Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
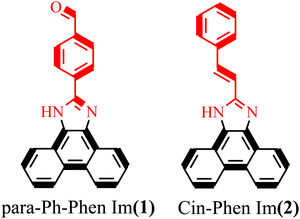
Dopant-free small-molecule hole-transport material for low-cost and stable perovskite solar cells†
Sahar
Majidi-Nezhad
a,
Negin
Sabahi
a,
Hashem
Shahroosvand
 *a,
Narges
Yaghoobi Nia
bc and
Aldo
Di Carlo
*a,
Narges
Yaghoobi Nia
bc and
Aldo
Di Carlo
 *d
*d
aGroup for Molecular Engineering of Advanced Functional Materials (GMA) Department of Chemistry, University of Zanjan, Zanjan, Iran. E-mail: shahroos@znu.ac.ir
bSchool of Aerospace Engineering, University of Rome “La Sapienza”, Via Salaria, 851 – 00138 Rome, Italy
cDepartment of Electronics Engineering, University of Rome Tor Vergata, Via del Politecnico 1, 00133 Roma, Italy
dCHOSE – Centre for Hybrid and Organic Solar Energy, University of Rome ‘‘Tor Vergata’’, via del Politecnico 1, Rome 00133, Italy
First published on 10th August 2023
Abstract
Dopant-free hole-transporting materials (HTMs) aim to improve efficiency and stability simultaneously, and are a promising direction for efficient perovskite solar cells (PSCs). To achieve dopant-free and low-cost HTMs, small organic molecules based on imidazole phenanthrene derivatives were easily prepared in a one-pot cycling reaction without the use of a catalyst or harsh conditions. The total cost of 1 g of this new HTM is about $5, which is 1/20th of the cost of the benchmark HTM spiro-OMeTAD (>$100). In particular, the power conversion efficiency (PCE) of PSCs based on the new HTM without using LiTFSI/4-tert-butylpyridine dopants is about 9.11%, which is higher than for PSCs based on spiro-OMeTAD, for which the PCE is 6.21% under the same conditions. Moreover, the light stability of PSCs based on the new additive-free HTM indicated good behaviour over 500 hours, resulting in only 10% loss of initial efficiency. The doped PSC also shows 14% efficiency, maintaining more than 80% of its initial efficiency after 500 hours of light exposure. The exclusive photovoltaic properties of the new HTM can be attributed to its high conductivity and hole mobility, which are related to the small size of the molecules compared to common HTMs known so far. These new HTMs represent a breakthrough in the engineering of additive-free PSCs based on organic HTMs, which will open up new avenues to achieve low-cost and high-stability PSCs.
1. Introduction
Following the COVID-19 crisis, the quest to replace fossil fuel energy with renewable and sustainable energy is an active field of research and a continuing problem.1 As the most promising candidate amongst the latest developments in solar energy, perovskite solar cells (PSCs) have successfully passed a large number of criteria from various consortia aimed at promoting low-cost PSCs into the marketplace.2–4 Their relatively low-cost abilities are due to the facile synthesis, tweaking, and tailoring of crystalline perovskite light absorbers, which are at the heart of the conversion of solar energy to electricity.5–7 The flexibility of PSCs and their thin-film abilities facilitate large-scale manufacturing processing techniques, such as inkjet printing, spraying,8,9 and zero-waste scalable blade spin-coating. However, what has surprised the photovoltaic community has been the dramatic growth in power conversion energy (PCE), from about 3.7% in 200910 to over 25.8% in 2023.11 Such incredible improvements in PCE can be attributed to a large number of modifications in layer deposition techniques,12–15 the type of electron transport (ETLs)16–22 and hole-transport materials (HTMs)23–29 used, the use of efficient additives30 and stabilizers,26,31–35 different perovskite compositions, and so on.36–38 In light of all of these, PSCs have attracted a high level of marketing activity by removing barriers to commercialization through the growth in PCE, increasing stability, environmental compatibility, and the possibility of scaling.38 One very promising option for decreasing the cost of PSCs is modification of the HTM, which has the highest price amongst the materials used to fabricate PSCs.39–41 For example, the total synthetic cost of 1 g of spiro-OMeTAD, hereafter designated as compound (3), as the most used HTM in efficient PSCs, is about $92, which rises dramatically to about $170–425 when brought to market.42–44Such a high cost for (3) has resulted from certain drawbacks in its manufacture, including: (1) use of a large number of synthesis steps (five in total); (2) a huge number of rounds of purification, which leads to lowering of the volume of the product; and (3) use of expensive catalysts, such as dichloro[2,2′-bis(diphenylphosphino)-1,1′-binaphthyl]palladium(II).45,46 Therefore, many attempts have been made to replace (3) with a low-cost HTM.46–48 However, (3) also suffers significantly from low electrical conductivity, and needs to be doped with dopants such as lithium bis(trifluoromethanesulfonyl)imide and 4-tert-butylpyridine to increase its electrical performance.49 However, by adding dopant to increase conductivity, the stability of PSCs based on (3) decreases. Hence, HTMs that are dopant-free are ideal candidates to replace (3).23,50,51 With respect to this, here we try to address the dilemma of the presence of dopant and the stability52,53 by using a dopant-free HTM. Thus, a very simple, cheap, and dopant-free HTM based on phenanthroimidazoles and having good stability is introduced.
Surprisingly, the phenanthroimidazole family has some competence as a new building block for blue fluorescent materials54 with high luminous efficiency,55 a relative balance of carrier injection, efficient transport properties,56,57 and excellent thermal stability.58 In addition, phenanthroimidazoles show favourable properties, such as anti-inflammatory,59 anti-lupus,60 anticancer,61 antibacterial,62 antitumor,63 and antiviral64 activity. In addition, the application of phenanthroimidazoles as HTMs in PSCs has recently been reported.65–67
Phenanthroimidazole compounds combine the desirable properties of imidazole and phenanthrene as block cores. Phenanthroline enhances intermolecular π–π packing and high cavity mobility, blocking the core with a planar π-bonded structure.68,69 Five-membered heterocyclic imidazoles have two different nitrogen atoms, one with electron-poor character, as in pyridine, and one with electron-rich character, as in pyrrole.70,71 A pyridine-like nitrogen atom as a Lewis base passivates perovskite defects. Furthermore, incorporation of the phenanthrene moiety improves the planarity of the imidazole-derived motif, thus promoting intermolecular π–π stacking. Moreover, due to the different electronic properties of the two nitrogen atoms in the imidazole moiety, the hybrid phenanthroimidazole core exhibits low symmetry, especially after incorporating different donors, and side edges, which leads to less apparent symmetry throughout the molecule. This low-symmetry structure favours maintaining amorphous thin films with high morphological uniformity, and avoids crystallization.69,72 Therefore, this hybrid core can provide not only high hole mobility and an interfacial passivation effect, but also excellent morphological uniformity of thin films.67
In view of all of these features, two very small HTMs based on phenanthroimidazole with different donors, with and without a carbonyl functional group and without using any dopant, were synthesized to investigate the effect of the presence of the carbonyl functional group and the reduction in cost and increase in stability of PSCs.
2. Results and discussion
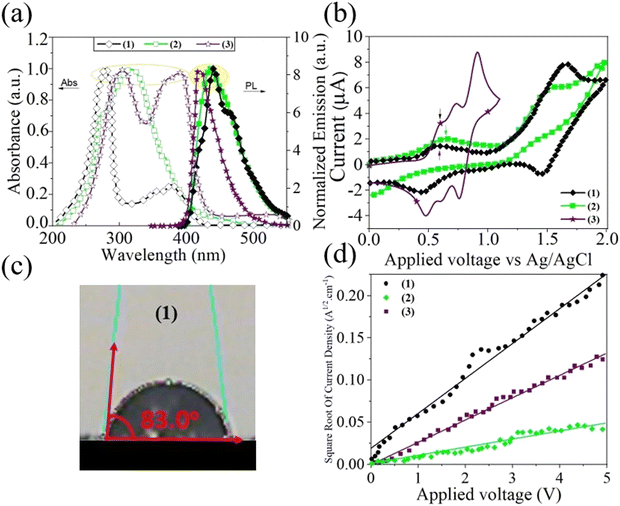
Preparation of phenanthroimidazole derivatives was accomplished using a one-pot cyclizing reaction via a very simple route without the use of a catalyst or column chromatography purification.73–77 The experimental details, including the methods and synthesis procedure for the HTMs, are presented in ESI,† S1 and S2. The molecular structures of the perovskite/HTMs are shown in Scheme 1. Synthesis reactions and molecular structures of the para-Ph-Phen Im, and Cin-Phen Im, which are designated as (1) and (2), are given in ESI,† Fig. S1. Moreover, all the spectroscopic and photovoltaic results obtained have been compared to benchmark values for the HTM spiro-OMeTAD, compound (3). The full characterizations, including Fourier-transform infrared spectroscopy, nuclear magnetic resonance, elemental analysis, and mass spectroscopy, are given in the ESI,† S1.Generally, the phenanthroimidazole compounds containing a phenyl ring in the 2-position exhibit an absorption band at around 350 nm and a strong fluorescence band at about 438 nm.78–81Fig. 1(a) shows the normalized UV-vis absorption and photoluminescence (PL) spectra of two new HTMs and compares them with (3) as a benchmark HTM, in dimethylformamide (DMF) as solvent (1.0 × 10−5 mol L−1). The absorption band at 300–400 nm is attributed to the intramolecular charge transfer of the π → π* transition, which agrees with earlier observations.82,83 As shown in Fig. 1(a), (1) exhibits a 20 nm blueshift in comparison to (3), while (2) exhibits a slight shift to a longer wavelength, which is probably related to an extended π-conjugation effect.84 Furthermore, the emission spectra of the HTMs in DMF solvent at room temperature were recorded and are shown in Fig. 1(a). All HTMs have a broad emission band in the range of 400–550 nm, which can be attributed to the π* → π transition.79,85 The emission maxima of the HTMs are shifted to lower energies (showing a redshift of about 15 to 23 nm), with respect to (3). The electrochemical behaviour of (1), (2), and (3) was analysed by cyclic voltammetry in DMF solvent from 0.0 V to 2 V, using Ag/AgCl as a reference electrode (Fig. 1(b)). Generally, the phenanthroimidazoles exhibited two, and in some cases four, peaks in the anodic scan and no activity in the negative potential range.86 As shown in Fig. 1(b), the onset oxidation potentials of (1) and (2) were observed at 0.57 and 0.65 V, respectively.87 For (2), the first oxidation value is 0.05 V higher than (3), while in the case of (1) this value is 0.03 V lower than for (3), confirming that (1) is more easily oxidized to (1)+ than (3) is to (3)+.88 Notably, a good energy balance between the highest occupied molecular orbital (HOMO) of the HTMs and the HOMO of the perovskite layer is a crucial feature to reach high efficiency PSCs.89,90 The HOMO and LUMOs of the HTMs were calculated according to the equation, EHOMO = −e(Eoxonset + 4.8) (eV), and ELUMO = EHOMO + E0–0, which is summarized in Table 1.91,92 Therefore, the HOMO energy levels were estimated to be −5.37 and −5.4 eV, and the LUMO levels −2.33 and −2.34 eV, respectively, for (1) and (2), which are in accordance with HOMO and LUMO energy levels of phenanthroimidazoles derivatives.93,94 As mentioned above, these HOMO values are deeper than that of (3) (−5.21 eV), which indicates their favourable level for hole extraction compared to (3), and with closer matching with the perovskite energy levels. This finding is promising for an efficient PSC based on the new HTMs because of the lower bandgap between the HOMO of the HTMs and the HOMO of the perovskite layer. By measuring the contact angle formed by a water droplet under the (1) film surface, we can obtain more information on the surface energetics and wettability of the hole conductors. The water contact angles of (1) coated on glass was 83.0° (see Fig. 1(c)), which indicated its good hydrophobicity and suitability as an HTM in PSCs. Hence, the hydrophobic properties of (1) can efficiently prevent water penetration into the perovskite layer (ESI,† Fig. S2).
| HTM | λ abs (nm) | λ em (nm) | E 0–0 (eV) | E OX (V) | E HOMO (eV) | E LUMO (eV) | η quenching |
|---|---|---|---|---|---|---|---|
| (1) | 278, 355 | 440 | 3.03 | 0.57 | −5.37 | −2.33 | 0.92 |
| (2) | 270, 319 | 435 | 3.06 | 0.65 | −5.4 | −2.34 | 0.90 |
| (3) | 304, 378, 525 (sh) | 421 | 3.04 | 0.6 | −5.21 | −2.17 | 0.96 |
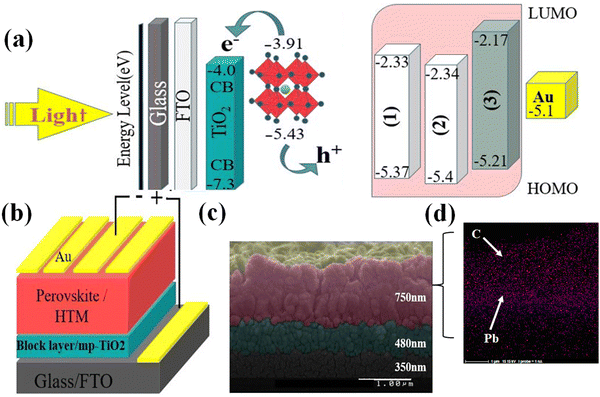
Important objectives for efficient dopant-free HTMs are the ability for charge carrier transport and for hole mobility.95 As shown in Fig. 1(d), the space-charge-limited currents technique96–98 was used to determine the hole mobility of the HTMs without using any dopant. Surprisingly, the estimated hole mobility value for (1), without the use of any additives, is about 9.5 × 10−4 cm2 V−1 s−1, which is more than (3), with a value of about 2.5 × 10−5 cm2 V−1 s−1. This means that the hole mobility of compound (1) is over 10 times that of (3) in the pristine sample.99–101 Such high hole mobility and conductivity for the HTM (1) is very promising for an efficient dopant-free PSC. Fig. 2(a) shows the frontier orbital energy levels of (1), (2), and (3), as well as the components used in the fabrication of the PSC. Fig. 2(a) clearly reveals that the energy level of the HOMO of (1) (−5.37 eV) is more closely matched with the perovskite energy level (−5.4 eV) than for (3) (−5.21 eV), resulting in (1) being more favourable than (3) for extracting holes from the perovskite layer to the HTM.
In fabricated PSCs, (1) and (2) were employed as new HTMs in an n–i–p structure in the configuration of FTO/compact TiO2 (∼350 nm)/thin mesoporous TiO2 (∼480 nm)/perovskite/HTM (∼750 nm)/Au. A schematic of the device is shown in Fig. 2(b). The PSC device structures were kept exactly constant and just the HTMs were changed. This allows us to monitor the influence of the different HTMs. We also fabricated a PSC using (3) as the HTM as a reference. Fig. 2(c) shows a cross-sectional scanning electron microscopy (SEM) image of the arrangement of deposited layers of the constructed PSCs. As shown in the SEM image, the layers were successfully deposited and there are no defects or diffusion between layers.
The current density–voltage (J–V) curves for the new HTMs and the conventional HTM (3) are shown in Fig. 3, and the corresponding photovoltaic parameters are summarized in Table 2. The J–V results indicated that using (1) for the HTM-based PSC showed a PCE of 14.06% with a VOC of 1 V, a short-circuit current density (JSC) of 19 mA cm−2, and a fill factor (FF) of 74%. This shows a higher efficiency compared to (2) and a slightly lower efficiency than (3) under the same device fabrication processes. Surprisingly, when dopants are removed from the HTMs, the photovoltaic parameters of (1) improved compared to (3). In fact, the dopant-free PSC based on (1) showed JSC, VOC, FF, and PCE values of 16.34 mA cm−2, 0.93 V, 0.69, and 9.11%, respectively, which are higher than for additive-free PSCs based on (3). The superior photovoltaic data for additive-free PSCs based on (1) can be attributed to the high mobility of (1), as discussed earlier.
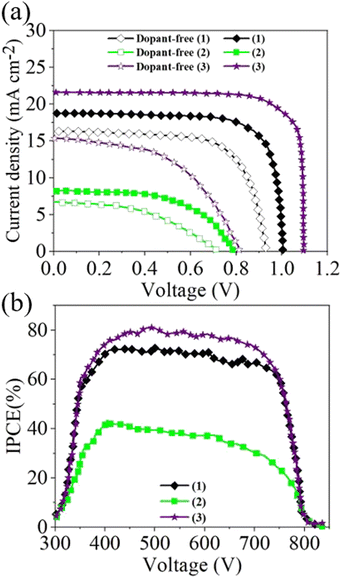 | ||
| Fig. 3 (a) J–V characteristics of PSCs based on the new HTMs containing dopant and free of dopant. (b) IPCE curves of the PSCs based on (1), (2) and (3). | ||
| HTM | V OC (V) | J SC (mA cm−2) | FF (%) | PCE (%)(±0.1) | Standard deviation (PCE) | Median (PCE) |
|---|---|---|---|---|---|---|
| (1) | 1 | 19 | 74 | 14.06 | 2.05 | 11.62 |
| Dopant-free (1) | 0.93 | 16.34 | 60 | 9.11 | 0.99 | 7.12 |
| (2) | 0.8 | 8 | 55 | 3.52 | 0.45 | 2.55 |
| Dopant-free (2) | 0.72 | 6.71 | 45 | 2.17 | 0.46 | 0.94 |
| (3) | 1.1 | 22 | 77 | 18.63 | 1.2 | 16.01 |
| Dopant-free (3) | 0.81 | 15.34 | 50 | 6.21 | 0.74 | 4.45 |
The incident photon to current efficiency (IPCE) at short-circuit conditions as a function of wavelength, and integration of the IPCE spectra over the AM1.5G solar spectrum, provide the expected JSC of the device under steady-state performance.102 As can be seen in Fig. 3(b), the PSC made using (1) presented a higher quantum yield between 300 nm and 850 nm than the PSC based on (2) and slightly lower than the benchmark PSC based on (3). The highest quantum yield reached was as high as 73% for (1), which agrees with the estimated JSC value from the I–V curve.
The statistical analysis of the photovoltaic performance of 15 PSCs based on (1), (2), and (3) is shown in Fig. 4. The standard deviation and median values of all parameters are given in Fig. 4 and in Table 2. Finally, the standard deviation and median values of the PCE of PSCs based on (1) are about 2.05% and 11.621%, which indicates the accuracy of results obtained.
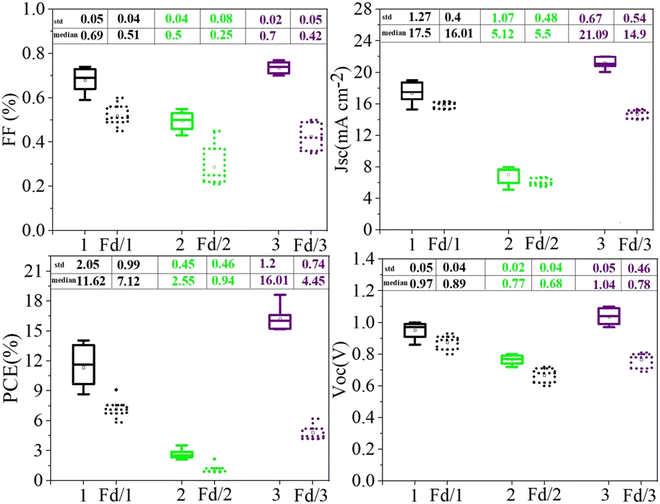 | ||
| Fig. 4 Statistical analysis of VOC, JSC, FF, and PCE of PSCs based on (1), (2), and (3) in the presence and absence of additives. Fd = additive-free. | ||
A further promising finding is the high efficiency and stability of PSCs based on the new HTMs in the absence of any additives. We also compared the key role of additive on the efficiency and stability, as shown in Fig. 5. The PCE of (3) in the absence of additive dramatically decreased, while no large differences in PCE was observed for PSCs based on the new HTMs, with and without additives. The encapsulation method and its details were as described in ref. 103.
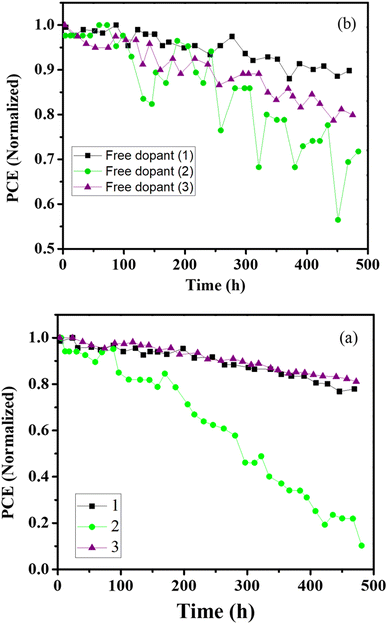 | ||
| Fig. 5 (a) The stability of PSCs over time based on (1), (2), and (3) and (b) based on additive-free (1), (2), and (3). | ||
As shown in Fig. 5(a), the PCEs of (1) and (3) are relatively similar, losing about 23% and 19% of their initial value, respectively, after 500 hours. In particular, the PCE of the additive-free PSC based on (1) showed less than 10% loss over 500 h. Finally, the dopant-free PSC based on (1) showed a PCE value of 9.11%, which is much more than the dopant-free PSC based on (3), with a PCE of 6.21%. The blueshift in the PL emission peak of the HTM coated on the perovskite can be attributed to the passivation process of the trap states close to the top surface of the perovskite, which dramatically decreases the stability and efficiency of PSCs.104 These results clearly indicated that HTM (1) is very promising for dopant-free PSCs because of its high conductivity, which removes the need for the presence of co-conductive additives, such as LiTFSI/4-tert-butylpyridine.
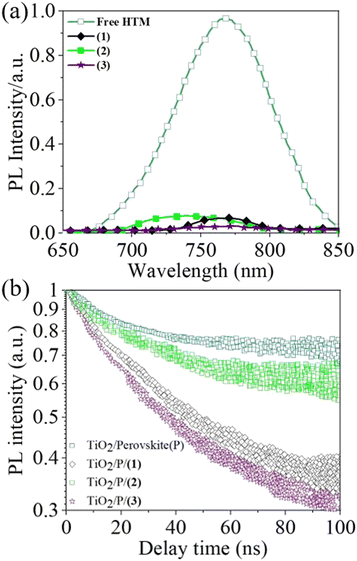
The steady-state PL spectra of perovskite films on the new HTMs, (3), and pristine perovskite, as reference, were measured to compare the ability of the HTM for extracting photon-holes. The pristine perovskite film gives an emission band peak at approximately ≈760 nm, which is in accordance with the literature.32,91,99 Clearly, strong PL quenching was achieved when the HTMs were coated on perovskite films, which suggested that (1) is a good quencher for perovskite films. Fig. 6(a) illustrates that perovskite/(3) and perovskite/(1) are more efficient than perovskite/(2), indicating their strong ability for extracting photo-holes from the perovskite active layer.
PL quenching efficiency was calculated using the following formula:
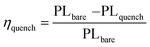 | (1) |
η quenching values for (1) and (3) were about 92% and 95%, respectively, which indicated that the (3)/perovskite film exhibited a slightly better PL quenching efficiency than (1)/perovskite. Interestingly, we proposed that the reason for the low efficiency of (2) is the unfavourable matching between the perovskite and the HTM. As shown in Fig. 6(a), the PL spectrum of perovskite film containing (2) shows a blueshift in wavelength, which can be attributed to the passivation of the trap states close to the top surface of the perovskite films.88,104
Time-resolved PL spectroscopy is a powerful technique to gain insight into the interface charge extraction behaviour and to estimate the photo-charge lifetime (τ2) of perovskite and perovskite/HTM.106 As shown in Fig. 6(b), the time-resolved PL decay patterns of TiO2/perovskite/(1) and TiO2/perovskite/(3) are relatively similar, with τ2 of 8.5 ns and 7.7 ns, respectively, while the τ2 of TiO2/perovskite without HTM is about 16.9 ns.107
Fitting of the data obtained using bi-exponential functions indicated that the faster decay kinetics of (1) and (3) is due to the decreasing hole population generated close to the perovskite/HTM interface,108 while the slower decay component of perovskite/(2) (τ2 = 15.2 ns) could be associated with the holes created in the bulk of the perovskite, which can be proposed as a reasonable origin for the low efficiency of PSCs based on (2).
Both the high and fast PL quenching values indicate that (1) does act as an ideal hole acceptor, and hole transfer to the HTM is faster than diffusion out of the interface.
Thus, superior results are seen for the new HTMs with dramatically reduced cost compared to (3). Surprisingly, unlike (3), which requires a costly synthesis process with complicated sublimation steps for purification (it is synthesized in five reaction steps at a very low temperature (−78 °C) and with high-cost Pd catalyst in an inert atmosphere conditions109,110), the new HTMs are synthesized in only one step and a simple low-cost process, without using any expensive Pd catalysts or other additives or column chromatography for purification.
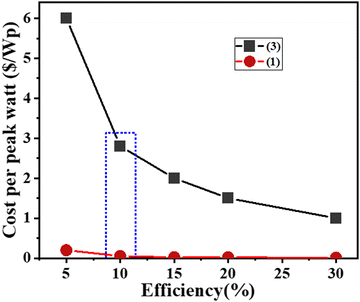
The synthesis pathway of these compounds does not require expensive synthesis procedures. The synthetic details and estimated chemical costs of the synthesized HTMs were calculated according to the cost model described in ESI,† Table S1. It is noteworthy that the cost of (1) and (2) are approximately $5.13 and $5.18 per g, respectively, which is much cheaper than for (3) ($92 per g).
Eqn (2) was used to further determine the cost per peak watt ($ per Wp, denoted here as Cw) for the utilization of the new HTM (1) and spiro-OMeTAD, where η is the solar cell efficiency in the range 5–30%, Cg is the cost-per-gram, p is the density which is assumed to be 1.1 g cm−3, t is the thickness of the donor material, and I is the solar insolation under peak conditions, which are assumed to be 100 nm and 1000 W m−2, respectively.
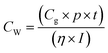 | (2) |
Of note, the total cost and efficiency of the new HTMs was compared with 20 pioneer HTMs, and clearly showed the outstanding properties of new the HTMs in terms of both cost and efficiency amongst all HTMs reported so far in this group.
A comparison between the current work and other HTMs, in terms of synthesis costs and PCE, is shown in Fig. 8. The molecular structure of all HTMs, as well as the photovoltaic parameters, are given in ESI,† Table S2. The most important points in this comparison are related to the cost of preparation and the catalysts used during the reaction. As can be clearly seen, the lowest prices among all the HTMs belong to (1) and (2), at $5.13 and $5.18, respectively, which is much lower than for the other HTMs. In fact, the number of experimental steps and use of catalyst causes this much bigger difference between the costs for the current HTMs versus the others.111
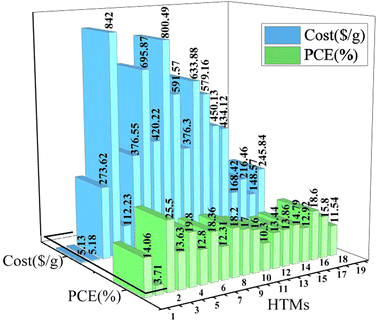 | ||
| Fig. 8 Comparison between costs of organic HTMs (blue histograms) and the PCE of the corresponding PSCs (green histograms), as reported in our work and in other relevant literature. The HTMs reported in previous work are designated with numbers from 3 to 19. The molecular structure and photovoltaic data of all HTMs are given in the ESI,† Table S2.88,99,112–118 | ||
3. Conclusion
In summary, a facile and easy synthesis pathway for a small HTM for use in low-cost PSCs is presented. The reduction of the total cost of the benchmark (3) from $100–400 per g to about $5 per g for the newly synthesized HTM is very promising for large-scale production. Moreover, dopant-free PSCs based on HTM (1) showed good PCE (≈9.1) along with remarkable stability over time (500 h), showing only a 10% loss of efficiency from the initial value. Moreover, the doped HTM also showed 14% efficiency and could retain more than 80% of its initial efficiency after 500 hours of light exposure. Additionally, the higher mobility of holes and greater conductivity of new HTM compared with (3) supported this photovoltaic performance. In particular, the PCE of the dopant-free PSC based on (1) is about two times that of (3) under the same conditions. In view of these results, one of the main aims of this paper is to encourage researchers to explore new avenues for the discovery of new, small HTMs that do not need support by a dopant for increasing conductivity, which should be of significant help in the future development and advancement of low-cost and high-stability PSCs for commercial applications.Author contributions
Sahar Majidi-Nezhad: methodology, investigation, formal analysis, and writing – original draft. Negin Sabahi: experimental analysis and writing – revision draft and editing. Hashem Shahroosvand: conceptualization, methodology, supervision, resources, and writing – review and editing. Narges Yaghoobi Nia and Aldo Di Carlo: writing – review and editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We deeply thank the University of Zanjan for financial support and NYN and ADC acknowledge the support of the Italian Ministry of Economic Development within the framework of the Operating Agreement with ENEA for Research on the Electric System.Notes and references
- A. R. Gollakota and C.-M. Shu, Gondwana Res., 2023, 114, 93 CrossRef CAS PubMed.
- J.-P. Correa-Baena, M. Saliba, T. Buonassisi, M. Grätzel, A. Abate, W. Tress and A. Hagfeldt, Science, 2017, 358, 739 CrossRef CAS PubMed.
- A. A. Brown, B. Damodaran, L. Jiang, J. N. Tey, S. H. Pu, N. Mathews and S. G. Mhaisalkar, Adv. Energy Mater., 2020, 10, 2001349 CrossRef CAS.
- N. Y. Nia, D. Saranin, A. L. Palma and A. Di Carlo, Solar Cells and Light Management, Elsevier, 2020, p. 163 Search PubMed.
- H. Zhou, Q. Chen, G. Li, S. Luo, T.-B. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542 CrossRef CAS PubMed.
- L. Yang, J. Feng, Z. Liu, Y. Duan, S. Zhan, S. Yang, K. He, Y. Li, Y. Zhou and N. Yuan, Adv. Mater., 2022, 34, 2201681 CrossRef CAS PubMed.
- S. Zhan, Y. Duan, Z. Liu, L. Yang, K. He, Y. Che, W. Zhao, Y. Han, S. Yang and G. Zhao, Adv. Energy Mater., 2022, 12, 2200867 CrossRef CAS.
- L. Fagiolari and F. Bella, Energy Environ. Sci., 2019, 12, 3437 RSC.
- J. W. Lee, D. K. Lee, D. N. Jeong and N. G. Park, Adv. Funct. Mater., 2019, 29, 1807047 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS PubMed.
- Y. Meng, C. Liu, R. Cao, J. Zhang, L. Xie, M. Yang, L. Xie, Y. Wang, X. Yin and C. Liu, Adv. Funct. Mater., 2023, 33, 2214788 CrossRef CAS.
- Y. Zhang, S.-W. Ng, X. Lu and Z. Zheng, Chem. Rev., 2020, 120, 2049 CrossRef CAS PubMed.
- P. Roy, N. K. Sinha, S. Tiwari and A. Khare, Sol. Energy, 2020, 198, 665 CrossRef CAS.
- N. Y. Nia, F. Giordano, M. Zendehdel, L. Cinà, A. L. Palma, P. G. Medaglia, S. M. Zakeeruddin, M. Grätzel and A. Di Carlo, Nano Energy, 2020, 69, 104441 CrossRef.
- Y. Han, T. Zuo, K. He, L. Yang, S. Zhan, Z. Liu, Z. Ma, J. Xu, Y. Che and W. Zhao, Mater. Today, 2022, 61, 54 CrossRef CAS.
- H. Wang, H. Li, W. Cai, P. Zhang, S. Cao, Z. Chen and Z. Zang, Nanoscale, 2020, 12, 14369 RSC.
- J.-F. Liao, W.-Q. Wu, Y. Jiang, J.-X. Zhong, L. Wang and D.-B. Kuang, Chem. Soc. Rev., 2020, 49, 354 RSC.
- L. Gao and G. Yang, Sol. RRL, 2020, 4, 1900200 CrossRef CAS.
- A. M. Elseman, C. Xu, Y. Yao, M. Elisabeth, L. Niu, L. Malavasi and Q. L. Song, Sol. RRL, 2020, 4, 2000136 CrossRef CAS.
- G. Yang, H. Tao, P. Qin, W. Ke and G. Fang, J. Mater. Chem. A, 2016, 4, 3970 RSC.
- S. Navazani, N. Y. Nia, M. Zendehdel, A. Shokuhfar and A. Di Carlo, Sol. Energy, 2020, 206, 181 CrossRef CAS.
- I. Ermanova, N. Yaghoobi Nia, E. Lamanna, E. Di Bartolomeo, E. Kolesnikov, L. Luchnikov and A. Di Carlo, Energies, 2021, 14, 1751 CrossRef CAS.
- H. D. Pham, T. C. J. Yang, S. M. Jain, G. J. Wilson and P. Sonar, Adv. Energy Mater., 2020, 10, 1903326 CrossRef CAS.
- L. Calió, S. Kazim, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2016, 55, 14522 CrossRef PubMed.
- J. Urieta-Mora, I. García-Benito, A. Molina-Ontoria and N. Martín, Chem. Soc. Rev., 2018, 47, 8541 RSC.
- N. Y. Nia, M. Zendehdel, M. Abdi-Jalebi, L. A. Castriotta, F. U. Kosasih, E. Lamanna, M. M. Abolhasani, Z. Zheng, Z. Andaji-Garmaroudi and K. Asadi, Nano Energy, 2021, 82, 105685 CrossRef.
- N. Y. Nia, F. Matteocci, L. Cina and A. Di Carlo, ChemSusChem, 2017, 10, 3854 CrossRef CAS PubMed.
- N. Irannejad, N. Yaghoobi Nia, S. Adhami, E. Lamanna, B. Rezaei and A. Di Carlo, Energies, 2020, 13, 2059 CrossRef CAS.
- N. Yaghoobi Nia, M. Bonomo, M. Zendehdel, E. Lamanna, M. M. Desoky, B. Paci, F. Zurlo, A. Generosi, C. Barolo and G. Viscardi, ACS Sustainable Chem. Eng., 2021, 9, 5061 CrossRef CAS.
- J. Chen and N. G. Park, Adv. Mater., 2019, 31, 1803019 CrossRef CAS PubMed.
- P. W. Liang, C. Y. Liao, C. C. Chueh, F. Zuo, S. T. Williams, X. K. Xin, J. Lin and A. K. Y. Jen, Adv. Mater., 2014, 26, 3748 CrossRef CAS PubMed.
- X. Li, M. Ibrahim Dar, C. Yi, J. Luo, M. Tschumi, S. M. Zakeeruddin, M. K. Nazeeruddin, H. Han and M. Grätzel, Nat. Chem., 2015, 7, 703 CrossRef CAS PubMed.
- L. Li, Y. Chen, Z. Liu, Q. Chen, X. Wang and H. Zhou, Adv. Mater., 2016, 28, 9862 CrossRef CAS PubMed.
- N. Yaghoobi Nia, E. Lamanna, M. Zendehdel, A. L. Palma, F. Zurlo, L. A. Castriotta and A. Di Carlo, Small, 2019, 15, 1904399 CrossRef CAS PubMed.
- M. Vasilopoulou, A. Fakharuddin, A. G. Coutsolelos, P. Falaras, P. Argitis, A. R. bin Mohd Yusoff and M. K. Nazeeruddin, Chem. Soc. Rev., 2020, 49, 4496 RSC.
- P. Schulz, D. Cahen and A. Kahn, Chem. Rev., 2019, 119, 3349 CrossRef CAS PubMed.
- C.-H. Chiang, M. K. Nazeeruddin, M. Grätzel and C.-G. Wu, Energy Environ. Sci., 2017, 10, 808 RSC.
- M. Zendehdel, N. Y. Nia and M. Yaghoubinia, Reliability and Ecological Aspects of Photovoltaic Modules, IntechOpen, 2020, vol. 1, p. 93 Search PubMed.
- Y. Zhang, F. Wu, L. Chen, F. Zhang, Y. Ji, W. Shen, M. Li, Q. Guo, W. Su and R. He, Sol. Energy Mater. Sol. Cells, 2020, 212, 110534 CrossRef CAS.
- Y. Cao, Y. Li, T. Morrissey, B. Lam, B. O. Patrick, D. J. Dvorak, Z. Xia, T. L. Kelly and C. P. Berlinguette, Energy Environ. Sci., 2019, 12, 3502 RSC.
- L. Hajikhanmirzaei, H. Shahroosvand, B. Pashaei, G. Delle Monache, M. K. Nazeeruddin and M. Pilkington, J. Mater. Chem. C, 2020, 8, 6221 RSC.
- A. J. Huckaba, P. Sanghyun, G. Grancini, E. Bastola, C. K. Taek, L. Younghui, K. P. Bhandari, C. Ballif, R. J. Ellingson and M. K. Nazeeruddin, ChemistrySelect, 2016, 1, 5316 CrossRef CAS.
- A. T. Murray, J. M. Frost, C. H. Hendon, C. D. Molloy, D. R. Carbery and A. Walsh, Chem. Commun., 2015, 51, 8935 RSC.
- J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 6054 CrossRef CAS.
- J. P. Wolfe, H. Tomori, J. P. Sadighi, J. Yin and S. L. Buchwald, J. Org. Chem., 2000, 65, 1158 CrossRef CAS PubMed.
- P. Ruiz-Castillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564 CrossRef CAS PubMed.
- M. L. Petrus, K. Schutt, M. T. Sirtl, E. M. Hutter, A. C. Closs, J. M. Ball, J. C. Bijleveld, A. Petrozza, T. Bein and T. J. Dingemans, Adv. Energy Mater., 2018, 8, 1801605 CrossRef.
- Š. Daškevičiūtė, N. Sakai, M. Franckevičius, M. Daškevičienė, A. Magomedov, V. Jankauskas, H. J. Snaith and V. Getautis, Adv. Sci., 2018, 5, 1700811 CrossRef PubMed.
- S. Wang, Z. Huang, X. Wang, Y. Li, M. Günther, S. Valenzuela, P. Parikh, A. Cabreros, W. Xiong and Y. S. Meng, J. Am. Chem. Soc., 2018, 140, 16720 CrossRef CAS PubMed.
- L. Zhang, X. Zhou, C. Liu, X. Wang and B. Xu, Small Methods, 2020, 4, 2000254 CrossRef CAS.
- T. Miyasaka, A. Kulkarni, G. M. Kim, S. Öz and A. K. Jena, Adv. Energy Mater., 2020, 10, 1902500 CrossRef CAS.
- G. Sathiyan, A. A. Syed, C. Chen, C. Wu, L. Tao, X. Ding, Y. Miao, G. Li, M. Cheng and L. Ding, Nano Energy, 2020, 72, 104673 CrossRef CAS.
- J. Zou, J. Wu, W. Sun, M. Zhang, X. Wang, P. Yuan, Q. Zhu, J. Yin, X. Liu and Y. Yang, Sol. Energy, 2019, 194, 321 CrossRef CAS.
- R. Sakamoto, N. Fukui, H. Maeda, R. Matsuoka, R. Toyoda and H. Nishihara, Adv. Mater., 2019, 31, 1804211 CrossRef CAS PubMed.
- S. Callaghan and M. O. Senge, Photochem. Photobiol. Sci., 2018, 17, 1490 CrossRef CAS PubMed.
- X. Ouyang, X.-L. Li, X. Zhang, A. Islam, Z. Ge and S.-J. Su, Dyes Pigm., 2015, 122, 264 CrossRef CAS.
- A. O. Eseola, O. Adepitan, H. Görls and W. Plass, New J. Chem., 2012, 36, 891 RSC.
- T. Jadhav, J. M. Choi, J. Shinde, J. Y. Lee and R. Misra, J. Mater. Chem. C, 2017, 5, 6014 RSC.
- N. G. Silva, R. O. Silva, S. R. Damasceno, N. S. Carvalho, R. S. Prudêncio, K. S. Aragão, M. A. Guimarães, S. A. Campos, L. M. Véras and M. Godejohann, J. Nat. Prod., 2013, 76, 1071 CrossRef PubMed.
- I. Singh, V. Luxami and K. Paul, Eur. J. Med. Chem., 2019, 180, 546 CrossRef CAS PubMed.
- N. Rani, P. Kumar, R. Singh and A. Sharma, Curr. Comput.-Aided Drug Des., 2015, 11, 8 CrossRef CAS PubMed.
- N. Rani, A. Sharma and R. Singh, Mini-Rev. Med. Chem., 2013, 13, 1812 CrossRef CAS PubMed.
- I. Ali, M. N. Lone and H. Y. Aboul-Enein, MedChemComm, 2017, 8, 1742 RSC.
- B. Su, C. Cai, M. Deng and Q. Wang, J. Agric. Food Chem., 2016, 64, 2039 CrossRef CAS PubMed.
- M. Sheokand, Y. Rout and R. Misra, J. Mater. Chem. C, 2022, 10, 6992 RSC.
- S. A. Ok, B. Jo, S. Somasundaram, H. J. Woo, D. W. Lee, Z. Li, B.-G. Kim, J. H. Kim, Y. J. Song and T. K. Ahn, Nat. Commun., 2018, 9, 4537 CrossRef PubMed.
- Y. Cheng, Q. Fu, X. Zong, Y. Dong, W. Zhang, Q. Wu, M. Liang, Z. Sun, Y. Liu and S. Xue, Chem. Eng. J., 2021, 421, 129823 CrossRef CAS.
- X. Zheng, Y. Hou, C. Bao, J. Yin, F. Yuan, Z. Huang, K. Song, J. Liu, J. Troughton and N. Gasparini, Nat. Energy, 2020, 5, 131 CrossRef CAS.
- C. Shen, Y. Wu, H. Zhang, E. Li, W. Zhang, X. Xu, W. Wu, H. Tian and W. H. Zhu, Angew. Chem., Int. Ed., 2019, 58, 3784 CrossRef CAS PubMed.
- J. Wang, H. Zhang, B. Wu, Z. Wang, Z. Sun, S. Xue, Y. Wu, A. Hagfeldt and M. Liang, Angew. Chem., Int. Ed., 2019, 131, 15868 CrossRef.
- F. Liu, F. Wu, W. Ling, Z. Tu, J. Zhang, Z. Wei, L. Zhu, Q. Li and Z. Li, ACS Energy Lett., 2019, 4, 2514 CrossRef CAS.
- B. Tu, Y. Wang, W. Chen, B. Liu, X. Feng, Y. Zhu, K. Yang, Z. Zhang, Y. Shi and X. Guo, ACS Appl. Mater. Interfaces, 2019, 11, 48556 CrossRef CAS PubMed.
- A. Clarke, R. Anderson and B. Stone, Phytochemistry, 1979, 18, 521 CrossRef CAS.
- A. Shienok, L. Kol’tsova, N. Zaichenko and V. Marevtsev, Russ. Chem. Bull., 2002, 51, 2050 CrossRef CAS.
- Y. Ooyama, H. Kumaoka, K. Uwada and K. Yoshida, Tetrahedron, 2009, 65, 8336 CrossRef CAS.
- K. Skonieczny, J. Jaźwiński and D. T. Gryko, Synthesis, 2017, 4651 CAS.
- N. L. Higuera, D. Peña-Solórzano and C. Ochoa-Puentes, Synlett, 2019, 225 CAS.
- W.-C. Chen, Y. Yuan, Y. Xiong, A. L. Rogach, Q.-X. Tong and C.-S. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 26268 CrossRef CAS PubMed.
- W. Li, D. Liu, F. Shen, D. Ma, Z. Wang, T. Feng, Y. Xu, B. Yang and Y. Ma, Adv. Funct. Mater., 2012, 22, 2797 CrossRef CAS.
- R. Sarkar, T. Chaudhuri, A. Karmakar and C. Mukhopadhyay, Org. Biomol. Chem., 2015, 13, 11674 RSC.
- K. Skonieczny, A. I. Ciuciu, E. M. Nichols, V. Hugues, M. Blanchard-Desce, L. Flamigni and D. T. Gryko, J. Mater. Chem., 2012, 22, 20649 RSC.
- M. Daskeviciene, S. Paek, A. Magomedov, K. T. Cho, M. Saliba, A. Kizeleviciute, T. Malinauskas, A. Gruodis, V. Jankauskas and E. Kamarauskas, J. Mater. Chem. C, 2019, 7, 2717 RSC.
- F. Zhang, S. Wang, H. Zhu, X. Liu, H. Liu, X. Li, Y. Xiao, S. M. Zakeeruddin and M. Grätzel, ACS Energy Lett., 2018, 3, 1145 CrossRef CAS.
- R. Shang, Z. Zhou, H. Nishioka, H. Halim, S. Furukawa, I. Takei, N. Ninomiya and E. Nakamura, J. Am. Chem. Soc., 2018, 140, 5018 CrossRef CAS PubMed.
- V. Govindan, K.-C. Yang, Y.-S. Fu and C.-G. Wu, New J. Chem., 2018, 42, 7332 RSC.
- R. Francke and R. D. Little, J. Am. Chem. Soc., 2014, 136, 427 CrossRef CAS PubMed.
- B. Rajamouli, R. Devi, A. Mohanty, V. Krishnan and S. Vaidyanathan, New J. Chem., 2017, 41, 9826 RSC.
- K. Rakstys, A. Abate, M. I. Dar, P. Gao, V. Jankauskas, G. N. Jacopin, E. Kamarauskas, S. Kazim, S. Ahmad and M. Grätzel, J. Am. Chem. Soc., 2015, 137, 16172 CrossRef CAS PubMed.
- K. Rakstys, C. Igci and M. K. Nazeeruddin, Chem. Sci., 2019, 10, 6748 RSC.
- L. Wang, J. Zhang, P. Liu, B. Xu, B. Zhang, H. Chen, A. K. Inge, Y. Li, H. Wang and Y.-B. Cheng, Chem. Commun., 2018, 54, 9571 RSC.
- Y. Li, Y. Cao, J. Gao, D. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243 CrossRef CAS.
- H. Zhu, F. Zhang, X. Liu, M. Sun, J. Han, J. You, S. Wang, Y. Xiao and X. Li, Energy Technol., 2017, 5, 1257 CrossRef.
- M. Idris, C. Coburn, T. Fleetham, J. Milam-Guerrero, P. I. Djurovich, S. R. Forrest and M. E. Thompson, Mater. Horiz., 2019, 6, 1179 RSC.
- D. Sylvinson MR, H.-F. Chen, L. M. Martin, P. J. Saris and M. E. Thompson, ACS Appl. Mater. Interfaces, 2019, 11, 5276 CrossRef CAS PubMed.
- L. Bai, Z. Wang, Y. Han, Z. Zuo, B. Liu, M. Yu, H. Zhang, J. Lin, Y. Xia and C. Yin, Nano Energy, 2018, 46, 241 CrossRef CAS.
- H. J. Snaith and M. Grätzel, Appl. Phys. Lett., 2006, 89, 262114 CrossRef.
- T. Leijtens, I.-K. Ding, T. Giovenzana, J. T. Bloking, M. D. McGehee and A. Sellinger, ACS Nano, 2012, 6, 1455 CrossRef CAS PubMed.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629 CrossRef CAS PubMed.
- P. Ganesan, K. Fu, P. Gao, I. Raabe, K. Schenk, R. Scopelliti, J. Luo, L. H. Wong, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2015, 8, 1986 RSC.
- A. Abate, S. Paek, F. Giordano, J.-P. Correa-Baena, M. Saliba, P. Gao, T. Matsui, J. Ko, S. M. Zakeeruddin and K. H. Dahmen, Energy Environ. Sci., 2015, 8, 2946 RSC.
- Z. Hawash, L. K. Ono, S. R. Raga, M. V. Lee and Y. Qi, Chem. Mater., 2015, 27, 562 CrossRef CAS.
- J. A. Christians, J. S. Manser and P. V. Kamat, J. Phys. Chem. Lett., 2015, 6, 852 CrossRef CAS PubMed.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316 CrossRef CAS PubMed.
- R. J. Westbrook, T. J. Macdonald, W. Xu, L. Lanzetta, J. M. Marin-Beloqui, T. M. Clarke and S. A. Haque, J. Am. Chem. Soc., 2021, 143, 12230 CrossRef CAS PubMed.
- B. Pashaei, H. Shahroosvand, M. Ameri, E. Mohajerani and M. K. Nazeeruddin, J. Mater. Chem. A, 2019, 7, 21867 RSC.
- N. Drigo, C. Roldan-Carmona, M. Franckevicius, K.-H. Lin, R. Gegevicius, H. Kim, P. A. Schouwink, A. A. Sutanto, S. Olthof and M. Sohail, J. Am. Chem. Soc., 2019, 142, 1792 CrossRef PubMed.
- T. Leijtens, G. E. Eperon, A. J. Barker, G. Grancini, W. Zhang, J. M. Ball, A. R. S. Kandada, H. J. Snaith and A. Petrozza, Energy Environ. Sci., 2016, 9, 3472 RSC.
- C. Motta, F. El-Mellouhi and S. Sanvito, Sci. Rep., 2015, 5, 1 Search PubMed.
- T. P. Saragi, T. Spehr, A. Siebert, T. Fuhrmann-Lieker and J. Salbeck, Chem. Rev., 2007, 107, 1011 CrossRef CAS PubMed.
- K. Rakstys, M. Saliba, P. Gao, P. Gratia, E. Kamarauskas, S. Paek, V. Jankauskas and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2016, 128, 7590 CrossRef.
- B. Pashaei, S. Bellani, H. Shahroosvand and F. Bonaccorso, Chem. Sci., 2020, 11, 2429 RSC.
- K. Rakstys, S. Paek, M. Sohail, P. Gao, K. T. Cho, P. Gratia, Y. Lee, K. H. Dahmen and M. K. Nazeeruddin, J. Mater. Chem. A, 2016, 4, 18259 RSC.
- H. Choi, K. Do, S. Park, J. S. Yu and J. Ko, Chem. – Eur. J., 2015, 21, 15919 CrossRef CAS PubMed.
- A. Molina-Ontoria, I. Zimmermann, I. Garcia-Benito, P. Gratia, C. Roldán-Carmona, S. Aghazada, M. Graetzel, M. K. Nazeeruddin and N. Martín, Angew. Chem., Int. Ed., 2016, 55, 6270 CrossRef CAS PubMed.
- M. Franckevičius, A. Mishra, F. Kreuzer, J. Luo, S. M. Zakeeruddin and M. Grätzel, Mater. Horiz., 2015, 2, 613 RSC.
- S. Do Sung, M. S. Kang, I. T. Choi, H. M. Kim, H. Kim, M. Hong, H. K. Kim and W. I. Lee, Chem. Commun., 2014, 50, 14161 RSC.
- P. Gratia, A. Magomedov, T. Malinauskas, M. Daskeviciene, A. Abate, S. Ahmad, M. Grätzel, V. Getautis and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2015, 54, 11409 CrossRef CAS PubMed.
- H. Choi, S. Paek, N. Lim, Y. H. Lee, M. K. Nazeeruddin and J. Ko, Chem. – Eur. J., 2014, 20, 10894 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ya00367a |
| This journal is © The Royal Society of Chemistry 2023 |