of the active compound, but it also enhances foliar adhesion and UV stability of the formulation, minimizing drift and obviating the need for repetitive applications, thus reducing the ecological footprint. Furthermore, we observed no foliar uptake of the applied nanocarrier at the cellular level, mitigating its potential entry into the food chain. |
Introduction
Zero hunger is set as the second sustainable development goal (SDG) of the United Nations towards 2030.1 One of the main crops that holds ∼20% of the global vegetable consumption per year, is tomato (Solanum Lycopersicum L.),2 which is a rich source of vitamins, macro-, and micronutrients, as well as phytochemicals.3 Yet, tomato production is greatly damaged by the soft rot disease caused by Erwinia carotovora (E. carotovora),4,5 manifesting as necrotic spots that rapidly expand on infected areas.6 Consequently, pesticides including copper cations are extensively used to mitigate this disease.7,8 However, a significant amount of the applied pesticide formulations is lost due to drift,9 which results in repeated pesticide application. Not only is this uneconomical, but it also has severe health and environmental implications,10–12 and may further induce pathogen resistance.13–15 This calls for the development of new sustainable formulations that include eco-friendly active ingredients and a carrier for sustained release.Essential oils (EOs) have emerged as promising candidates for serving as sustainable pesticides that had already evolved through natural selection to protect plants against insects, and pathogenic fungi or bacteria.16–18 Among EOs, thymol (TY) has attracted significant attention due to its broad-spectrum antimicrobial and insecticidal properties.19–21 Moreover, several TY-containing formulations are registered as pesticides in beehives by the U.S. Environmental Protection Agency (EPA),22 and TY is categorized as GRAS (generally recognized as safe) by the U.S. Food and Drug Administration.23 Nonetheless, the efficacy of TY as a pesticide is limited since it is highly volatile, light sensitive, and poorly soluble in water, resulting in low residual activity.20,24 To overcome these limitations, TY has been incorporated into various micro- and nano-delivery systems such as starch microspheres, liposomes, and β-cyclodextrin complexes.20,24–26
In contrast to such synthetic approaches, natural nanoclays, which are abundant and intrinsically mesoporous, constitute a sustainable and cost-effective alternative.27,28 One prominent candidate clay is halloysite nanotubes (HNTs), which have been successfully utilized as carriers for TY and other EOs allowing their sustained release.29,30 HNTs are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aluminosilicate clay minerals and exhibit a unique tubular morphology with characteristic dimensions of 600–900 nm, 50 nm, and 15 nm in length, outer and inner diameter, respectively.31,32 Recent studies have revealed that HNTs are not more toxic than other high-aspect ratio nanomaterials.33,34 In addition, HNTs (<1 mg mL−1) were found to be nonphytotoxic to wheat seedlings and even beneficial for the proliferation of tobacco cells.35 Owing to the abundance of HNTs combined with their beneficial attributes, they have been extensively studied as nanocarriers for numerous bioactive compounds.36 In addition, HNTs can be physically and chemically modified to endow them with new functionalities and tune the loading and release behavior of guest molecules.33,37–39
1 aluminosilicate clay minerals and exhibit a unique tubular morphology with characteristic dimensions of 600–900 nm, 50 nm, and 15 nm in length, outer and inner diameter, respectively.31,32 Recent studies have revealed that HNTs are not more toxic than other high-aspect ratio nanomaterials.33,34 In addition, HNTs (<1 mg mL−1) were found to be nonphytotoxic to wheat seedlings and even beneficial for the proliferation of tobacco cells.35 Owing to the abundance of HNTs combined with their beneficial attributes, they have been extensively studied as nanocarriers for numerous bioactive compounds.36 In addition, HNTs can be physically and chemically modified to endow them with new functionalities and tune the loading and release behavior of guest molecules.33,37–39
Polydopamine (PDA), a mussel-inspired biocompatible polymer,40,41 has been widely investigated in combination with HNTs.42–44 The catechol side group of PDA chain strongly adheres to both organic and inorganic surfaces through chemical and physical interactions;45 thus, PDA-coated particles strongly attach to plant leaves, minimizing drift loss,46 even under humid conditions.47 In addition, the melanin-like domains in PDA absorb near-infrared (NIR) irradiation,48 and exhibit photothermal activity that could trigger a pesticidal effect,43 or the release of active compounds.49 At the same time, a PDA coating can also shield the active ingredient against UV radiation.50 Moreover, the polymer degradation under acidic pH,48,51 facilitates triggered release at low pH values,52 characteristic of pathogenic infection of plants.53–57
In this study, we combine for the first time the three components HNTs, TY, and PDA into an all-natural nano-formulation for plant disease control using tomato as a model plant and E. carotovora as a relevant model pathogen responsible for the soft rot disease. HNTs were first loaded with TY and then coated with PDA through the self-polymerization of dopamine in TY-loaded HNTs dispersion. The investigated multifunctional hybrids are shown to promote the following: (1) sustained and pH-triggered release of TY, (2) photo-triggered antibacterial effect, (3) prevention of TY loss under UV radiation, and (4) prolonged pesticidal activity by superior foliar adhesion tested in planta.
Experimental
Materials
Halloysite nanotubes were purchased from NaturalNano (USA). Thymol (≥98.5%), tween 80, dopamine hydrochloride (98%), Tris-HCl (>99%), disodium hydrogen phosphate (ACS reagent, ≥99.0%), sodium citrate, (3-aminopropyl)triethoxysilane (APTES), fluorescein isothiocyanate (FITC), propidium iodide (PI), and agar were obtained from Sigma-Aldrich, Israel. Acetone, absolute ethanol, citric acid, and sodium chloride were obtained from BioLab, Israel. Tryptone and yeast extract for Luria-Bertani (LB) medium were supplied by Becton Dickinson (USA). Magnesium chloride (anhydrous, 99%) was purchased from Alfa Aesar, Israel. Sodium dihydrogen phosphate (Reag. Ph Eur) was purchased from Merck, Israel. Citric acid and sodium citrate were used for the pH 5 buffer preparation, and disodium hydrogen phosphate and sodium dihydrogen phosphate were used for the pH 7 buffer preparation. Milli-Q water (18 MΩ cm) was used to prepare all the solutions and is referred to as double-distilled water (DDW) in this work.Bacterial culture: E. carotovora Subsp. brasiliensis was isolated from potatoes and kindly supplied by the Maon Region Communities Cooperative, Israel.
Synthesis of TY-loaded HNTs/PDA hybrids
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min and the supernatant was discarded. The obtained pellet was washed 3 times with distilled water by centrifugation and dried overnight under vacuum at room temperature. The same procedure was also followed for in situ polymerization of dopamine onto pristine HNTs.
000 × g for 10 min and the supernatant was discarded. The obtained pellet was washed 3 times with distilled water by centrifugation and dried overnight under vacuum at room temperature. The same procedure was also followed for in situ polymerization of dopamine onto pristine HNTs.
Characterization
The morphology of pristine HNTs and HNTs/PDA hybrids was characterized using an FEI Tecnai G2 T20 S-Twin transmission electron microscope (TEM) at an accelerating voltage of 200 keV. The samples were prepared by mounting 5 μL the respective HNTs dispersion (1 mg mL−1) on a carbon type-B grid and dried overnight in a desiccator. Scanning electron microscopy-energy dispersive X-ray (STEM-EDX) measurement was performed by an EDAX EDS detector on samples mounted on nickel grids with a silicon oxide support. EDX data were processed by TIA (TEM Imaging & Analysis) software version 4.12, FEI Company, OR, USA.Thermogravimetric analysis (TGA) was carried out using a TGA Q5000 instrument (TA Instruments, USA) in a dynamic high-resolution mode (resolution number: 6; sensitivity value: 1). the samples were heated at a rate of 10 °C min−1 up to 600 °C. TGA results were analyzed by Universal Analysis 200 version 4.5A build 4.5.0.5 software.
The chemical composition of HNTs before and after modification was investigated by attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy using a Thermo 6700 FTIR spectrometer (USA) equipped with a Smart iTR diamond ATR device.
The zeta potential values of pristine and modified HNTs dispersions were measured in aqueous conditions at neutral pH (0.5 mg mL−1 in DDW) by a Malvern Zetasizer Nano ZSP instrument (UK) at 25 °C.
The optical absorbance of pristine and modified HNTs dispersions (following each stage of modification) were recorded at a concentration of 1 mg mL−1 using a multimode plate reader (Varioskan, Thermo Fisher Scientific, USA).
Quantification of thymol loading
TY content in TY-loaded HNTs and TY-loaded HNTs/PDA hybrids was quantified by its extraction in absolute ethanol. Briefly, TY-loaded samples were dispersed in 1 mL absolute ethanol (5 mg mL−1) and sonicated for 1 h on ice. Afterwards, the samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min and the supernatant was collected. Extraction and centrifugation were repeated for two successive cycles, and the collected supernatants were measured for their absorbance at 278 nm using a multimode plate reader. The concentration of TY in respective samples was determined using a calibration curve generated from different TY concentrations (see ESI,† Fig. S1). The TY loading capacity (LC) and encapsulation efficiency (EE) were determined by using the following equations:
000 × g for 10 min and the supernatant was collected. Extraction and centrifugation were repeated for two successive cycles, and the collected supernatants were measured for their absorbance at 278 nm using a multimode plate reader. The concentration of TY in respective samples was determined using a calibration curve generated from different TY concentrations (see ESI,† Fig. S1). The TY loading capacity (LC) and encapsulation efficiency (EE) were determined by using the following equations: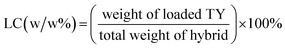 | (1) |
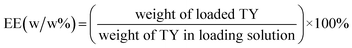 | (2) |
Stimuli-responsive thymol release
The release behavior of TY from the different carriers was investigated in different pH buffers at 30 °C under shaking (200 rpm).60,61 In brief, TY-loaded HNTs/PDA hybrids (1 mg) and TY-loaded HNTs (1.85 mg) were dispersed separately in 1 mL of pH 5 and pH 7 buffers. Note that due to the significant difference in the TY content in these systems, we used different amounts of loaded HNTs and kept the TY content constant at 0.14 mg. At different time intervals, the whole release medium was collected by centrifugation and replaced with an equal volume of fresh buffers. The amount of TY released in different pH buffers was quantified by measuring the TY absorbance at 278 nm.62 The release experiments were performed in triplicates and the data were plotted as % cumulative release vs. time. A double exponential saturation model was fitted for TY release profiles by Prism software (version 9.5.0 (730), GraphPad Software LLC) according to the following equation:| R(t) = Rinf + (R0 − Rinf)afaste−kfast·t + (R0 − Rinf)(1 − afast)e−kslow·t | (3) |
| R(t) = Rinf[1 − afaste−kfast·t − (1 − afast)e−kslow·t] | (4) |
Antibacterial assay
The antibacterial properties of TY-loaded HNTs/PDA hybrid against E. carotovora were characterized in vitro by the standard plate count method.59 To study the effect of pH on bacterial growth, the TY-loaded HNTs/PDA hybrid (0.67 mg) was dispersed in 0.4 mL buffer (pH 5 or pH 7) and mixed with 0.1 mL of bacterial suspension (108 CFU mL−1). The effect of NIR irradiation was studied by irradiating (at 808 nm wavelength and a power density of 1.5 W cm−2 for 15 min) the latter dispersions using a laser (WSLS-808-007-H, Wavespectrum Laser Group, Beijing, China) equipped with a fiber collimator (Thorlabs, Newton, NJ, USA). Following the different treatments, the bacterial dispersions were subjected to shaking at 200 rpm for 1 h at 28 °C, decimally diluted, and uniformly spread onto LB agar plates. The plates were then incubated at 28 °C for 16–18 h, and the bacterial colonies were counted using a CLC-330 colony counter (MRC Lab, Israel). Control experiments followed the same protocols for TY-loaded HNTs, TY emulsion (prepared by dispersing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of TY and Tween 80 in water at a concentration of 2 mg mL−1 using probe sonication at 40% amplitude), pristine HNTs, and PDA-coated HNTs. Note that the concentration of TY in TY-loaded HNTs (1.1 mg) and TY emulsion was 80 μg/0.5 mL (equivalent to the conc. of TY in 0.67 mg of TY-loaded HNTs/PDA hybrid) and the concentration of HNTs in pristine HNT and PDA-coated HNTs was 0.6 mg/0.5 mL (equivalent to the conc. of HNTs in 0.67 mg of TY-loaded HNTs/PDA hybrid).
1 ratio of TY and Tween 80 in water at a concentration of 2 mg mL−1 using probe sonication at 40% amplitude), pristine HNTs, and PDA-coated HNTs. Note that the concentration of TY in TY-loaded HNTs (1.1 mg) and TY emulsion was 80 μg/0.5 mL (equivalent to the conc. of TY in 0.67 mg of TY-loaded HNTs/PDA hybrid) and the concentration of HNTs in pristine HNT and PDA-coated HNTs was 0.6 mg/0.5 mL (equivalent to the conc. of HNTs in 0.67 mg of TY-loaded HNTs/PDA hybrid).
Imaging of fluorescently-labelled HNTs in plants
Phytotoxicity study
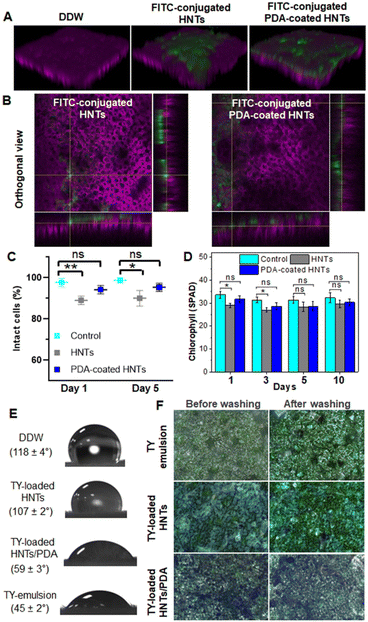
The phytotoxicity of HNTs and PDA-coated HNTs in tomato leaves was studied by assessing the integrity of the leaf cells membrane, by propidium iodide (PI) staining and subsequent confocal microscopy imaging. The leaves of live plants were treated (by spraying) with HNTs and PDA-coated HNTs dispersions at a concentration of 2.5 mg mL−1. After 1 and 5 days of treatment, leaf discs were collected with a cork borer and incubated in PI solution (10 μg mL−1) for 15 min, after which the samples were thoroughly washed with DDW to remove excess unbound dye. The stained disks were mounted in a gel chamber created on a microscope slide, filled with glycerol as a mounting medium, and sealed with a glass coverslip. The samples were imaged using a 20× objective lens by using a multiphoton multispectral laser-scanning microscope (Zeiss LSM 510 META NLO) at an excitation wavelength of 488 nm and emission was collected between 500–600 nm. The confocal images were used to calculate the percentage of intact cells (depicting no PI-stained nuclei) with respect to the total number of cells.The effect of the nanotubes on the chlorophyll content was studied for up to 10 days by using a chlorophyll meter (Spad 502 chlorophyll meter).
Wetting and adhesion studies
The wetting behavior of the different aqueous dispersions of HNTs, TY-emulsion, TY-loaded HNTs, and TY-loaded HNTs/PDA hybrid was studied on the tomato leaf surface using a contact angle optical tensiometer (Attension Theta Flow, Biolin Scientific). For measurement, the selected leaves were carefully fixed onto a glass slide using an adhesive double-sided tape.64 The contact angle (CA) was recorded through the sessile drop method by placing a droplet of 7 μL of the studied dispersion on the adaxial side of the leaf surface. To observe the change in the CA with time, the shape of the droplet was recorded by a camera for up to 10 min with an interval of 60 s and analyzed using the Attension software. The measurements for every sample were recorded in triplicates.The retention of the different formulations onto the leaf surface was investigated following a previously published procedure;65,66 where tomato leaves were sprayed with 5 mL of the respective formulations (i.e., pristine HNTs, TY emulsion, TY-loaded HNTs, and TY-loaded HNTs/PDA hybrid) and allowed to dry. The surface of the treated leaves was observed using an upright light microscope (ZEISS Axio Scope A1, Germany) equipped with an Axiocom MRc (ZEISS, Germany) camera. The treated leaves were washed by spraying 5 mL of DDW for 30 s at an angle of 60° and imaged. The data was processed using ZEN blue (Carl Zeiss Microscopy) software and Z-stack projections images are presented.
In planta antibacterial studies
 | (5) |
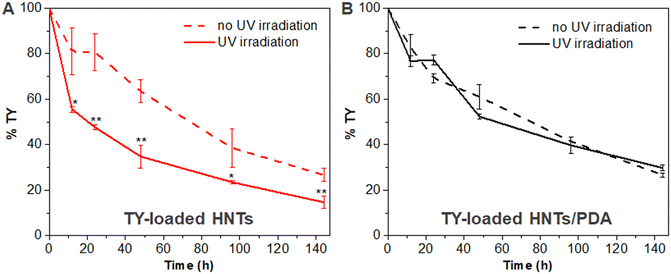
Thymol content following UV irradiation
The capability of TY-loaded HNTs and TY-loaded HNTs/PDA hybrid to retain the TY content was studied with and without irradiation with UV light.70 The respective samples were dispersed in DDW at a TY concentration of 0.5 mg mL−1. Subsequently, the samples were placed 10 cm under a UV lamp (Hamamatsu, Lightningcure LC8, Japan) at a wavelength of 365 nm and irradiated at 20% intensity of 138 mW cm−2 for different periods (12, 24, 48, 96, and 144 h). After irradiation, the residual TY content was extracted from the respective sample using ethanol and quantified by absorbance measurements at 278 nm (Varioskan, Thermo Fisher Scientific, USA). The control dispersions (non-irradiated) were investigated using the same protocol.Statistical analysis
Origin software (Origin Pro 8.5 Corporation, U.S.) was used to plot the data. Student t-test (two-tailed) using GraphPad Prism 8.0 software was performed at a confidence level of 99% to calculate the significance of the difference between groups. The experiments were performed in triplicates and plotted as mean ± standard error. Plots showing no asterisks or “ns” indicate the absence of a significant difference.Results
Synthesis and characterization of thymol-loaded HNTs/PDA hybrids
The synthesis of TY-loaded HNTs and TY-loaded HNTs/PDA hybrids is schematically illustrated in Fig. 1A. HNTs were loaded with TY using the solvent evaporation method under vacuum,38 and subsequently subjected to an in situ dopamine polymerization. Dopamine self-polymerizes through a series of oxidation, cyclization, and isomerization reactions in mild-basic conditions to form PDA,71 while the reactions are accompanied by a distinct color change of the TY-loaded HNTs dispersion from whitish to deep brown (Fig. 1A).48,72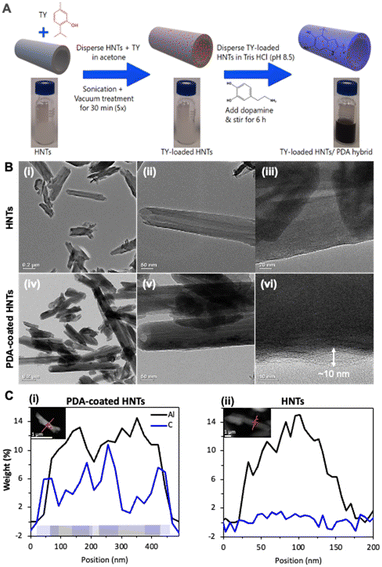 | ||
| Fig. 1 (A) A schematic illustration summarizing the steps followed for the preparation of TY-loaded HNTs/PDA hybrid. (B) TEM images of pristine HNTs (i–iii), showing their characteristic tubular morphology and open-ended lumen, and the HNTs/PDA hybrid (iv–vi) obtained following the synthetic steps in A, depicting a thin layer that coats the nanotubes. (C) Elemental profile of Al (black) and C (blue) for (i) PDA-coated HNTs and (ii) HNTs measured by STEM-EDX. The presence of the analyzed elements is schematically illustrated at the bottom of (i). Insets depicting the analyzed clay nanotubes with the profile trace indicated in orange. For further details please refer to Fig. S3 in the ESI.† | ||
The morphology and structure of the resulting particles were characterized by TEM and the respective micrographs are presented in Fig. 1B. Indeed, the modified nanotubes show a thin polymer shell that coats their external surface (Fig. 1B-vi), which cannot be observed for the pristine HNTs (Fig. 1B-iii). The pristine HNTs exhibit cylindrical shape with an irregular inner and outer diameter of 10–30 nm and 40–100 nm, respectively (Fig. 1B-i). The HNT lumen is clearly observable as a brighter strip running along the nanotubes longitudinally, revealing open-ended features (Fig. 1B-ii).73,74 Following polymerization the contrast between the HNT lumen and its external surface is more pronounced in comparison to the neat HNTs, see Fig. 1B-v in comparison to Fig. 1B-iii, as was previously observed.74 Importantly, higher magnification images, see Fig. 1B-vi and S2 in the ESI,† reveal a thin ∼10 nm layer that coats the nanotubes, ascribed to the PDA, and confirms the formation of core–shell nanostructures. STEM-EDX analysis, presented in Fig. 1C-i and S3,† indicates the colocalization of the aluminum compound of the coated-HNTs and a carbonaceous layer. The latter is observed to further extend a few nanometers beyond the outer surface of the HNTs wall and also into the middle section of the profile (position 180–260 nm), characterized by a lower aluminum composition and thus identified as the tube lumen. No elevated carbon content was observed in the profile of the control pristine HNTs (Fig. 1C-ii). Thus, we suggest that the mechanism of the shell formation first involves the adherence of dopamine catechol anchors onto the HNTs surface, via various interactions viz., H-bonding and electrostatic,75,76 followed by polymerization. The presence of a PDA shell on various nanomaterials in the size range of 3–10 nm has been observed and reported before.48,77–79
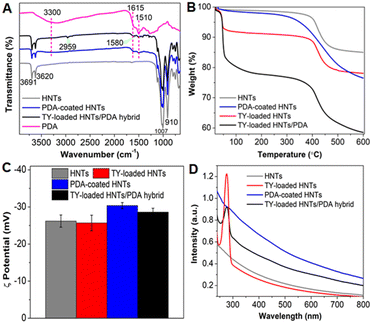
The chemical composition of the hybrid following each of the synthetic steps, described in Fig. 1A, was studied using FTIR-ATR (Fig. 2A). The spectrum of HNTs depicts the signature bands at 910 cm−1, 1007 cm−1, 3620 cm−1, and 3691 cm−1 corresponding to the O-H bending of inner hydroxyl groups, in-plane Si–O–Si symmetric stretching vibrations, inner AlO–H stretching vibrations, and O–H stretching of inner-surface hydroxyl groups, respectively (Fig. 2A black curve).80 The spectrum of TY-loaded HNTs (Fig. S4A in the ESI,† red curve) depicts two new peaks at 2959 cm−1 and 1580 cm−1 corresponding to the CH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of TY, respectively,62,81 which matches with the spectrum of pure TY (Fig. S4A in the ESI,† grey trace). After dopamine polymerization, the following additional peaks were observed: (i) a broad peak between 3140–3400 cm−1 corresponding to the -OH vibrations of catechol groups, (ii) less intense peaks at 1510 cm−1 and 1615 cm−1 ascribed to the shearing vibration of N-H in amide group and aromatic rings of PDA (see also Fig. S4B† for a detailed spectrum from 2000 to 600 cm−1),52 all of which match with the spectrum of pure PDA (Fig. 2A, magenta curve). In addition, the spectrum of TY-loaded HNTs/PDA hybrid (see also Fig. S4B† for a detailed spectrum from 2000 to 600 cm−1) depicts all characteristic peaks that well correspond to HNTs, TY, and PDA, confirming the presence of all three components.
C stretching vibrations of TY, respectively,62,81 which matches with the spectrum of pure TY (Fig. S4A in the ESI,† grey trace). After dopamine polymerization, the following additional peaks were observed: (i) a broad peak between 3140–3400 cm−1 corresponding to the -OH vibrations of catechol groups, (ii) less intense peaks at 1510 cm−1 and 1615 cm−1 ascribed to the shearing vibration of N-H in amide group and aromatic rings of PDA (see also Fig. S4B† for a detailed spectrum from 2000 to 600 cm−1),52 all of which match with the spectrum of pure PDA (Fig. 2A, magenta curve). In addition, the spectrum of TY-loaded HNTs/PDA hybrid (see also Fig. S4B† for a detailed spectrum from 2000 to 600 cm−1) depicts all characteristic peaks that well correspond to HNTs, TY, and PDA, confirming the presence of all three components.
To complement these findings, we studied the weight change following each of the synthetic steps used for the fabrication of the TY-loaded HNTs/PDA hybrid by thermogravimetry and the results are presented in Fig. 2B. The pristine HNTs thermogram shows weight losses of 1% at a temperature range of 22 °C to 40 °C and of 13% between 300 °C and 530 °C, corresponding to the loss of moisture and dehydroxylation of structural Al-OH and Si-OH groups from HNTs, respectively.82 After TY loading into the HNTs (TY-loaded HNTs), an additional weight loss of 6.6% (between 37 °C to 65 °C) is observed, confirming the successful loading of the EO (Fig. 2B and S5A† for pure TY). The thermogram of the TY-loaded HNTs/PDA hybrid (Fig. 2B, black curve) depicts a complex behavior, where weight losses at 37–65 °C (16.5%) and 300–530 °C (∼13%) are ascribed to TY evaporation and HNT hydroxyls, respectively (Fig. S5B in the ESI† for the corresponding DTG curves for clarity). Since PDA decomposes over a wide temperature range52,83 (see Fig. S5B ESI† for DTG curve of neat PDA), we have quantified the polymer content in the hybrid according to its residue on a dry basis compared to that of pristine HNTs as depicted in Fig. S5C, ESI.† Accordingly, the PDA content is 8% and is in agreement with other studies,42,75,84 confirming the successful formation of a PDA coating onto both pristine HNTs and TY-loaded HNTs.
The thermograms also reveal that the TY content in the hybrid is 2.7-fold higher than in the case of the TY-loaded HNTs (no PDA, see Table S1†). This can be attributed to the PDA layer which encapsulates the TY payload within the clay lumen and entraps the surface-physiosorbed TY molecules, retaining the EO during the washing step following the in-situ polymerization. Moreover, this can be also facilitated by non-covalent interactions (π–π and H-bonding) between the TY cargo and PDA, as was previously reported for several aromatic payloads such as the anticancer drug doxorubicin and the pesticide imidacloprid.52,85 The DTG curves, presented in Fig. S3A-i and S3B (ESI†), reveal the profound effect of loading on the evaporation endpoint temperature of TY. The TY payload in the hybrid exhibits the highest value compared to TY-loaded HNTs (without PDA) and neat TY with values of 200, 65 and 55 °C, respectively. Thus, the substantial increase of the thermal stability of TY in the PDA-coated hybrid is mainly associated with former's encapsulation and entrapment by the polymer shell, while the adsorptive interactions at the clay surface29,86 play a lesser role.
Zeta potential measurements of the HNTs aqueous dispersions (under neural pH) following each of the synthetic steps, detailed in Fig. 1A, were carried out to complement the surface characterization of the modified HNTs and the results are presented in Fig. 2C. Following dopamine polymerization, the zeta potential value of the HNTs (−26.2 ± 1 mV for pristine HNTs, attributed to the presence of negatively charged silica groups at the tubes' external surface)87,88 is found to decrease to a value of −30.3 ± 0.7 mV. This may be ascribed to the deprotonated catechol–OH groups of PDA,89,90 further supporting the formation of a PDA coating onto the nanotubes. A similar decrease was also obtained for the TY-loaded HNTs/PDA hybrid (−28.6 ± 0.1 mV), where it should be noted that TY loading resulted in only minor zeta potential change in comparison to the pristine HNTs, ascribed to the uncharged nature of TY.91
The optical properties for aqueous dispersions of the HNTs-based hybrids (1 mg mL−1) were investigated by measuring their absorption spectra (Fig. 2D). In comparison to pristine HNTs, PDA-coated HNTs and TY-loaded HNTs/PDA hybrid show stronger absorbance in the UV-vis-NIR region, as contributed by the PDA shell.78,92 For PDA-coated HNTs, the observed absorbance peak at 280 nm is assigned to the presence of a considerable fraction of dopamine in PDA.79 The broad absorbance specifically towards the NIR region is attributed to the electronic properties of π–π* transition of polymeric backbone benzenoid ring,93,94 and is favorable for photothermal therapy.78 In addition, TY-loaded HNTs/PDA hybrid shows an intense absorbance peak at 278 nm, ascribed to the presence of TY.62 A similar peak is also detected in the spectrum of the TY-loaded HNTs but without the pronounced PDA absorbance.
Thymol loading and release
Thymol was loaded into HNTs using the solvent evaporation method under vacuum and the resulting loaded HNTs were used for the subsequent dopamine polymerization followed by a washing step in DDW. Note that the control sample, TY-loaded HNTs without PDA, underwent a similar washing step using DDW following the loading process. Subsequently, thymol extraction was used to characterize the loading capacity of the modified HNTs. After careful composition tailoring, we found that for a ratio of 1.25 (HNTs)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5(TY)
2.5(TY)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(PDA), the highest TY loading capacity of 14.7 ± 1.9% and encapsulation efficiency of 7.4 ± 1.0% is obtained. Notably, the latter TY content is ∼2.5-fold higher in comparison to the corresponding TY-loaded HNTs (no PDA), where a loading capacity of only 5.6 ± 1.8% and encapsulation efficiency of 2.8 ± 0.9% is attained. These measured TY loading values are in good agreement with the calculated loading capacity based on the TGA data (see Fig. 2B and Table S1 in the ESI†). The loaded TY molecules can be confined within the clay lumen and also physiosorbed onto the HNTs surface. During the in situ polymerization, PDA is suggested to encapsulate the lumen-confined TY molecules and sterically entrap those physiosorbed onto the clay surface,85 reducing their loss during the consecutive washing step, and thus improving the loading capacity. Molecular interactions, including H-bonding and π–π interactions between the TY molecules and the catechol as well as the primary amine groups of PDA further facilitate the reattainment of these volatile molecules.85,95
1(PDA), the highest TY loading capacity of 14.7 ± 1.9% and encapsulation efficiency of 7.4 ± 1.0% is obtained. Notably, the latter TY content is ∼2.5-fold higher in comparison to the corresponding TY-loaded HNTs (no PDA), where a loading capacity of only 5.6 ± 1.8% and encapsulation efficiency of 2.8 ± 0.9% is attained. These measured TY loading values are in good agreement with the calculated loading capacity based on the TGA data (see Fig. 2B and Table S1 in the ESI†). The loaded TY molecules can be confined within the clay lumen and also physiosorbed onto the HNTs surface. During the in situ polymerization, PDA is suggested to encapsulate the lumen-confined TY molecules and sterically entrap those physiosorbed onto the clay surface,85 reducing their loss during the consecutive washing step, and thus improving the loading capacity. Molecular interactions, including H-bonding and π–π interactions between the TY molecules and the catechol as well as the primary amine groups of PDA further facilitate the reattainment of these volatile molecules.85,95
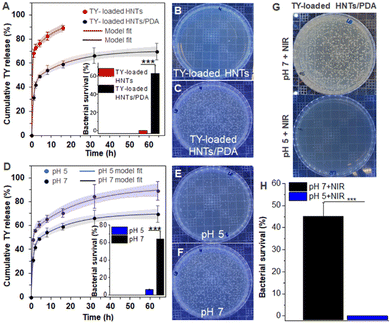
The release of TY from the TY-loaded HNTs and the TY-loaded HNTs/PDA hybrid was investigated in a pH 7 buffer at 37 °C and the obtained profile is presented in Fig. 3A; where a double exponential model is applied with a very good fit to the experimental results (R2 > 0.97 for TY-loaded HNTs/PDA and >0.9 for TY-loaded HNTs). The double exponential model describes a mass transfer regime comprising two different release sites simultaneously, one of them is more rapid than the other.96,97 In our system, the fast and slow-release mechanisms are attributed to the TY adsorbed onto the clay surface and inside the HNTs lumen, respectively. For the PDA-coated hybrid (Fig. 3A), a 3-fold decrease in the rate constant of the fast-release component and a consecutive increase in its half-time (from 3 to 1 h) is obtained. Thus, the PDA shell polymerized on the nanotubes (see Fig. 1B-vi) hinders the burst release of the surface-adsorbed TY molecules. This assumption is further supported by the fact that the calculated parameters of the slow-release component, including the partition between fast and slow components were not significantly altered following the PDA coating. The slower release of TY from the PDA-coated hybrid can be also corroborated by its exerted antimicrobial properties as will be discussed in the next section.
PDA is well known for its stimuli-responsive properties, specifically pH and NIR irradiation,98,99 and therefore its coating onto the loaded HNTs is expected to affect the TY release under different environmental conditions. First, the effect of pH on the TY release from TY-loaded HNTs/PDA hybrid was investigated and Fig. 3D depicts the accumulated release in pH 5 and 7. Under acidic conditions, the observed value of cumulative release was 20–30% higher in comparison to neutral conditions through most of the release study, as well as for the calculated release at infinite time. Nonetheless, no significant differences were observed in the release kinetics or partition between fast and slow components. A similar effect of acidic conditions on the plateau release value of other drugs and active compounds from PDA-coated hybrids has been reported before.100,101 The acid-triggered release behavior of the TY-loaded HNTs/PDA hybrid further underscores its core–shell structure and is ascribed to conformational changes of the PDA shell due to the electrostatic repulsion of protonated amine groups possibly enabling higher equilibrium concentration of the released cargo.89
Antibacterial properties
The in vitro antibacterial activity against E. carotovora exerted by the TY-loaded HNTs and TY-loaded HNTs/PDA hybrids was investigated in a neutral buffer (pH 7) and the results (after 1 h incubation) are depicted in Fig. 3B and C, respectively (see also inset in Fig. 3A). The TY-loaded HNTs induced 100% bacterial inhibition, similar to the effect of a TY emulsion at an equivalent concentration (Fig. S6 in the ESI†). These results correspond to the burst release of TY from the nanotubes, where >70% of the TY was released within 1 h, as presented in Fig. 3A. The attained antibacterial effect of the TY-loaded HNTs/PDA hybrid after 1 h was moderate, compared to that of the TY-loaded HNTs, and consistent with the sustained release of TY from these coated nanotubes.The effect of the PDA layer on the release of TY is further manifested when comparing the exerted antibacterial activity of the TY-loaded HNTs/PDA hybrid at different pH conditions. At pH 5, the hybrid's antibacterial activity was significantly enhanced, and 93% inhibition was obtained (Fig. 3E), demonstrating a 2.6-fold increase compared to neutral conditions, see inset in Fig. 3D. This finding is ascribed to accelerated TY release at a lower pH value as discussed in the previous section. It should be noted that pH alone has a negligible effect on the growth of E. carotovora, as shown in Fig. S6 in the ESI.†102
PDA's strong absorption in the NIR region and photothermal properties have motivated its exploitation as a coating material rendering surfaces with photo-responsive antimicrobial properties.93,103,104 Thus, the effect of NIR irradiation on the exerted antibacterial properties of the developed hybrid was investigated. First, the photothermal properties of both the PDA-coated HNTs and the TY-loaded hybrid were characterized under irradiation with a NIR laser (15 min at 808 nm), and a significant temperature increase of >20 °C was detected, see Fig. S7 in the ESI.† Next, the antibacterial properties following irradiation were measured and the results are presented in Fig. 3G and H, where for the TY-loaded HNTs/PDA hybrid a complete bacterial inhibition at pH 5 is observed. At pH 7, the irradiation effect was weaker, and a 1.5-fold enhancement in bacterial inhibition was attained. Note that for the control PDA-coated HNTs (no TY), NIR irradiation induced only a 20% increase in bacterial inhibition, see Fig. S8 in the ESI.† Therefore, the superior photo-induced antibacterial efficacy of the TY-loaded hybrid can be ascribed to the combined effect of the photothermal activity of the PDA coating,93,105,106 which was previously shown to damage bacterial cell-membrane and its constituents,107,108 and the released TY payload. Thus, in the context of agriculture, the combination of solar NIR irradiation and naturally induced acidic conditions in plants may be harnessed to trigger the dual stimuli-responsive property of the loaded hybrids. Lower pH at bacterial infection sites in plants could be elicited by various factors including acidic cell-wall degrading enzymes secreted by bacteria, such as polygalacturonase of E. carotovora55,56,109,110 or pathogen-induced acidification of plant organelles,57 as well as acidic signaling compounds including ethylene produced by plants in response to infection.111
Interactions of HNT-based carriers with tomato leaf surface
Taking these observations into consideration, we investigated the potential uptake of HNTs via foliar routes for model tomato plants. Both pristine HNTs and PDA-coated HNTs were labeled with FITC, see ESI† for fluorescence spectra in Fig. S9, and imaged by confocal microscopy. Fig. 4A depicts the reconstructed 3D z-stacking images, taken from the leaf epidermis to mesophyll cells. The labeled HNTs and hybrid particles are observed only on the leaf surface (where the chloroplast autofluorescence is observed as magenta). Moreover, Pearson's coefficient analysis (P = 0) confirms no correlation between the fluorescence signal of the nanotubes and that of the chloroplasts, see Fig. S10 in the ESI† for additional information. The orthogonal views, presented in Fig. 4B, allow further localization of the particles in deeper x–z and y–z directions of the leaf, where again no overlapping of the FITC fluorescence and chloroplast autofluorescence is observed. The fluorescence from both labeled HNTs and PDA-coated HNTs is detected only from the epidermal cell layer (Fig. S11 in the ESI†) and no FITC fluorescence can be observed in the mesophyll cells (see Fig. 4A and S10 in the ESI†). Thus, these results indicate that the nanotubes are localized on and within the epidermis layer, without penetrating inside the leaf, suggesting their potential as a safe nanocarrier for the delivery of active payloads.
A complementary light microscopy study (using an upright reflectance microscope) reveals the accumulation of the different HNTs-based carriers on the leaf cuticle following their application via spraying (Fig. 4F, left panel). Note that the deposition of the hybrid particles on the leaf surface can be easily observed by their distinct dark color in comparison to the corresponding whitish TY-loaded HNTs. Nanocarriers with high-aspect ratio, such as HNTs, are suggested as advantageous for foliar applications given their higher contact area in comparison to spherical particles.117 The retention of the loaded carriers onto the plant leaves was qualitatively evaluated following their wash with water and the respective micrographs are depicted in Fig. 4F, right panel. The PDA-coated carriers exhibit greater retention on the leaf surface in comparison to TY-loaded HNTs, which can be ascribed to the enhanced wettability provided by the PDA as well as to the strong adhesion provided by the catechol side chains of PDA, which can strongly bind to various types of surfaces.46,47,120 Moreover, the formation of different interactions (H-bonding, electrostatic, and covalent Schiff base) between the PDA hydroxyls and amines and functional groups on leaf surface (complex fatty acids, fatty alcohols, and fatty aldehydes) have been previously suggested.65,120 Thus, these results demonstrate the superior foliar adhesion and retention of the hybrid carriers, which can potentially provide long-term efficacy.
Efficacy of TY-loaded HNTs/PDA hybrid against soft rot disease
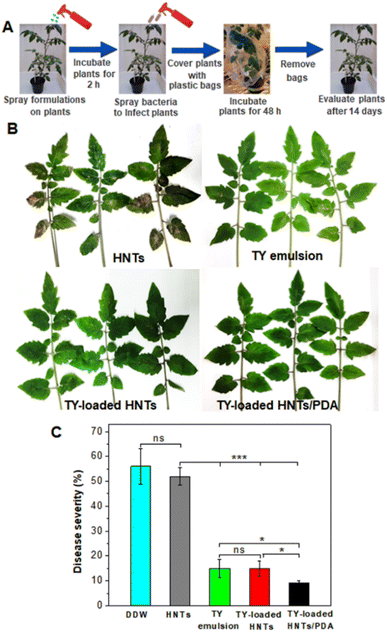
The potential of the TY-loaded HNTs/PDA hybrid to suppress the soft rot disease of tomato plants was investigated and compared to that of TY-loaded HNTs and TY emulsion. The latter was used as a positive control at an optimized concentration of 0.4 mg mL−1, corresponding to previously reported values which were demonstrated as effective in reducing >50% bacterial infection on plants.121,122 The concept of the study is presented in Fig. 5A, where tomato plants were first treated with the respective dispersion and then inoculated with E. carotovora bacterial suspensions. Plants treated with just DDW (see Fig. S13 in the ESI†) exhibit bacterial spots with coalescence on leaflets with a disease severity index (DSI) of >50%, demonstrating the ability of E. carotovora to severely infect the leaves.Fig. 5B displays representative images of foliage treated with the respective formulations. All TY-containing treatments are shown to suppress to some extent the disease visual symptoms in comparison to plants treated with HNTs dispersion (or DDW). Quantitative analysis of the treatment's efficacy on disease severity (Fig. 5C), expressed in terms of % DSI, reveals that pristine HNTs have no significant antibacterial activity. In the case of TY-loaded HNTs (as well as TY emulsion used as a positive control), a significant reduction in DSI to ∼15% is realized, where leaflets do not display any coalescence of bacterial spots but show sporadic necrotic symptoms (see Fig. 5B). Interestingly, analysis of leaves treated with TY-loaded HNTs/PDA confirms the superior efficacy of the developed nano-formulation in mitigating the soft rot disease in comparison to other treatments, which may be attributed to the controlled release behavior of the hybrid carrier. Furthermore, the hybrid could retain its disease control effect under drift conditions due to the good wettability and exceptional adhesion ability provided by the catechol moieties of the PDA even under wet conditions.47,123 We note that the photothermal activity of the hybrid nanocarriers could potentially enhance even further their antibacterial activity of the released TY under sunlight, yet this was challenging to demonstrate in a greenhouse experiment.
TY Stability under UV irradiation
A wide range of volatile EOs, including TY, have been reported to be chemically unstable under various environmental conditions (e.g., light, heat, air) resulting in deteriorated biological activity.124 Several studies reported that encapsulation of EOs in different nanocarriers allows them to retain their exerted biological activity by reducing their volatility.26,124–127 As the designed HNT-based carriers should function under varying UV conditions, we studied their chemical stability under UV irradiation and the results are depicted in Fig. 6. The TY-loaded HNTs/PDA hybrid (Fig. 6B) is found to exhibit superior retention of TY in comparison to the loaded HNTs (Fig. 6A) throughout the 144 h-long study. Notably, there are no significant differences in the extracted TY content w/ and w/o UV irradiation for the TY-loaded HNTs/PDA hybrid. However, in the case of the TY-loaded HNTs, UV irradiation is found to induce a profound and significant decrease in the residual TY content. After 48 h of UV exposure, the TY content decreased to a value of 34.8 ± 5.1% for the TY-loaded HNTs, and the residual TY content at the end of the experiment was lower than 15%, see Fig. 6A. In comparison, for the HNTs/PDA hybrid, TY content after 48 h was 52.4 ± 0.9% and 29.8 ± 1.3% at the end of the study (144 h), see Fig. 6B. This 1.5-fold difference in TY content between the carriers is attributed to the UV-shielding property of the PDA shell which covers the HNTs,50 and minimizes TY loss, which is highly advantageous for foliar application of these carrier.Conclusions
This study presents a stimuli-responsive core–shell agro-nanocarrier which is easily fabricated from natural compounds via in situ polymerization of dopamine on clay HNTs loaded with the antibacterial essential oil, thymol (TY). The resulting TY-loaded HNTs/PDA hybrid demonstrates superior efficiency in protecting tomato plants from soft rot disease, as demonstrated in planta by real-scenario greenhouse experiments. This superior crop protection performance is ascribed to the advantageous attributes of the PDA-coated nanocarrier, as detailed below.The 10 nm thick layer of PDA, polymerized on the clay nanotubes, is shown to significantly improve the loading capacity of TY and allows for its sustained and prolonged release in comparison to TY-loaded HNTs lacking the PDA compound. Moreover, the unique chemical composition of PDA allows for a dual-stimuli-triggered TY release under acidic conditions (characteristic of plant bacterial infection site) and via a photothermal effect upon exposure to NIR irradiation (which mimics the NIR irradiation in sunlight). This is corroborated by in vitro results demonstrating that both stimuli induce an intensification of the antibacterial effect exerted by the developed hybrid against E. carotovora, the bacterial pathogen responsible for the soft rot disease.
Regarding formulation stability, a rain simulation experiment reveals that the formulation is highly resistant to wash-out from the leaf surface. In addition, the PDA layer enhances TY's chemical stability under UV irradiation, potentially minimizing the loss of the active ingredient under sunlight. Both of these attributes can maintain the long-term bioactivity of the loaded compound by reducing its loss to the environment, thereby rendering the formulation more sustainable and cost-effective.
Furthermore, 3D confocal images demonstrate that the PDA-coated clay particles remain on the leaf's epidermis layer without being internalized through foliar uptake routes, obviating the formulation's phytotoxicity and potential entry into the food chain.
Our findings underscore the significant potential of the designed PDA-coated clay-based nanocarrier to enhance the effectiveness, stability, and safety of biocide application in plant pathogen control. In addition to antimicrobial agents, the presented system could be utilized in the future as a generic platform for the controlled release of a broader range of bioactive compounds, including plant growth regulators, protecting crops from diseases, promoting healthy plant growth, and improving overall yield.
Author contributions
S. S. conceived the idea of PDA coating; synthesized and characterized the properties of the nanocarriers; performed: loading and release experiments, stability study, in vitro antibacterial assay, plant-HNTs interactions experiments, and in-planta efficacy studies of the nano-formulations. He also wrote the first draft. O. P. S. carried out all the electron microscopy studies, analysed the release and thermogravimetry data, and participated in writing and revising the manuscript. H. A. H. assisted in performing the in-vitro antibacterial assay and synthesizing the nanocarriers. E. S. supervised the study, and was responsible for funding acquisition, as well as contributed to data analysis, writing and revising the manuscript. All authors have read and agreed to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Israel Innovation Authority in the framework of the SMART consortium. The authors thank Dr. Michal Leshem for editing the manuscript.References
- SDG 2, Zero hunger | Sustainable Development Goals | Food and Agriculture Organization of the United Nations, https://www.fao.org/sustainable-development-goals/goals/goal-2/en/, (accessed 4 February 2023) Search PubMed.
- FAOSTAT, https://fenix.fao.org/faostat/internal/en/#home, (accessed 4 February 2023) Search PubMed.
- C. Erika, S. Griebel, M. Naumann and E. Pawelzik, Front. Plant Sci., 2020, 11, 589692 CrossRef PubMed.
- Y. Aysan, F. Sahin, R. Cetinkaya-Yildiz, M. Mirik and F. Yucel, J. Plant Dis. Prot., 2005, 112(1), 42–51 Search PubMed.
- A. Akbar, S. ud Din, M. Ahmad, G. daraz Khan and S. Alam, J. Nat. Sci. Res., 2014, 4(11), 99–102 Search PubMed.
- A. M. Alippi, E. Dal Bó, L. B. Ronco, P. E. Casanova and O. M. Aguilar, Plant Dis., 1997, 81, 230 CAS.
- N. Pradnyarani, M. S. Kulkarni, K. C. Kirankumar, R. K. Mesta and C. K. Chethankumar, Int. J. Chem. Stud., 2018, 6(4), 75–78 Search PubMed.
- Y. Kolomiiets, I. Grygoryuk, L. Butsenko, V. Bohoslavets, Y. Blume and A. Yemets, Open Agric. J., 2020, 14, 290–298 Search PubMed.
- W. Aktar, D. Sengupta and A. Chowdhury, Interdiscip. Toxicol., 2009, 2, 1 Search PubMed.
- G. W. Sundin, L. F. Castiblanco, X. Yuan, Q. Zeng and C. H. Yang, Mol. Plant Pathol., 2016, 17, 1506 CrossRef CAS PubMed.
- F. H. M. Tang, M. Lenzen, A. McBratney and F. Maggi, Nat. Geosci., 2021, 14, 206–210 CrossRef CAS.
- R. Nair, S. H. Varghese, B. G. Nair, T. Maekawa, Y. Yoshida and D. S. Kumar, Plant Sci., 2010, 179, 154–163 CrossRef CAS.
- T. C. Hoang, E. C. Rogevich, G. M. Rand, P. R. Gardinali, R. A. Frakes and T. A. Bargar, Environ. Pollut., 2008, 154, 338–347 CrossRef CAS PubMed.
- S. Sharma, B. Singh, P. Bindra, P. Panneerselvam, N. Dwivedi, A. Senapati, A. Adholeya and V. Shanmugam, ACS Appl. Mater. Interfaces, 2021, 13, 9143–9155 CrossRef CAS PubMed.
- X. Zhao, H. Cui, Y. Wang, C. Sun, B. Cui and Z. Zeng, J. Agric. Food Chem., 2018, 66, 6504–6512 CrossRef CAS PubMed.
- R. C. Fierascu, I. C. Fierascu, C. E. Dinu-Pirvu, I. Fierascu and A. Paunescu, Z. Naturforsch., C: J. Biosci., 2020, 75, 183–204 CrossRef CAS PubMed.
- C. Regnault-Roger, C. Vincent and J. T. Arnason, Annu. Rev. Entomol., 2012, 57, 405–424 CrossRef CAS PubMed.
- A. T. H. Mossa, J. Environ. Sci. Technol., 2016, 9, 354–378 CrossRef CAS.
- A. Escobar, M. Pérez, G. Romanelli and G. Blustein, Arabian J. Chem., 2020, 13, 9243–9269 CrossRef CAS.
- F. Tao, L. E. Hill, Y. Peng and C. L. Gomes, LWT--Food Sci. Technol., 2014, 59, 247–255 CrossRef CAS.
- A. Marchese, I. E. Orhan, M. Daglia, R. Barbieri, A. Di Lorenzo, S. F. Nabavi, O. Gortzi, M. Izadi and S. M. Nabavi, Food Chem., 2016, 210, 402–414 CrossRef CAS PubMed.
- Federal Register :: Thymol; Exemption from the Requirement of a Tolerance, https://www.federalregister.gov/documents/2006/01/18/06-436/thymol-exemption-from-the-requirement-of-a-tolerance, (accessed 4 February 2023) Search PubMed.
- eCFR :: 21 CFR Part 172 -- Food Additives Permitted for Direct Addition to Food for Human Consumption, https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172, (accessed 4 February 2023) Search PubMed.
- M. Coimbra, B. Isacchi, L. Van Bloois, J. S. Torano, A. Ket, X. Wu, F. Broere, J. M. Metselaar, C. J. F. Rijcken, G. Storm, R. Bilia and R. M. Schiffelers, Int. J. Pharm., 2011, 416, 433–442 CrossRef CAS PubMed.
- G. M. Glenn, A. P. Klamczynski, D. F. Woods, B. Chiou, W. J. Orts and S. H. Imam, J. Agric. Food Chem., 2010, 58, 4180–4184 CrossRef CAS PubMed.
- A. R. Bilia, C. Guccione, B. Isacchi, C. Righeschi, F. Firenzuoli and M. C. Bergonzi, J. Evidence-Based Complementary Altern. Med., 2014, 2014, 651593 Search PubMed.
- A. Giannakas, I. Tsagkalias, D. S. Achilias and A. Ladavos, Appl. Clay Sci., 2017, 146, 362–370 CrossRef CAS.
- M. A. Kinninmonth, C. M. Liauw, J. Verran, R. Taylor, V. Edwards-Jones, D. Shaw and M. Webb, Appl. Clay Sci., 2013, 83–84, 415–425 CrossRef CAS.
- M. Krepker, R. Shemesh, Y. Danin Poleg, Y. Kashi, A. Vaxman and E. Segal, Food Control, 2017, 76, 117–126 CrossRef CAS.
- M. H. Lee, H. S. Seo and H. J. Park, J. Food Sci., 2017, 82, 922–932 Search PubMed.
- E. Joussein, Dev. Clay Sci., 2016, 7, 12–48 CAS.
- F. W. Degrazia, V. C. B. Leitune, A. S. Takimi, F. M. Collares and S. Sauro, Dent. Mater., 2016, 32, 1133–1143 CrossRef CAS PubMed.
- O. Prinz Setter and E. Segal, Nanoscale, 2020, 12, 23444–23460 Search PubMed.
- H. Wei, H. Wang, H. Chu and J. Li, Int. J. Biol. Macromol., 2019, 133, 1210–1218 CrossRef CAS PubMed.
- L. Chen, Z. Guo, B. Lao, C. Li, J. Zhu, R. Yu and M. Liu, Environ. Sci.: Nano, 2021, 8, 3015–3027 RSC.
- A. C. Santos, C. Ferreira, F. Veiga, A. J. Ribeiro, A. Panchal, Y. Lvov and A. Agarwal, Adv. Colloid Interface Sci., 2018, 257, 58–70 Search PubMed.
- M. R. Dzamukova, E. A. Naumenko, Y. M. Lvov and R. F. Fakhrullin, Sci. Rep., 2015, 5, 1–11 Search PubMed.
- G. Fakhrullina, E. Khakimova, F. Akhatova, G. Lazzara, F. Parisi and R. Fakhrullin, ACS Appl. Mater. Interfaces, 2019, 11, 23050–23064 CrossRef CAS PubMed.
- M. Massaro, G. Cavallaro, C. G. Colletti, G. Lazzara, S. Milioto, R. Noto and S. Riela, J. Mater. Chem. B, 2018, 6, 3415–3433 Search PubMed.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- C. J. Bettinger, J. P. Bruggeman, A. Misra, J. T. Borenstein and R. Langer, Biomaterials, 2009, 30, 3050–3057 Search PubMed.
- M. L. Alfieri, M. Massaro, M. d'Ischia, G. D'Errico, N. Gallucci, M. Gruttadauria, M. Licciardi, L. F. Liotta, G. Nicotra, G. Sfuncia and S. Riela, J. Colloid Interface Sci., 2022, 606, 1779–1791 CrossRef CAS PubMed.
- S. Yuce, O. Demirel, B. Alkan Tas, P. Sungur and H. Unal, ACS Appl. Nano Mater., 2021, 4, 13432–13439 CrossRef CAS.
- C. E. Taş, S. O. Gundogdu and H. Ünal, ACS Appl. Nano Mater., 2022, 5, 5407–5415 CrossRef.
- M. J. Harrington, A. Masic, N. Holten-Andersen, J. H. Waite and P. Fratzl, Science, 2010, 328, 216–220 CrossRef CAS PubMed.
- Y. Tong, L. Shao, X. Li, J. Lu, H. Sun, S. Xiang, Z. Zhang, Y. Wu and X. Wu, J. Agric. Food Chem., 2018, 66, 2616–2622 CrossRef CAS PubMed.
- J. Liebscher, Eur. J. Org. Chem., 2019, 2019, 4976–4994 Search PubMed.
- X. Li, C. Xie, H. Xia and Z. Wang, Langmuir, 2018, 34, 9974–9981 CrossRef CAS PubMed.
- W. Nong, W. Guan, Y. Yin, C. Lu, Q. Wang, Y. Luo, B. Zhang, Z. Xu, J. Wu and Y. Guan, Chem. Eng. J., 2021, 420, 129874 CrossRef CAS.
- W. B. Sheng, W. Li, G. X. Zhang, Y. Bin Tong, Z. Y. Liu and X. Jia, New J. Chem., 2015, 39, 2752–2757 RSC.
- Q. Zheng, T. Lin, H. Wu, L. Guo, P. Ye, Y. Hao, Q. Guo, J. Jiang, F. Fu and G. Chen, Int. J. Pharm., 2014, 463, 22–26 CrossRef CAS PubMed.
- Y. Sun and E. W. Davis, J. Mater. Chem. B, 2019, 7, 6828–6839 Search PubMed.
- Y. Liang, C. Fan, H. Dong, W. Zhang, G. Tang, J. Yang, N. Jiang and Y. Cao, ACS Sustainable Chem. Eng., 2018, 6, 10211–10220 CrossRef CAS.
- Y. Shan, C. Xu, H. Zhang, H. Chen, M. Bilal, S. Niu, L. Cao and Q. Huang, Nanomater., 2020, 10, 2000 CrossRef CAS PubMed.
- W. Pagel and R. Heitefuss, Physiol. Mol. Plant Pathol., 1990, 37, 9–25 Search PubMed.
- S. R. Herron, J. A. E. Benen, R. D. Scavetta, J. Visser and F. Jurnak, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 8762–8769 CrossRef CAS PubMed.
- C. Kesten, F. M. Gámez-Arjona, A. Menna, S. Scholl, S. Dora, A. I. Huerta, H.-Y. Huang, N. Tintor, T. Kinoshita, M. Rep, M. Krebs, K. Schumacher and C. Sánchez-Rodríguez, EMBO J., 2019, 38, e101822 CrossRef CAS PubMed.
- Y. Liu, H. Guan, J. Zhang, Y. Zhao, J. H. Yang and B. Zhang, Int. J. Hydrogen Energy, 2018, 43, 2754–2762 Search PubMed.
- X. Yu, D. He, X. Zhang, H. Zhang, J. Song, D. Shi, Y. Fan, G. Luo and J. Deng, ACS Appl. Mater. Interfaces, 2019, 11, 1766–1781 CrossRef CAS PubMed.
- A. P. Tiwari, D. P. Bhattarai, B. Maharjan, S. W. Ko, H. Y. Kim, C. H. Park and C. S. Kim, Sci. Rep., 2019, 9, 1–13 CrossRef CAS PubMed.
- L. Keawchaoon and R. Yoksan, Colloids Surf., B, 2011, 84, 163–171 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhang, Z. Zhu, X. Jiao, Y. Shang and Y. Wen, J. Agric. Food Chem., 2019, 67, 1736–1741 Search PubMed.
- V. Vergaro, E. Abdullayev, Y. M. Lvov, A. Zeitoun, R. Cingolani, R. Rinaldi and S. Leporatti, Biomacromolecules, 2010, 11, 820–826 CrossRef CAS PubMed.
- E. Papierowska, S. Szporak-Wasilewska, J. Szewińska, J. Szatyłowicz, G. Debaene and M. Utratna, Trees, 2018, 32, 1253–1266 CrossRef.
- M. Zhao, P. Li, H. Zhou, L. Hao, H. Chen and X. Zhou, Chem. Eng. J., 2022, 435, 134861 CrossRef CAS.
- S. Sharma, S. Singh, A. K. Ganguli and V. Shanmugam, Carbon, 2017, 115, 781–790 CrossRef CAS.
- I. Ocsoy, M. L. Paret, M. A. Ocsoy, S. Kunwar, T. Chen, M. You and W. Tan, ACS Nano, 2013, 7, 8972–8980 CrossRef CAS PubMed.
- K. D. Le, J. Kim, N. H. Yu, B. Kim, C. W. Lee and J. C. Kim, Front. Plant Sci., 2020, 11, 775 CrossRef PubMed.
- K. S. Chiang, H. I. Liu and C. H. Bock, Ann. Appl. Biol., 2017, 171, 139–154 CrossRef.
- J. L. D. Oliveira, E. V. R. Campos, A. E. S. Pereira, T. Pasquoto, R. Lima, R. Grillo, D. J. De Andrade, F. A. Dos Santos and L. F. Fraceto, J. Agric. Food Chem., 2018, 66, 1330–1340 CrossRef PubMed.
- N. F. Della Vecchia, R. Avolio, M. Alfè, M. E. Errico, A. Napolitano and M. D'Ischia, Adv. Funct. Mater., 2013, 23, 1331–1340 CrossRef CAS.
- C. Battistella, N. C. Mccallum, K. Gnanasekaran, X. Zhou, V. Caponetti, M. Montalti and N. C. Gianneschi, ACS Cent. Sci., 2020, 6, 1179–1188 Search PubMed.
- V. S. Raman, S. Rooj, A. Das, K. W. Stöckelhuber, F. Simon, G. B. Nando and G. Heinrich, J. Macromol. Sci., Part A: Pure Appl.Chem., 2013, 50, 1091–1106 CrossRef CAS.
- H. Kang, X. Liu, S. Zhang and J. Li, RSC Adv., 2017, 7, 24140–24148 RSC.
- S. Ganguly, T. K. Das, S. Mondal and N. C. Das, RSC Adv., 2016, 6, 105350–105362 RSC.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- A. Kumar, S. Kumar, W. K. Rhim, G. H. Kim and J. M. Nam, J. Am. Chem. Soc., 2014, 136, 16317–16325 CrossRef CAS PubMed.
- L. Sen Lin, Z. X. Cong, J. B. Cao, K. M. Ke, Q. L. Peng, J. Gao, H. H. Yang, G. Liu and X. Chen, ACS Nano, 2014, 8, 3876–3883 CrossRef PubMed.
- J. J. Feng, P. P. Zhang, A. J. Wang, Q. C. Liao, J. L. Xi and J. R. Chen, New J. Chem., 2011, 36, 148–154 RSC.
- X. Zhao, C. Zhou, Y. Lvov and M. Liu, Small, 2019, 15, 1900357 CrossRef PubMed.
- A. C. Solano Valderrama, G. Cecilia and R. De, Am. J. Anal. Chem., 2017, 08, 726–741 CrossRef.
- S. Hamedi and M. Koosha, Appl. Clay Sci., 2020, 197, 105770 CrossRef CAS.
- H. Luo, C. Gu, W. Zheng, F. Dai, X. Wang and Z. Zheng, RSC Adv., 2015, 5, 13470–13477 RSC.
- C. Chao, J. Liu, J. Wang, Y. Zhang, B. Zhang, Y. Zhang, X. Xiang and R. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 10559–10564 CrossRef CAS PubMed.
- X. Xu, B. Bai, H. Wang and Y. Suo, ACS Appl. Mater. Interfaces, 2017, 9, 6424–6432 CrossRef CAS PubMed.
- R. Shemesh, M. Krepker, M. Natan, Y. Danin-Poleg, E. Banin, Y. Kashi, N. Nitzan, A. Vaxman and E. Segal, RSC Adv., 2015, 5, 87108–87117 RSC.
- C. Wang, Z. He, Y. Liu, C. Zhou, J. Jiao, P. Li, D. Sun, L. Lin and Z. Yang, Appl. Clay Sci., 2020, 198, 105802 Search PubMed.
- O. Prinz Setter, A. Movsowitz, S. Goldberg and E. Segal, ACS Appl. Bio Mater., 2021, 4, 4094–4104 Search PubMed.
- L. Zha, J. Qian, B. Wang, H. Liu, C. Zhang, Q. Dong, W. Chen and L. Hong, Int. J. Pharm., 2020, 587, 119665 CrossRef CAS PubMed.
- M. Wu, C. Zhong, Q. Zhang, L. Wang, L. Wang, Y. Liu, X. Zhang and X. Zhao, J. Nanobiotechnol., 2021, 19, 1–17 Search PubMed.
- National Center for Biotechnology Information, PubChem Compound Summary for CID 6989, Thymol, https://pubchem.ncbi.nlm.nih.gov/compound/Thymol, Accessed Jan. 15, 2024.
- J. H. Lin, C. J. Yu, Y. C. Yang and W. L. Tseng, Phys. Chem. Chem. Phys., 2015, 17, 15124–15130 Search PubMed.
- W. Lei, K. Ren, T. Chen, X. Chen, B. Li, H. Chang, J. Ji, W. X. Lei, K. Ren, T. T. Chen, X. C. Chen, B. C. Li, H. Chang and J. Ji, Adv. Mater. Interfaces, 2016, 3, 1600767 CrossRef.
- S. H. Kim, E. B. Kang, C. J. Jeong, S. M. Sharker, I. In and S. Y. Park, ACS Appl. Mater. Interfaces, 2015, 7, 15600–15606 CrossRef CAS PubMed.
- X. Wang, J. Zhang, Y. Wang, C. Wang, J. Xiao, Q. Zhang and Y. Cheng, Biomaterials, 2016, 81, 114–124 CrossRef CAS PubMed.
- M. Massaro, A. Borrego-Sánchez, R. Sánchez-Espejo, C. Viseras Iborra, G. Cavallaro, F. García-Villén, S. Guernelli, G. Lazzara, D. Miele, C. I. Sainz-Díaz, G. Sandri and S. Riela, Appl. Clay Sci., 2021, 215, 106310 Search PubMed.
- G. Lazzara, S. Riela and R. F. Fakhrullin, Ther. Delivery, 2017, 8, 633–646 CrossRef PubMed.
- H. Ma, S. Li, H. Zhang, Y. Wei and L. Jiang, Colloids Surf., A, 2019, 561, 332–340 Search PubMed.
- H. Zhang, X. Wang, P. Wang, R. Liu, X. Hou, W. Cao, R. Zhong, X. Liu and Y. Zhang, RSC Adv., 2018, 8, 37433–37440 RSC.
- Z. Zhu and M. Su, Nanomater., 2017, 7(7), 160 CrossRef PubMed.
- C. Wang, J. Bai, Y. Liu, X. Jia and X. Jiang, ACS Biomater. Sci. Eng., 2016, 2, 2011–2017 CrossRef CAS PubMed.
- P. Laurent, L. Buchon, J. F. Burini and N. Orange, Biotechnol. Lett., 2001, 23, 753–756 CrossRef CAS.
- T. S. Sileika, H. Do Kim, P. Maniak and P. B. Messersmith, ACS Appl. Mater. Interfaces, 2011, 3, 4602–4610 CrossRef CAS PubMed.
- C. Y. Liu and C. J. Huang, Langmuir, 2016, 32, 5019–5028 CrossRef CAS PubMed.
- D. Hu, L. Zou, B. Li, M. Hu, W. Ye and J. Ji, ACS Biomater. Sci. Eng., 2019, 5169–5179 CrossRef CAS PubMed.
- N. M. O. Andoy, K. Jeon, C. T. Kreis and R. M. A. Sullan, Adv. Funct. Mater., 2020, 30, 2004503 CrossRef CAS.
- P. Chandra Ray, S. Afrin Khan, A. Kumar Singh, D. Senapati and Z. Fan, Chem. Soc. Rev., 2012, 41, 3193–3209 RSC.
- E. Ju, Z. Li, M. Li, K. Dong, J. Ren and X. Qu, Chem. Commun., 2013, 49, 9048–9050 RSC.
- S. P. Lei, H. C. Lin, L. Heffernan and G. Wilcox, J. Bacteriol., 1985, 164, 831–835 CrossRef CAS PubMed.
- G. N. Agrios, Plant Pathol., 2005, 124–174 Search PubMed.
- I. P. De León, J. P. Oliver, A. Castro, C. Gaggero, M. Bentancor and S. Vidal, BMC Plant Biol., 2007, 7, 1–11 CrossRef PubMed.
- Y. Su, V. Ashworth, C. Kim, A. S. Adeleye, P. Rolshausen, C. Roper, J. White and D. Jassby, Environ. Sci.: Nano, 2019, 6, 2311 Search PubMed.
- S. Sharma, B. K. Sahu, L. Cao, P. Bindra, K. Kaur, M. Chandel, N. Koratkar, Q. Huang and V. Shanmugam, Prog. Mater. Sci., 2021, 121, 100812 CrossRef CAS.
- E. González-Grandío, G. S. Demirer, C. T. Jackson, D. Yang, S. Ebert, K. Molawi, H. Keller and M. P. Landry, J. Nanobiotechnol., 2021, 19, 1–15 Search PubMed.
- J. Yanga, W. Cao and Y. Rui, J. Plant Interact., 2017, 12, 158–169 CrossRef.
- S. Hong, K. Y. Kim, H. J. Wook, S. Y. Park, K. D. Lee, D. Y. Lee and H. Lee, Nanomedicine, 2011, 6, 793–801 CrossRef CAS PubMed.
- R. Grillo, B. D. Mattos, D. R. Antunes, M. M. L. Forini, F. A. Monikh and O. J. Rojas, Nano Today, 2021, 37, 101078 CrossRef CAS.
- J. J. Nairn and W. A. Forster, Pest Manage. Sci., 2024, 80(2), 212–219 CrossRef CAS PubMed.
- G. Yokoyama, D. Yasutake, T. Tanizaki and M. Kitano, Photosynthetica, 2019, 57(3), 740–747 CrossRef CAS.
- T. Wu, X. Fang, Y. Yang, W. Meng, P. Yao, Q. Liu, B. Zhang, F. Liu, A. Zou and J. Cheng, J. Agric. Food Chem., 2020, 68, 12549–12557 CrossRef CAS PubMed.
- S. Kumari, R. C. Choudhary, R. V. Kumaraswamy, D. Bhagat, A. Pal, R. Raliya, P. Biswas and V. Saharan, Plant Physiol. Biochem., 2019, 145, 64–74 CrossRef CAS PubMed.
- S. Kumari, R. V. Kumaraswamy, R. C. Choudhary, S. S. Sharma, A. Pal, R. Raliya, P. Biswas and V. Saharan, Sci. Rep., 2018, 8, 1–12 CAS.
- M. L. Alfieri, L. Panzella, S. L. Oscurato, M. Salvatore, R. Avolio, M. E. Errico, P. Maddalena, A. Napolitano and M. d'Ischia, Biomimetics, 2018, 3, 26 CrossRef CAS PubMed.
- H. M. C. Marques, Flavour Fragrance J., 2010, 25, 313–326 CrossRef.
- A. Celebioglu, Z. I. Yildiz and T. Uyar, Food Res. Int., 2018, 106, 280–290 CrossRef CAS PubMed.
- M. Christofoli, E. C. C. Costa, K. U. Bicalho, V. de Cássia Domingues, M. F. Peixoto, C. C. F. Alves, W. L. Araújo and C. de Melo Cazal, Ind. Crops Prod., 2015, 70, 301–308 CrossRef CAS.
- N. Massad-Ivanir, A. Sand, N. Nitzan, E. Valderama, M. Kurczewski, H. Remde, A. Wegenberger, K. Shlosman, R. Shemesh, A. Störmer and E. Segal, Food Packag. Shelf Life, 2023, 37, 101079 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional material in ESI includes the following: standard curve for TY absorbance in ethanol, FTIR spectra of TY and TY-loaded HNTs, TGA and DTG curves of HNTs-based hybrids, TY loading capacity of TY-loaded HNTs and TY-loaded HNTs/PDA hybrid, images showing the effect of TY emulsion, and plain buffer solutions at pH 7 and pH 5 on E. carotovora growth, temp. profile of deionized water, HNTs, PDA-coated HNTs, and TY-loaded HNTs/PDA hybrid after irradiation with 808 nm NIR laser, antibacterial activity HNTs and PDA-coated HNTs lacking TY with and without NIR irradiation, fluorescence spectra of FITC-conjugated HNTs and FITC-conjugated PDA-coated HNTs, confocal images of leaves (mesophyll cells and leaf epidermis) after 24 h incubation with FITC-conjugated HNTs and FITC-conjugated PDA-coated HNTs, confocal images of HNTs and PDA-coated HNTs treated leaves after PI-staining, and photographs of uninfected and bacterial infected leaflets treated with deionized water. (PDF). See DOI: https://doi.org/10.1039/d3en00934c |
| ‡ Authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |