Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-, two- and three-dimensional interlocked polymers based on hybrid inorganic–organic rotaxanes†
Selena J.
Lockyer
*,
George F. S.
Whitehead
 ,
Grigore A.
Timco
,
Grigore A.
Timco
 ,
Eric J. L.
McInnes
and
Richard E. P.
Winpenny
,
Eric J. L.
McInnes
and
Richard E. P.
Winpenny
 *
*
Department of Chemistry, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: selena.lockyer@manchester.ac.uk; richard.winpenny@manchester.ac.uk
First published on 9th August 2024
Abstract
We report three new polymers, based on mechanically interlocked inorganic–organic rotaxanes. They are made in very mild conditions and involve pyrimidine head groups binding to copper(ii) linking units. A two-dimensional 6,3 net and a three-dimensional 10,3b net are found depending on the solvent used in the reaction.
Metal–organic frameworks (MOFs) are one of the most active areas of chemistry with around 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 publications a year.1 They have been proposed for application in catalysis,2 separations3 and sensors.4 One class of MOF are mechanically interlocked molecules (MIMs); these can consist of one-,5 two-6 and three-dimensional7–10 coordination polymers, with non-covalently attached rings around the links that form the MOF. As the rings are held in place mechanically these MOFs are also known as rotaxane coordination polymers (RCP).
000 publications a year.1 They have been proposed for application in catalysis,2 separations3 and sensors.4 One class of MOF are mechanically interlocked molecules (MIMs); these can consist of one-,5 two-6 and three-dimensional7–10 coordination polymers, with non-covalently attached rings around the links that form the MOF. As the rings are held in place mechanically these MOFs are also known as rotaxane coordination polymers (RCP).
Most commonly, organic linkers are used between transition metal or lanthanide nodes.11 In almost all MIMS an organic molecule forms the macrocyclic component of a rotaxane.12 Sometimes a metal ion is incorporated to template the wheel to the axle, such as a Cu ion,13,14 but there is a recent report of MIMs based on {Al8} macrocycles.15 Many MIMs present challenges due to solubility, leading to use of polar solvents and/or solvothermal conditions. In our compounds the macrocyclic component is [Cr7NiF8(O2CtBu)16]−1, and the presence of sixteen pivalates increases solubility in non-polar solvents, allowing structures to form in a range of organic solvents and under mild conditions, which is a current target for the development of MOFs.1 The {Cr7Ni} ring forms around an secondary ammonium, which can incorporate functionalised stoppers to directly bind to other molecules or can be further adapted once the ring has been formed. We have reported polymeric structures based on {Cr7Ni} rings, including 1D chains linked by CuII ions16 or {Fe2Co} triangles17 and a 10,3b net with {Fe2Co} nodes.18
Here, we report three new polymeric structures using two [2]rotaxanes containing 1 as the ring. The dimensionality of the resulting MOF is controlled by functionalisation of the thread with pyrimidine end groups, leading to a 1D-zig-zag chain, a 2D 6,3 net and a 3D 10,3b net. The nodes within these MIMs are all [Cu2(O2CtBu)4], where the pivalates give high solubility.19,20
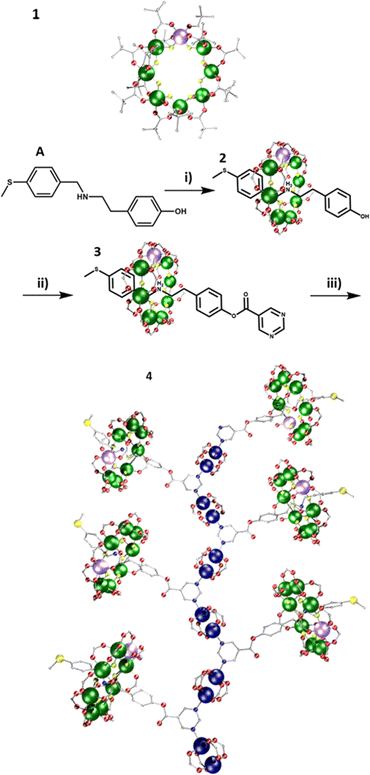
A {Cr7Ni} was grown around thread A (Fig. 1) to produce [2]rotaxane [(AH)1], 2; this can undergo a Steglich esterification to form the rotaxane [(CH)1], 3 (Fig. S1, ESI†). Compound 3 was dissolved in acetone, and one equivalent of [Cu2(O2CtBu)4(HO2CtBu)2] added, followed by addition of toluene. Slow evaporation gave crystals of the 1D polymer; {(3)[Cu2(O2CtBu)4]}, 4. 4 crystallises in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group and forms a 1D chain (Fig. 1). The pyrimidine end group from the [2]rotaxane threads bridge between two {Cu2} paddlewheels. The pyrimidine-{Cu2} chains form the backbone of the structure, with the rotaxanes branching out in alternate directions. The distance between adjacent {Cr7Ni} rings (centre-to-centre) is 17.31(2) Å; that between opposing {Cr7Ni} rings is 27.09(3) Å.
space group and forms a 1D chain (Fig. 1). The pyrimidine end group from the [2]rotaxane threads bridge between two {Cu2} paddlewheels. The pyrimidine-{Cu2} chains form the backbone of the structure, with the rotaxanes branching out in alternate directions. The distance between adjacent {Cr7Ni} rings (centre-to-centre) is 17.31(2) Å; that between opposing {Cr7Ni} rings is 27.09(3) Å.
A dual functionalised rotaxane was grown around thread B, to produce [(BH)1], 5 (Fig. 2). A double Steglich esterification gives the [2]rotaxane [(DH)1], 6 which includes a pyrimidine group at both ends of the thread (Fig. S2, ESI†). Compound 6 was dissolved in THF, and two equivalents of [Cu2(O2CtBu)4(HO2CtBu)2] added. MeCN was added, and slow evaporation gave crystals in 48 h of a 2D polymer {(6)[Cu2(O2CtBu)4]2}, 7. 7 crystallises in the P21/n monoclinic crystal system and forms 2D sheets. As in 4, the pyrimidine end groups coordinate to two {Cu2} units, and the pyrimidines and {Cu2} units produce a 1D-zig-zag chain. However, in 7 the chains are linked by [2]rotaxanes leading to a hexagonal 6,3 net (Fig. 2 and Fig. S14, ESI†). Hexagons are formed from two rotaxanes and four {Cu2} units. The 2D layers are stacked in an ABAB fashion. Disordered solvent molecules are found between the layers. The distance between equivalent {Cr7Ni} centres in neighbouring planes is 18.45(1) Å (Fig. S5, ESI†).
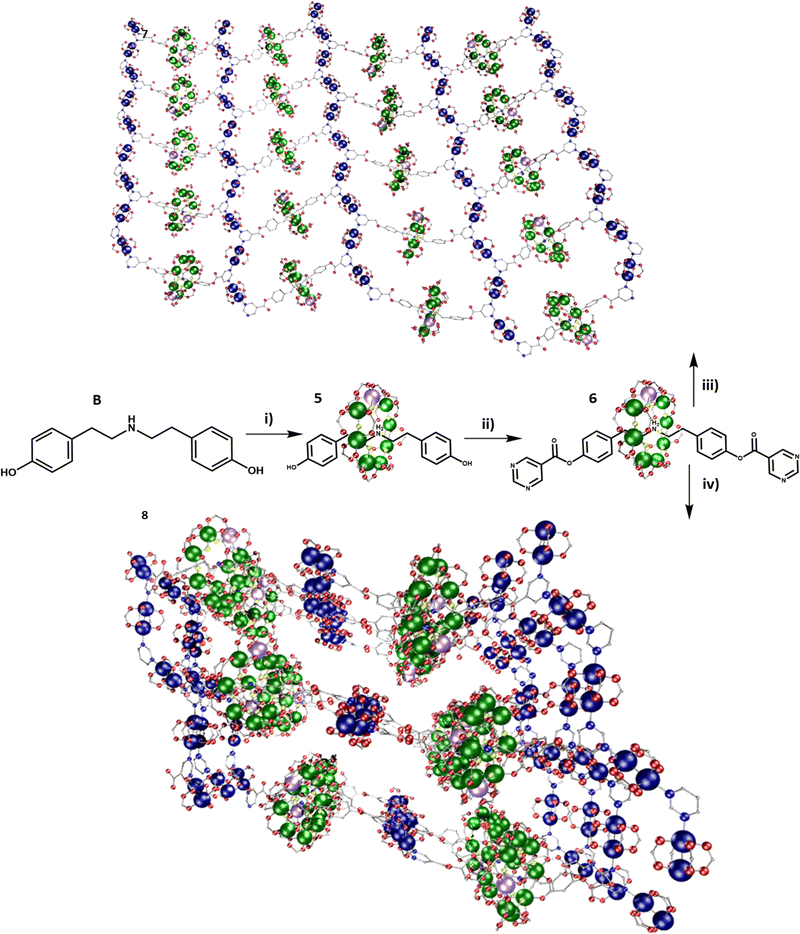 | ||
Fig. 2 Scheme for synthesis of the 2D and 3D polymers; 7 and 8. Reaction conditions: (i) pivalic acid, 2NiCO3·3Ni(OH)2·4H2O, CrF3·4H2O, 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, 24 °C, 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h; (ii) pyrimidine-5-carboxylic acid, DCC, DMAP, DCM, RT, 20 h; (ii) pyrimidine-5-carboxylic acid, DCC, DMAP, DCM, RT, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h; (iii) [Cu(O2CtBu)4(HO2CtBu)2], thf, acetonitrile, RT; (iv) [Cu(O2CtBu)4(HO2CtBu)2], acetone, RT. Colours as Fig. 1. h; (iii) [Cu(O2CtBu)4(HO2CtBu)2], thf, acetonitrile, RT; (iv) [Cu(O2CtBu)4(HO2CtBu)2], acetone, RT. Colours as Fig. 1. | ||
The same reaction that formed 7 was carried out using acetone to dissolve the rotaxane and {Cu2}. In this case small crystals formed within a few seconds; slow evaporation over 24 h allowed further crystal growth of the 3D polymer; {(6)2[Cu2(O2CtBu)4]3}, 8 (Fig. 2). 8 crystallises in the P21/n monoclinic crystal system and forms a 3D MOF (Fig. S10–S12, ESI†). As in 4 and 7 the pyrimidine groups bind to two {Cu2}, however in 8 there are two distinct rows of {Cu2} units which are perpendicular to one another. This leads to a grid-like pattern of the {Cu2} framework. In order to complete a loop from one end of a rotaxane to the other end, the chain traverses an adjacent layer (Fig. S15, ESI†). Each circuit is made from four complete rotaxanes, six {Cu2} units and two pyrimidines from neighbouring rotaxanes, giving a 10,3b net. The ratio of {Cu2}:rotaxane did not affect whether 7 or 8 is formed, this is only controlled by the solvent. Compound 8 can be dissolved in THF (with addition of MeCN) to give 7. Compound 7 does not dissolve in acetone to give 8. This suggest that the formation of 8 is a result of the limited solubility that the {Cu2} and rotaxanes have in acetone when they initially bind. However, in THF the {Cu2} and rotaxane combination is much more soluble and allows time to rearrange to form the better packed structure of 7. While the three structures 4, 7 and 8 are 1-, 2- and 3-D respectively, the distances between the building blocks are consistent (Fig. 3 and Fig. S16, ESI,†Table 1). Firstly, the contacts from the {Cr7Ni} centroid to the nearest Cu(II) have two distinct distances, one between 11.7–12.7 Å and one between 14.1–14.4 Å. Secondly, the {Cr7Ni}⋯{Cr7Ni} distance via {Cu2} is around 27 Å in all cases. The shortest {Cr7Ni}⋯{Cr7Ni} distance is through space in all three compounds, and is 16.6–17.3 Å. The inter-layer contacts between adjacent {Cr7Ni} rings in 4 is 17 Å, for 7 there are two distinct distances between layers, of 16 and 19 Å. Compound 8 does not contain individual layers. These contacts are short enough no spin-echo is seen in EPR experiments (see below).
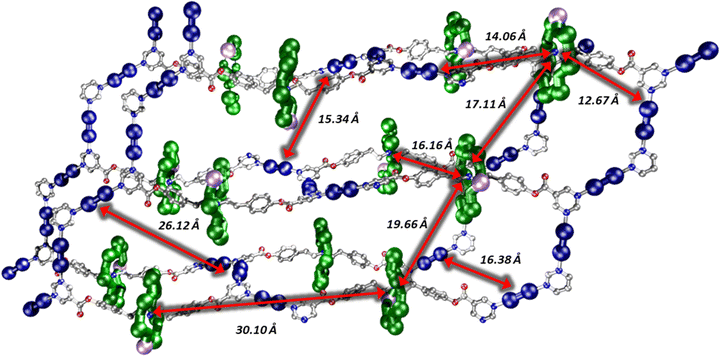 | ||
| Fig. 3 Section for the structure of 8, showing intra; ring–ring (center-centre) distances, ring-copper (centre to nearest contact) and copper paddle wheel-copper paddle wheel (nearest contacts). | ||
| Compound | Contacts/Å | ||
|---|---|---|---|
| Ring centroid⋯Cu | Compound | Ring centroid⋯Cu | |
| 4 | 11.73, 14.37 | 4 | 11.73, 14.37 |
| 7 | 12.60, 14.16 | 7 | 12.60, 14.16 |
| 8 | 12.67, 14.06 | 8 | 12.67, 14.06 |
To study magnetic interactions between the building blocks, continuous wave (c.w.) EPR spectroscopy was performed at Q-band (ca. 34 GHz) on powder samples (Fig. S18, ESI†). At 5 K all three show only the well-defined spin S = 1/2 ground state of {Cr7Ni}.16 Strong anti-ferromagnetic coupling between the CuII ions within each {Cu2} renders this unit diamagnetic at 5 K. Above 10 K the resonance due to the S = 1/2 ground state of {Cr7Ni} decreases in intensity and a broad feature centred at g = 2.0 due to {Cr7Ni} excited states grows in. At 150 K the spectra are dominated by this broad feature, and a signal due to the S = 1 excited state of the {Cu2}17 is visible which grows in intensity on further warming. For 8 hyperfine coupling to the two I = 3/2 nuclear spins of the {Cu2} is clearly resolved on the ΔmS = 2 transition at ca. 500 mT. The spectra for 4, 7 and 8 are almost identical, and are the sum of the spectra of the {Cr7Ni} and {Cu2} building blocks. No echo was observed in pulse EPR experiments on 4, 7 and 8 as powder samples at 3K. The EPR results indicate that although the interaction energies are too weak to be measured by c.w. EPR, they are still influencing the dynamic behaviour of the electron spins.
This work was supported by an EPSRC Established Career Fellowship (EP/R011079/1) to R.E.P.W. who also thanks the European Research Council for an Advanced Grant (ERC-2017-ADG-786734). We thank Diamond Light Source for access to synchrotron X-ray facilities. We also thank the EPSRC EPR NRF (EP/W014521/1, EP/X034623) and the Leverhulme trust for Grant RPG-2023-020.
Data availability
The supporting data, including full experimental details, X-ray crystallography, CW EPR and EPR simulations have been included as part of the ESI.† Crystallographic data for 4, 7 and 8 have been deposited at the CCDC deposition service, under deposition numbers 2367045, 2367046 and 2367047, respectively.Conflicts of interest
The authors declare no conflict of interest.Notes and references
- S. Dai, A. Tissot and C. Serre, Bull. Chem. Soc. Jpn., 2021, 94, 2623–2636 CrossRef CAS.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS PubMed.
- R. B. Lin, S. C. Xiang, W. Zhou and B. L. Chen, Chem, 2020, 6, 337–363 CAS.
- J. Q. Liu, Z. D. Luo, Y. Pan, A. K. Singh, M. Trivedi and A. Kumar, Coord. Chem. Rev., 2020, 406, 213145 CrossRef CAS.
- D. Whang and K. Kim, J. Am. Chem. Soc., 1997, 119, 451–452 CrossRef CAS.
- K. Park, D. Whang, E. Lee, J. Heo and K. Kim, Chem. – Eur. J., 2002, 8, 498 CrossRef CAS.
- D. J. Hoffart and S. J. Loeb, Supramol. Chem., 2007, 19, 89 CrossRef CAS.
- S. J. Bao, Y. Zou, H.-N. Zhang and G.-X. Jin, PNAS, 2024, 121, e2407570121 CrossRef PubMed.
- W.-X. Gao, H.-J. Feng, B.-B. Guo, Y. Lu and G.-X. Jin, Chem. Rev., 2020, 120, 6288–6325 CrossRef CAS PubMed.
- Q.-S. Mu, X. Gao, Z. Cui, Y.-J. Lin and G.-X. Jin, Sci. China: Chem., 2023, 66, 2885–2891 CrossRef CAS.
- E. Lee, J. Heo and K. Kim, Angew. Chem., Int. Ed., 2000, 39, 2699–2701 CrossRef CAS PubMed.
- V. N. Vukotic and S. J. Loeb, Chem. Soc. Rev., 2012, 41, 5896–5906 RSC.
- A. Coskun, M. Hmadeh, G. Barin, F. Gándara, Q. Li, E. Choi, N. L. Strutt, D. B. Cordes, A. M. Z. Slawin, J. F. Stoddart, J. P. Sauvage and O. M. Yaghi, Angew. Chem., Int. Ed., 2012, 51, 2160–2163 CrossRef CAS PubMed.
- J. D. Crowley, S. M. Goldup, A. Lee, D. A. Leigh and R. T. McBurney, Chem. Soc. Rev., 2009, 38, 1530–1541 RSC.
- W. Fang, Y. Liu, C. Zheng, H. Xiao, Z. Wang, C. Zhang, X. Zhang, S. Wang, R. Chen and J. Zhang, Angew. Chem., Int. Ed., 2024, e202411576 Search PubMed.
- G. F. S. Whitehead, B. Cross, L. Carthy, V. A. Milway, H. Rath, A. Fernandez, S. L. Heath, C. A. Muryn, R. G. Prichard, S. J. Teat, G. A. Timco and R. E. P. Winpenny, Chem. Commun., 2013, 49, 7195–7197 RSC.
- J. Ferrando-Soria, A. Fernandez, I. J. Vitorica-Yrezabal, D. Asthana, C. A. Muryn, F. Tuna, G. A. Timco and R. E. P. Winpenny, Chem. Commun., 2019, 55, 2960–2963 RSC.
- G. F. S. Whitehead, S. J. Teat, K. J. Gagnon, G. A. Timco and R. E. P. Winpenny, Chem. Commun., 2015, 51, 3533–3536 RSC.
- S. Piligkos, H. Weihe, E. Bill, F. Neese, H. El Mkami, G. M. Smith, D. Collison, G. Rajaraman, G. T. Timco, R. E. P. Winpenny and E. J. L. McInnes, Chem. – Eur. J., 2009, 15, 3152–3167 CrossRef CAS PubMed.
- B. Bleaney and K. D. Bowers, Proc. R. Soc. A, 1952, 214, 451–465 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2367045, 2367046 and 2367047. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc03566f |
| This journal is © The Royal Society of Chemistry 2024 |