Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermoresponsive polycations
Vikram
Baddam
 and
Heikki
Tenhu
and
Heikki
Tenhu
 *
*
Department of Chemistry, University of Helsinki, Finland. E-mail: heikki.tenhu@helsinki.fi
First published on 24th July 2023
Abstract
Aqueous solutions of polyelectrolytes are known to be complex; however, when the charged polymer carries a simple counterion such as sodium or chloride it is usually soluble in water. By modifying the hydrophobic/hydrophilic balance of the polymer structure with substituents in the charged repeating unit or by changing the counterion, the solubility and thermal behavior of the polymer can be varied. Recent interest in polymeric ionic liquids has moved much of the focus towards asymmetric systems where the polymer may bind a bulky, often hydrophobic, counterion. Polyelectrolytes with hydrophobic substituents and/or counterions are often thermoresponsive and, interestingly, by refining the structure, it is possible to change the LCST behavior into UCST, and even the coexistence of both LCST and UCST is possible. In this review, we summarize recent progress made on investigating the thermoresponsive behavior of polycations and cationic copolymers in aqueous and non-aqueous solvents. The main emphases are on tuning the solution properties of polycations with alkyl substituents, counterions, and copolymer structures.
1. Introduction
Polyelectrolyte solutions have been extensively studied for years, and many theoretical and experimental investigations have been conducted to understand the conformations of polyelectrolyte chains in solution.1–5 Compared to the solutions of non-charged polymers, polyelectrolyte solutions are complicated because of several variable interactions.6 So far, discussions on polyelectrolytes have mainly concentrated on the polymer chain conformation and its dependence on simple counterions, such as Na+, Cl−, etc.7–12 The chain conformation is dictated by coulombic interactions and added salts determine the functionality of a polyelectrolyte and play a crucial role when employing the polyelectrolyte for its applications.13–22The importance of counterions on the properties of polyelectrolytes has only recently been realized. Counterions affect the solubilities of the charged chains and can make them stimuli-responsive in aqueous or non-aqueous solvents. The solubility of a permanently charged polymer is not prominently affected by pH but this may change when using hydrophobic counterions.23–28 Ionic interactions can be modulated by an external stimulus like temperature, and this is how a polyelectrolyte may become thermoresponsive in aqueous or non-aqueous systems.
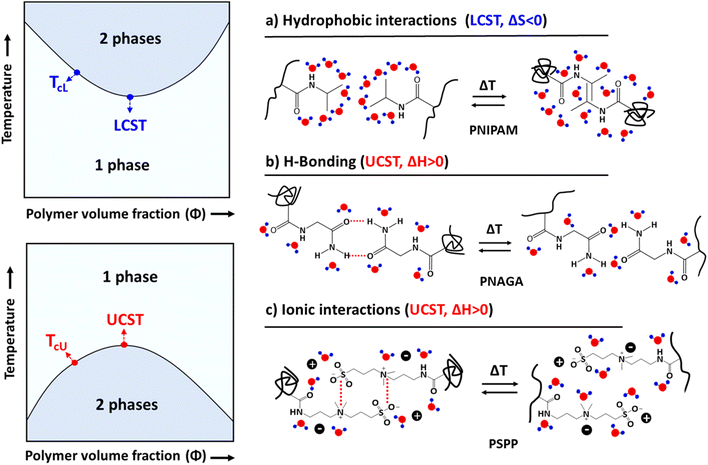
Thermoresponsive polymers that undergo phase separation either below the upper critical solution temperature (UCST) or above the lower critical solution temperature (LCST) are well documented in the literature.29–34 The phase separation mechanism of nonionic LCST polymers is mainly based on intra- or intermolecular hydrophobic interactions, which change when the hydration layer around the polymer breaks down upon heating.29,35 The UCST behavior is due to intra- and intermolecular H-bonding or electrostatic interactions.30,36–39 The thermoresponsive behavior of polyelectrolytes is different from that of non-charged polymers, as the phase separation process depends on three body interactions, including polymer–polymer, polymer–water, and strong polymer–counterion interactions. The behavior of a non-ionic polymer is mainly due to the polymer–polymer and polymer–water interactions. In non-ionic systems, one can manipulate the phase transition behavior by adding hydrophilic or hydrophobic units to the main polymer chain or to the repeating units. Zhao et al. proposed the thermodynamic mapping of thermoresponsive polymer systems based on various interactions and polymer chain architectures.40
A few reviews already discussed some polyelectrolytes that exhibited thermoresponsive behavior in aqueous or non-aqueous systems,36,41,42 however, structural variation of polycations and copolymer architectures was not reported in detail. In the present short review, we first discuss certain non-ionic, zwitterionic and anionic thermoresponsive polymers and their phase separation mechanisms. Recently, interest in thermoresponsive polycation systems has noticeably increased43–46 and the main focus of the present review is on novel studies of cationic polymers. The main aim of the review is to summarize observations concerning how detailed chemical structures of the cationic structural units and the type of counterion affect the phase separation processes in aqueous or non-aqueous polycation systems.
2. Thermoresponsive polymers
2.1. Nonionic polymers
Polymers that phase separate upon heating or cooling are already well-known.33,34,47–49 In a LCST system, polymers are miscible with a solvent below a certain temperature, but phase separation occurs above it. Poly(N-isopropylacrylamide) (PNIPAM) is the most studied polymer, and phase separation takes place above the LCST (about 32 °C), or simply TcL, see Fig. 1. The phase separation of such non-ionic systems is mainly dependent on the negative entropy of mixing (ΔSm < 0 when T < LCST) due to the polymer–solvent interactions. As the water cages surrounding the repeating units are disrupted by heating, the polymer chains start to aggregate above the cloud point temperature.UCST polymers behave oppositely. Polymers are soluble above certain critical temperatures (TcU), but phase separate below that. Poly(N-acryloyl glycinamide) (PNAGA) is an example of a nonionic polymer that shows the UCST-type phase transition in aqueous solutions. The polymers dissolve above the UCST because of the positive enthalpy of mixing (ΔHm > 0).31 The UCST-type phase separation can be observed in several hydrogen-bonding polymers, such as polymethacrylamide, acrylamide-, ureido-, and imidazole-based polymers.37,39,40,50–54 When the polymer chain carries both H-bond donors and acceptors, inter- and intra-chain H-bonds build up. Solubilizing such a polymer requires the disruption of the H-bonds by thermal energy, and this is the origin of the UCST behavior in nonionic polymer systems. Electrostatic interactions trigger similar processes in the case of oppositely charged polyelectrolytes.55,56
2.2. Polyzwitterions and polyanions
Polyzwitterions and polyampholytes exhibit UCST or LCST-type phase transitions in aqueous or non-aqueous systems due to intra- and intermolecular electrostatic interactions and dipole–dipole interactions between oppositely charged ionic groups.30,44,57–62 Sulfobetaine-based zwitterionic polymers undergo UCST-type phase transitions in aqueous solutions. The behavior depends on the molar mass of the polymer, salt, and polymer concentrations. A few studies have been conducted to elucidate the effects of the molecular structure of polysulfobetaines, such as S1, on their thermoresponsive behavior.56,63 Hildebrand et al. studied polysulfobetaines in which the anionic and cationic charges were separated with various different spacers. They observed that polymers where the spacers were substituted with hydroxyl groups underwent phase separation at lower temperatures compared to the polymers with alkyl spacers (S2 and S3 in Fig. 2).56 The cloud point temperatures can be tuned with alkyl substituents in the sulfobetaine repeating units. It has been observed that with sulfobetaines with long or bulky alkyl spacers, the solubilities of the polymers increase in water (S4 and S5). This is because the steric hindrance of larger alkyl groups decreases the electrostatic interactions between the ionic groups.64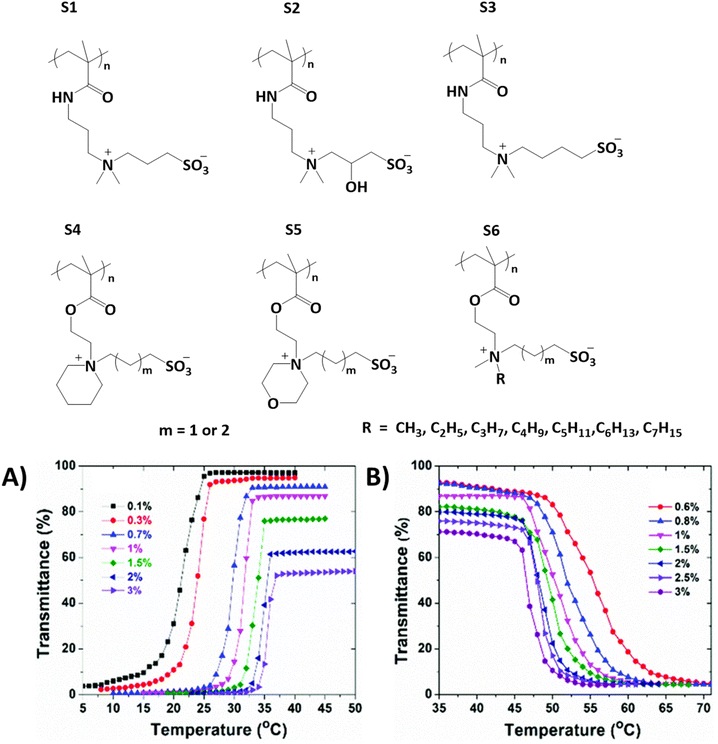 | ||
| Fig. 2 Series of zwitterionic polysulfobetaines. (A) Transmittance data collected upon cooling S6 with methyl substituents; (B) collected upon heating S6 with a n-pentyl substituent at various polymer concentrations. Reproduced from ref. 64. Copyright 2018 Royal Society of Chemistry. | ||
Wang et al. studied polysulfobetaines S6 by changing the length of one of the alkyl substituents of the quaternary nitrogen.64 Polymers with methyl substituents undergo UCST-type phase separation in water, whereas those with ethyl, propyl, or butyl substituents are soluble. Interestingly, polymers with long alkyl substituents (pentyl or hexyl) undergo LCST phase transitions upon heating (Fig. 2A and B). By increasing the alkyl length to n-heptyl, polymers become totally insoluble in water.64 This intriguing solution behavior is due to the increase in the entropic penalty (ordering of water molecules) as well as weakening of the electrostatic interactions between zwitterionic groups by introducing hydrophobic units.
Few thermoresponsive polyanions in aqueous systems have been reported.19,41 For instance, anionic styrene sulfonate (PSS) and 3-sulfopropyl methacrylate polymers (PMPS) undergo LCST-type phase transitions in aqueous solutions, see Fig. 3. The phase separation of these polymers is mainly due to hydration changes around the anionic repeating units and the hydrophobic balance of the counterions. Upon heating, polymers form random aggregates that are surrounded by cationic counterions.41,65,66
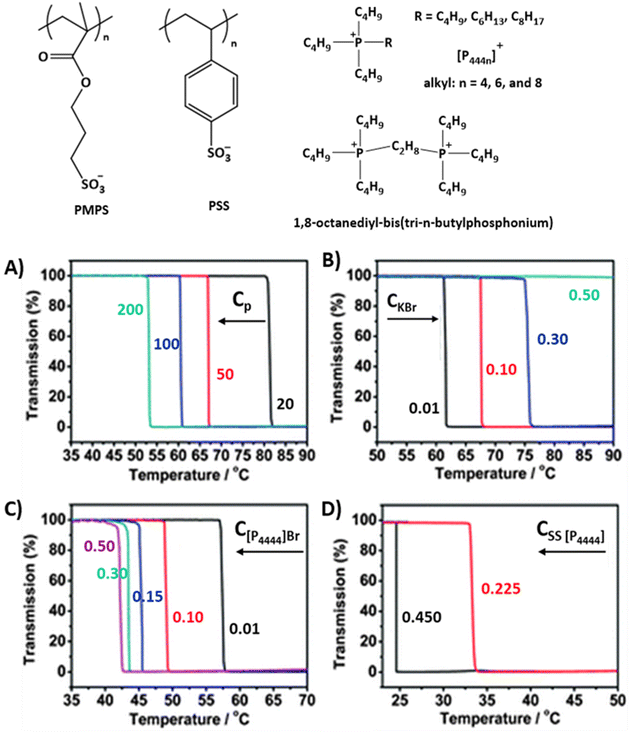 | ||
| Fig. 3 Strong polyanions that show LCST-type phase transitions with corresponding hydrophobic counterions. (A) Transmittance curves of PSS [P4444] solutions with various polymer concentrations (Cp), (B) PSS [P4444] (100 g L−1) in aqueous KBr solutions (0.01–0.5 M), (C) PSS [P4444] (100 g L−1) in [P4444]Br (0.01–0.5 M) aqueous solutions, (D) PSS [P4444] (100 g L−1) in SS [P4444] aqueous solutions. Reproduced from ref. 67. Copyright 2012 Royal Society of Chemistry. | ||
In the case of PMPS, the polymer does not phase separate when using [P4444]+ as a counter ion.66–69 However, by changing the alkyl substituent from butyl to hexyl in the tetraalkylphosphonium ions ([P4444]+ to [P4446]+), the polymers undergo LCST-type phase separation upon heating. The cloud point TcL shifts to lower temperatures with the addition of more hydrophobic [P4448]+ ions with n-octyl substituents.68 For styrene-based poly styrene sulfonate tetrabutylphosphonium, PSS [P4444], LCST-type phase separation can be induced using the same [P4444]+ counter ions and TcL can be tuned with counterions, the monomer SS[P4444], and polymer concentrations (see Fig. 3).67,70 When increasing the polymer, counterion, or monomer concentration, the TcL shifts to lower temperatures (Fig. 3A, C and D). Alternatively, with the addition of simple salts like KBr, the cloud point shifts to higher temperatures, or the LCST-type phase transitions disappear at high salt concentrations (see Fig. 3B). The phase separation processes of the same PSS systems were studied using gemini-cationic counterions.71,72 In these cases, the phase transitions of the solutions are sensitive to the counterion and polymer concentrations, similar to the monocationic PSS [P4444] systems.
Zhang et al. conducted in-depth studies on the phase separation of PSS.72 They observed that the polymers underwent liquid–liquid phase separation upon heating. This was reasoned to be the effect of polymer dynamic crosslinking by the gemini counterions. Based on variable-temperature 1H NMR and two-dimensional correlation analysis, the authors concluded that dehydration of anionic chains mainly served as the driving force for phase separation in the PSS aqueous solution. The polymerized anion tends to form aggregated cores with dications distributed around the globules at the end of the transition process. The LCST-type phase transitions were also observed in covalently cross-linked gels of PSS and acrylate-based sulfonate polymers.73 The cloud points of the gels decrease with increasing crosslinker concentration. The behavior of the gels differed from that reported for linear polymers. The endotherms measured calorimetrically upon heating were very broad.73
Aside from polysulfonates, a few carboxylic polyanions that show LCST or UCST-type phase transitions have been reported.38,74,75 Polyanions made of borate (–B−–) and sulfonylimide (–SO2N(−)SO2−) have been studied as single-ion conducting polymers.76 However, to the best of our knowledge, there have been no studies on the thermoresponsive behavior of such polyanions.
Thermoresponsive polyelectrolytes or poly(ionic liquid)s have only recently gained attention, and polycation systems are presently being explored much more intensely than polyanions. This could be owing to the larger structural variances of polymerizable cationic ionic liquids or ionomers than the anionic ones. This gives us a good reason to discuss the polycations separately.
3. Thermoresponsive behavior of polycations
The solution properties of polycations change significantly if simple ions (Cl−, Br−, etc.) are replaced with bulkier counterions. The change is especially noticeable for fluorinated counterions, such as tetrafluoroborate (BF4−), hexafluorophosphate (PF6−), trifluoromethanesulfonate (OTf−), and bis(trifluoromethylsulfonyl)amide (NTf2−). Polycations can undergo phase separation by modulating the coulombic interactions between the cationic repeating units and the counterions with temperature. Several polycations have been reported to show phase separation in aqueous or non-aqueous solutions either above the cloud point temperature (TcL) upon heating (LCST-type) or below the cloud point temperature (TcU) upon cooling (UCST-type). However, the type of phase separation process mainly depends on the hydrophilic–hydrophobic balance of cationic repeating units and of the counterions.Interesting research has already been conducted on the phase separation of polycations with hydrophobic counterions. For example, aqueous solutions of the cationic poly(allylammonium chloride) (PAAC) phase separate when introducing hydrophobic counterions like p-ethylbenzenesulfonate (EBS−) ions.24 This phenomenon was also investigated for solutions of the styrene-based polycation poly((vinylbenzyl)trimethylammonium chloride) (PVBTMAC) with hydrophobic fluorescent ions such as pyrene sulfonate (PSA−). Excimer emission of PSA− ions in the presence of PVBTMAC confirmed the formation of hydrophobic domains.25 Analogous to nonionic polymers, polycations may phase separate from aqueous solutions when introducing a cosolvent. The strong polycation poly(methacryl oxyethyl trimethylammonium) (PMOTA) with its methanesulfonate counterion undergoes a reversible coil-to-globule transition upon increasing the acetone content in aqueous polymer solutions. It was concluded that the free energy of the system, determined by coulombic interactions and strong ion pairing, led to intra- and intermolecular associations when the dielectric constant of the solution was lowered with the addition of acetone.77,78 Throughout the following discussion on responsive polycations, we refer to numbering given in Scheme 1.
Poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA; P1) is a weak polycation and besides its pH responsiveness, it can show LCST in aqueous solutions. Both UCST and LCST behaviors may be observed by adjusting the pH of PDMAEMA solutions when using either mono- or multivalent counterions.79–81 Other tertiary amine polymers have been reported to show thermoresponsive behavior in aqueous solutions. Pang et al. recently reviewed the thermoresponsive behavior of tertiary amine functionalized poly((meth)acrylamide)s, poly((meth)acrylates), poly(styrenes), poly(vinyl alcohols), and poly(ethylene oxides).82
3.1. UCST transition
Strong polycations with UCST-type phase transitions have been studied much more than those with a LCST. Noh et al. studied the thermoresponsive behavior of the branched polyethylene imine (PEI) and methylated PEI, MPEI (P2).83 They observed the UCST-type phase transition for PEI in aqueous solutions with simple ions like Cl−, Br−, and I−. When the ionic interactions between the counterion and PEI are strong enough to overcome the water–ion interaction, conditions are created that lead to the UCST behavior. However, this behavior was only observed under extreme pH conditions. In the latter case, it was observed that the hydrophobicity of the counterion of methylated PEI was an important parameter to induce the UCST transition. When I− or BF4− were used as counterions, MPEI underwent phase separation, whereas with Br− and Cl− MPEI was soluble over the temperature range 0–100 °C. The behavior also depended on the molar mass of the polymers: higher TcU values were recorded for a high molar mass polymer compared to the short one.83 Such properties were also observed for PDMAEMA under certain pH conditions. When using hydrophobic counterions like bistrifluoromethane sulfonylimide (NTf2−), PDMAEMA undergoes an UCST-type phase transition in aqueous solutions.81Strongly charged PMOTA (P3) with different alkyl substituents and BF4− as a counterion displays an UCST-type transition.84 However, the same polymer with an iodide counterion (PMOTAI) is water-soluble at any temperature. The addition of the hydrophobic counterion NTf2− to an aqueous solution of PMOTA leads to complete phase separation due to strong ion pairing.85 In the presence of NaCl or changing the counterions to less hydrophobic trifluoromethanesulfonate (OTf−) ions, an UCST-type phase transition can be induced. Similarly, a styrene-based polycation with imidazolium pendants (P4) also undergoes an UCST-type transition and the reported cloud point temperatures are higher than those for P3 under the same conditions. A low amount of triflate counterions is needed to make P4 thermoresponsive due to the hydrophobic phenyl units. For both polymers, a sufficient ionic strength (NaCl) is required to observe the cloud/clearing point when using the NTf2− counterions. The cloud points can be tuned over a wide temperature window by changing the ionic strength of solutions. In the case of P3, the maximum TcU was observed at around 500 mM in both LiOTf and KOTf aqueous solutions (Fig. 4A).85 The same styrene-based imidazolium polycation P4 with BF4− counterions was reported to show an UCST-type phase transition in methanol/water (80/20) mixtures. The TcU strongly depends on the molar mass and concentration of the polymer. TcU increased from 14 to 21.4 °C when the DP of P4 increased from 36 to 107. In addition, the TcU can be tuned to lower temperatures with increasing water content in solvent mixtures.86
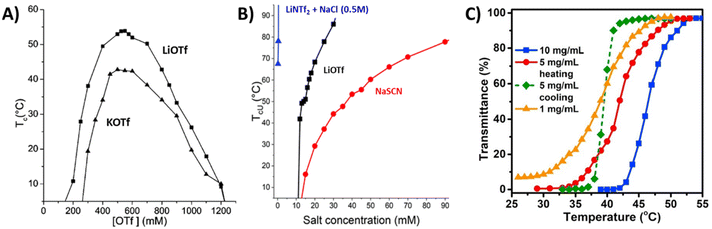 | ||
| Fig. 4 (A) TcU values of PMOTAI (P3) solution (1 mg mL−1) as a function of the concentration of OTf ions with either LiOTf or KOTf. Adapted from ref. 85. Copyright 2014 American Chemical Society. (B) The TcU points for P5b in various salt solutions. Reproduced from ref. 88. Copyright 2020 Royal Society of Chemistry. (C) Transmittance curves for aqueous P7 solutions at different polymer concentrations. Reproduced from ref. 89. Copyright 2019 with permission from Elsevier. | ||
Styrene-based quaternary ammonium polycations (P5a) carrying either OTf− or Cl− as counterions are well soluble in water, however, they become thermoresponsive with an UCST-type phase transition upon the addition of a small amount of triflate ions. In the presence of 0.1 M of LiOTf, the polycations with a low molar mass (DP = 24) undergo phase separation below 35 °C. However, with increasing molar mass of P5a, the TcU shifts to higher temperatures (DP = 61, TcU = 51 °C) when the LiOTf concentration remains constant. The other two acrylamide-based polycations poly(3-(acrylamido)propyl)trimethylammonium chloride (PAMPTMAC; P6) or methacrylate-based P3 do not phase separate at any temperature under similar conditions (DP = 24, 1 g L−1 and LiOTf = 0.1 M).87
Recently, the influence of alkyl substituents on the phase separation of styrene-based polycations was investigated in several salt solutions.88 The polycation with ethyl substituents, P5b, undergoes an UCST-type phase transition in the presence of the counterions SCN−, OTf−, and NTf2− (Fig. 4B). As in the case of P3, when NTf2− was the counterion, a secondary salt, NaCl (0.5 M), was needed to induce the UCST-type behavior. Interestingly, at certain NTf2− concentrations, P5b phase separation may take place in two steps. At room temperature P5b forms particles. Upon heating, the transmittance decreases (LCST-type transition), but solutions become clear again upon further heating above TcU (UCST-type transition). On the other hand, only UCST-type phase transitions were observed upon cooling. The TcU can be tuned with other counterions (OTf−, SCN−) without using NaCl (Fig. 4B). The UCST-type phase transition was also observed in the presence of NO3−, however, a high amount of salt (2.4 M of NaNO3) was needed when compared to the hydrophobic ions. It was concluded that the minimum concentration of salt inducing the salting-out effect decreased in the following order: NaNO3 > NaSCN > LiOTf > LiNTf2 with 500 mM NaCl. P5c with n-propyl substituents exhibits UCST-type phase transitions in NaH2PO4 solutions. Similar to P5b, the polymer undergoes stepwise phase separation (LCST and UCST-type) in NaSCN or NaNO3 solutions, and the process can be reversible upon cooling at certain NaNO3 salt concentrations. In addition, the same polymer undergoes a LCST-phase transition in concentrated salt solutions (3.5 M NaCl). Polymers with longer n-alkyl substituents show the LCST-type transition in various salt solutions (this is discussed in the next section). The switching of the phase separation behavior from UCST to LCST-type was mainly attributed to changes in the hydrophobic–hydrophilic balance of the cationic structures. Short alkyl chain polycations are well soluble in water but become insoluble when using hydrophobic counterions. Additionally, due to hydration changes around the long alkyl substituents, the polymers become insoluble at elevated temperatures. The polymer–water interactions are overruled by polymer–ion interactions.88
The UCST-type phase transitions can also be observed for other styrene-based polycations P7, which contain reactive aldehyde groups (Fig. 4C).89 In the presence of BF4− counterions, the TcU increases with increasing polymer concentration. The phase behavior can be tuned via the post-modification of aldehyde substituents on the aromatic groups. With the formation of oxime, the cloud point shifts to lower temperatures. These polymers with a secondary amine as a weak cationic group undergo the LCST-type phase transition in water/alcohol mixtures.
Biswas et al. studied the thermoresponsive behavior of styrene-based phosphonium polycations (P8a), which carry hydrophobic tri-phenyl substituents.91 The polycation exhibited the UCST-type phase transition in aqueous solutions in the presence of the sodium salts NaBr or NaCl (Fig. 5). The cloud point temperature TcU was dependent on the polymer molar mass, concentration, and the size of the counterions. The phase transition could be induced with a small amount of NaBr, whereas in the case of Cl− ions 450 mM of NaCl was needed (Fig. 5C). TcU increased with increasing salt concentration. Large anions with high polarizability interact strongly with the larger number of cationic phosphonium groups. Thus, polymers became insoluble below 100 °C in the presence of I−, SCN−, and CN− ions. A co-nonsolvency effect was observed for P8a; TcU decreased linearly with increasing fractions of MeOH in the water–MeOH mixtures (polymer Mn = 40 kDa, 0.5 wt% with 550 mM NaCl) (Fig. 5D). A similar decrease in TcU with increasing volume percent of DMF and DMSO was observed in the mixed solvent systems. The same research group also reported imidazolium-based polycation P9, which showed the UCST-type phase transition in aqueous NaI solutions.92
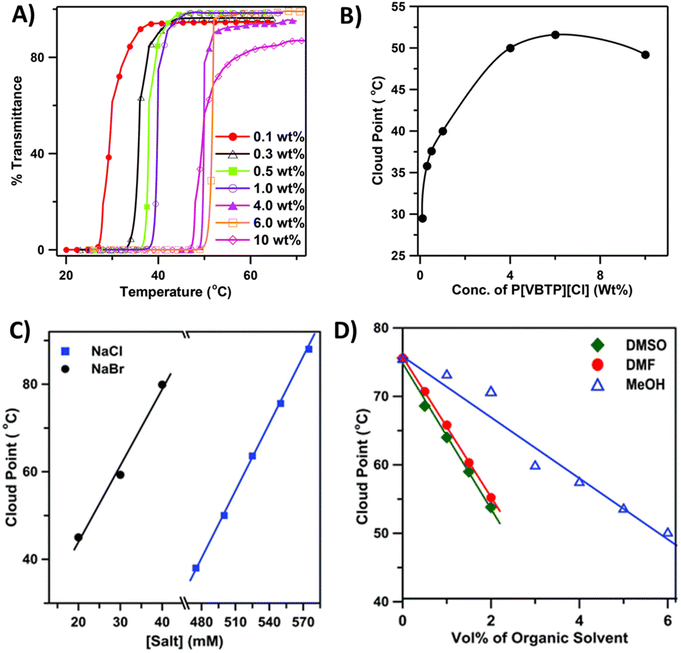 | ||
| Fig. 5 (A) Transmittance cooling curves of aqueous solutions of P8a containing 475 mM NaCl and (B) corresponding phase diagram. (C) Plot of Tcp values as a function of NaCl and NaBr concentration for 0.5 wt% aqueous P8a (Mn = 40 kDa) solution. (D) Effect of organic cosolvent composition on the Tcp values of P8a (Mn = 40 kDa, 0.5 wt%) in a water–organic solvent binary mixture containing 550 mM NaCl. Reproduced from ref. 90. Copyright 2016 Royal Society of Chemistry. | ||
Imidazolium-based polycations with ether backbones P10 were reported to exhibit UCST-type transitions in aqueous solutions.93,94 The polycations with BF4− counterions undergo UCST-type transitions when less hydrophobic substituents (Me = P10a or Me2 = P10b) are present on the imidazolium rings. If the alkyl substituents are long like n-butyl (P10c) or n-pentyl (P10d), the polymer solubility may change, and UCST-type phase transitions are possible in methanol or ethanol depending on the counterion (BF4− or NTf2−).93
3.2. LCST transition
So far, we have discussed the UCST-type phase separation process of polycations driven by strong polymer–counterion interactions. Interestingly, the phase separation process can be changed to LCST by changing the alkyl chain substituents and the charged moieties to more hydrophobic ones. Ritter et al. studied the LCST-type phase separation of imidazolium-based polycations P9 with NTf2− counterions in aqueous cyclodextrin (CD) solutions.95 The polycations undergo the LCST-type phase transition in aqueous solutions when ionic interactions between the bulky counterions and butyl imidazolium repeating units are weakened by CDs (Fig. 6). The behavior is similar to nonionic LCST polymer systems; however, in the case of P9, it is due to entropy driven decomplexation of the anions and CDs at higher temperatures. In the presence of CDs at T < LCST, the polymers are well soluble in water as the NTf2− ions are bound to the host–guest complexes with CDs. The phase transition temperatures TcL increase significantly with increasing CD concentrations as higher energies are needed to break the host–guest complexes.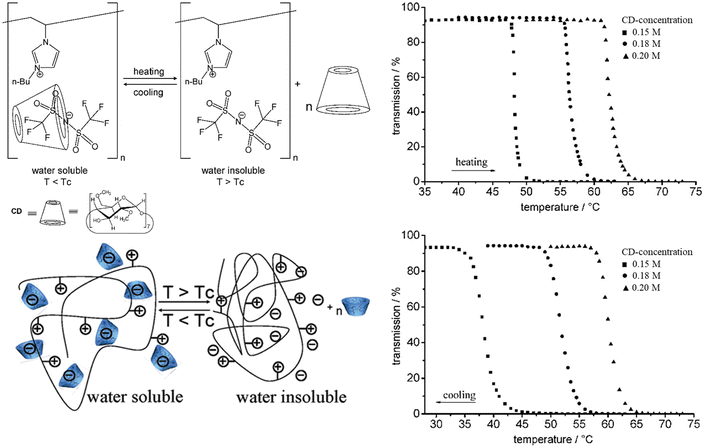 | ||
| Fig. 6 Pseudo-LCST behavior of P9. Transmittance curves for aqueous P9 solutions at different CD concentrations. Reproduced from ref. 95. Copyright 2008 American Chemical Society. | ||
Yuan et al. studied cationic poly(tributyl-4-vinylbenzylphosphonium) (PVBTBP) bearing alkyl sulfonate counterion P8b.43 Similar to polyanions PSS [P4444], this polymer shows a LCST-type phase transition in aqueous solutions. The phase transition behavior in either trialkyl ammonium or phosphonium-based polycation systems is similar to the aforementioned polyanion systems.96,97 The phase separation temperatures of the polymers are strongly dependent on the salt and polymer concentrations, as well as on the length of the alkyl chain substituents both on the counterions and on the cationic repeating units. Polycation P8b bearing n-pentylsulfonate counterions (C5S−) undergoes a LCST-type phase separation in pure water (TcL = 45 °C, polymer concentration 3 wt%).43 The cloud point temperature increases with the addition of NaCl, and decreases when sodium sulfonate salts (NaCnS) with different alkyl chain lengths are introduced (0.02 M in all cases). As shown in Fig. 7A, the shift in the cloud point temperature follows the hydrophilicity order of anions, Cl− > C3S− > C4S− > C5S− > C6S−. Addition of NaCl leads to a continuous increase of the TcL, which is ∼44 °C in pure water and 87 °C in 0.5 M NaCl, see Fig. 7B. When NaC5S was added, the TcL decreased sharply down to below 20 °C at 0.07 M. In the case of NaC3S, a constant TcL ∼ 40 °C was observed over a low salt concentration range (0.002 to 0.6 M), indicating that an anion exchange process was taking place. However, above 0.6 M, similar behavior was observed to that in the case of NaC5S. Ohno et al. reported different cloud point temperatures for similar polymer systems bearing phosphonium or ammonium cationic groups. Ammonium-based polycations (P5e) phase separate above 65 °C, whereas phosphonium ones (P8b) do it at 45 °C when the cation is substituted with n-pentyl units.98 The cloud point shifts to lower temperature in the case of hexyl substituents (P5f) and the hydrophobic polycations form particles in aqueous solutions at room temperature. In the case of P5d, the LCST-type phase separation can be induced by anion exchange from Cl− to C6S− and the TcL shifts to lower temperature with increasing polymer concentration (Fig. 7c).99
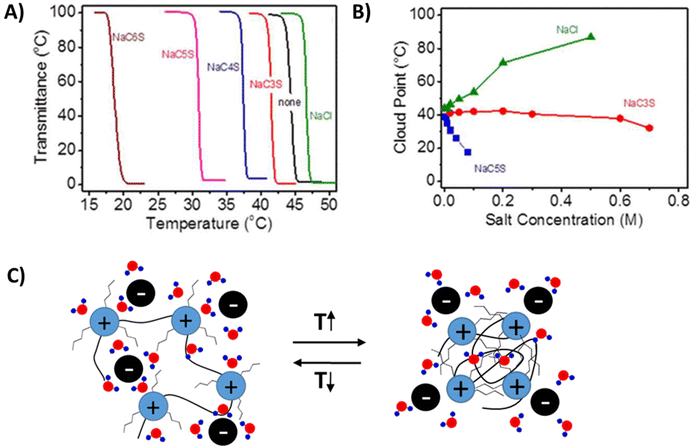 | ||
| Fig. 7 (A) Turbidity curves of 3 wt% aqueous solution of P5e in the presence of sodium salts (0.02 M). (B) Plot of the cloud point temperature versus salt concentration. Reprinted from ref. 43. Copyright 2013 American Chemical Society. (C) Cartoon representation of the LCST-type phase separation mechanism of polycations. | ||
More recently, Karjalainen et al. studied styrene-based systems in various aqueous salt solutions.88 The polycations P5d and P5e underwent LCST-type phase transitions. With increasing NaC5S concentration, the cloud point of P5d shifted first to lower temperatures and then started to increase. The behavior was similar when NaCl (0.25 M) was introduced as a secondary salt to screen the main ionic interactions. The LCST-type phase separation can also be induced using high concentrations of NaCl; the TcL shifts to lower temperatures upon increasing the salt concentration from 1 to 3 M. Interestingly, using other sodium salts like NaNO3, Na2HPO4, Na2SO4, and Na2CO3, LCST-type phase separation can be induced in P5d and P5e with or without NaCl as a secondary salt. However, a high amount of salt is needed compared to NaCnS systems.
Charged polymers are often observed to phase separate from organic solvents with increasing temperature. Reasons behind these observations are very different from what is known to take place in water. The main driving force is the balance of polymer–solvent and polymer–polymer interactions, which depend on the dielectric constant of the solvent.78 Organic solutions are briefly discussed here because this also opens a view to develop systems where charged polymers are dissolved in mixed solvents.
Polycations with vinyl ether backbones and imidazolium or pyridinium salt pendants show LCST-type phase separation in various organic solvents.94 Polycation P10c with butyl imidazolium units is insoluble in nonpolar solvents (hexane and ethyl acetate) but soluble in polar solvents, such as water and methanol. However, it undergoes a LCST-type phase separation in chloroform when the counterion is chloride. Phase separation was observed for pyridinium polycations P11 in chloroform/methanol mixtures with a low methanol content [chloroform/methanol 97/3 (w/w)]. The TcL is dependent on the polymer molar mass and concentration. In addition, the polymer solubility can be changed by changing the alkyl substituents or counterions. When two methyl or one n-pentyl substituents were bound to the imidazolium ring, the polycation with the SbF6− counterion underwent a LCST-type phase transition in acetone or THF solutions. Such behavior was mainly due to the counter-anion-mediated weakening of interactions between the polymer pendants and the solvent upon heating.
Styrene-based polycations with chiral cationic imidazolium units (P12) also undergo the LCST-type phase transition in CHCl3.99 The cloud point temperature increased with changing the substituent in the imidazolium ring from butyl to phenyl. In contrast, when the substituent in the imidazole ring was changed from (L)-valine to (L)-phenylalanine, the transition temperature decreased from 48.5 to 12.5 °C. Furthermore, TcL decreases when counterions are changed from Cl− to NTf2− ions.
The thiazolium-based polycation (P13) with PF6− counterions was reported to exhibit a LCST-type phase transition in acetone.100 The TcL shifts to lower temperatures with increasing polymer concentration (Fig. 8A). In addition, TcL can be tuned by external salts (LiBF4, LiNTf2, and KPF6) as well as with cosolvents (THF, DMF, or DCM) (Fig. 8C and D). In the presence of bulky counterions, the cloud point shifted from 37 °C (pure acetone) to a higher temperature of 45 °C with 0.01 M of LiNTf2. In contrast, a gradual decrease in TcL down to 30 °C and 23 °C was observed in the presence of PF6− and BF4− ions (Fig. 8C). Alternatively, the addition of 2–6 wt% of DMF (a good solvent for the polymer) resulted in a high shift in TcL to 49 °C or even beyond the investigated temperature range. In addition to the increased solvent quality, hydrogen bonding between the C2 proton of the thiazolium ring and solvent molecules may affect the polymer behavior in the presence of DMF. Therefore, the cloud point shifted toward higher temperatures. Opposite behavior was observed for the same system when mixing the polymer solution with a poor solvent, such as THF or DCM. With the addition of THF or DCM, the cloud point temperature decreased gradually, similarly to the case of adding bulky counterions (Fig. 8D).
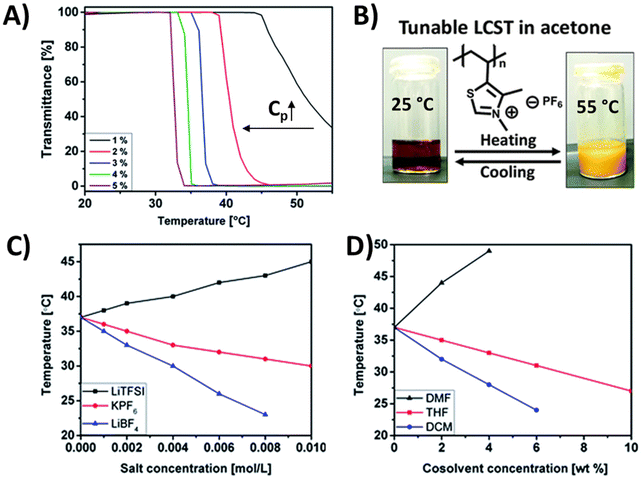 | ||
| Fig. 8 (A) Turbidity curves for acetone solutions of P13 measured at various polymer concentrations. (B) Photographs of a 3 wt% P13 solution in acetone below the Tc (left) and above the Tc (right). TcL measured for 3 wt% P13 solutions in acetone: (C) at different concentrations of external salts; (D) at different concentrations of cosolvents. Reproduced from ref. 100 with permission from the Royal Society of Chemistry, copyright 2016. | ||
Based on the above observations, it is possible to draw conclusions on the structural characteristics of cationic moieties, as well as counterions, which are crucial in designing thermoresponsive polycations. With short alkyl substituents, most polycations show UCST-type phase transitions in the presence of counterions that bring hydrophobicity to the polymers. Polycations or counterions with long alkyl chain substituents undergo LCST-type phase transitions, similarly to the polyanions discussed earlier. Polycations and the types of their phase transitions in aqueous or non-aqueous solutions are summarized in Table 1.
| Polycation | Compound number | R | R1 | Xl | XT | Type of transition |
|---|---|---|---|---|---|---|
| XI− = initial counterion from synthesis, XT− = counterion added to induce thermoresponsive behavior. | ||||||
| PDMAEMA | P1 | — | — | — | [Co(CN)6]3− | LCST or UCST |
| [Fe(CN)6]4− | ||||||
| [Cr(CN)6]3− | ||||||
| NTf2− | ||||||
| PEI | P2a | — | — | Cl−, Br−, I− | UCST | |
| M-PEI | P2b | — | Cl−, Br−, I− | Cl−, Br−, I− | ||
| PMOTA | P3a | Methyl | Cl−, Br−, I−, BF4− | BF4−, OTf−, NTf2− | UCST | |
| P3b | Ethyl | Br− | BF4− | |||
| P3b | n-Butyl | Br− | BF4− | |||
| PVBMIm | P4 | — | Cl−, BF4− | BF4−, OTf−, NTf2− | UCST | |
| PVBTAA | P5a | Methyl | Cl−, OTf− | OTf− | UCST | |
| P5b | Methyl | Cl− | H2PO4−, SO4−2, Cl−, NO3−, SCN −, OTf−, NTf2− | UCST or LCST | ||
| P5c | n-Propyl | Cl− | H2PO4−, Cl−, NO3−, SCN − | UCST or LCST | ||
| P5d | n-Butyl | Cl− | H2PO4−, CO3−, SO4−2, Cl−, NO3−, SCN −, OTf−, NTf2−, n-C4H9SO3−, n-C5H11SO3−, n-C6H13SO3−, n-C12H25SO3− | LCST | ||
| P5e | n-Pentyl | Cl− | H2PO4−, CO3−, SO4−2, Cl−, n-C4H9SO3−, n-C5H11SO3− | LCST | ||
| P5f | n-Hexyl | n-C4H9SO3− | LCST | |||
| PAMPTMAC | P6 | — | — | Cl− | OTf−, NTf2− | UCST |
| PDEVB | P7 | — | — | I−, BF4− | BF4− | UCST |
| PVBTAP | P8a | Phenyl | Cl− | Cl−, Br− | UCST | |
| P8b | n-Butyl | — | Cl−, n-C3H7SO3−, n-C4H9SO3−, n-C5H11SO3−, n-C6H13SO3− | LCST | ||
| P8c | n-Pentyl | — | Cl− | n-C4H9SO3− | LCST | |
| PVBuIm | P9 | — | — | Br−, NTf2− | I−, NTf2− | UCST or LCST |
| PVEIm | P10a | Methyl | H | Cl− | BF4− | UCST |
| P10b | Methyl | Methyl | Cl− | BF4−, NTf2−, SBF6− | ||
| P10c | n-Butyl | H | Cl− | Cl−, BF4−, NTf2− | LCST | |
| P10d | n-Pentyl | H | Cl− | Cl−, BF4−, NTf2−, SBF6− | ||
| PVEPy | P11 | — | — | Cl− | LCST | |
| PVBVIm | P12a | Val | Phenyl | Cl− | Cl−, NTf2− | LCST |
| P12b | Val | n-Butyl | Cl− | Cl− | LCST | |
| PVBPIm | P12c | Phe | Phenyl | Cl− | Cl− | LCST |
| PMVTh | P13 | — | — | PF6− | PF6−, BF4−, NTf2− | LCST |
3.3. UCST or LCST of cationic polypeptides
Synthetic polypeptides are structurally more complicated than vinyl polymers. They mimic natural peptides with excellent biocompatibility and unique secondary structures (e.g., α-helix and β-sheet) and are widely used for biological or biomedical applications.101 A few nonionic synthetic polypeptides that show UCST behavior under physiologically relevant conditions have been reported in the literature, and in this section we discuss some cationic derivatives of those.64,65 The compound numbers refer to Scheme 2.Tang's group synthesized several polyglutamate derivatives with cationic pendants. Helical L-glutamate polypeptides bearing various imidazolium or pyridinium side groups (P14, P17) are thermoresponsive in aqueous solutions in the presence of I− or BF4− counterions.102 This behavior is very dependent on the alkyl substituents of the cationic groups and counterion. Polycation P14b goes through an UCST-type phase transition in NaI or NaBF4 aqueous solutions, whereas P14a with methyl substituents is well soluble. Interestingly, P14b with BF4− counterions can undergo a LCST-type transition in acetone, and with I− ions an UCST transition in methanol or ethanol. The effect of salt concentrations was studied on P15, another type of imidazolium-based polypeptide with n-alkyl spacers.103 With increasing the NaBF4 or NaI concentration, TcU shifts to higher temperatures, whereas the opposite trend is observed with the addition of NaCl.67 Polypeptide P16 with thioether spacer units exhibits UCST behavior when using I− or BF4− ions.104
Similar observations have been made for pyridinium-based polycations, with either n-alkyl or benzyl linkers between the cationic group and the backbone.105,106 Polymers P17a and b with either 2-methyl or 4-methyl pyridinium groups are insoluble in water. However, the polymers undergo UCST-type phase transitions below 30 °C in the case of 3-methyl pyridinium.105 In polymers with aromatic spacers, P18, the solution behavior is independent of the position of methyl substituents. Polymers exhibit UCST-type phase behavior in water in the presence of BF4− ions.106 The cloud point temperatures TcU were tuned by either changing the polymer or salt concentration. Tang's group also synthesized more complex di-cationic polypeptides containing cationic n-butyl imidazolium pendants (P19) or ammonium pendants with sulfonium linkages (P20). These dicationic polymers underwent UCST-type phase transitions in aqueous BF4− solutions. The TcU of the polymers can be adjusted through varying the polymer or NaBF4 concentrations. The cloud point shifts to a lower temperature in the presence of halides (Cl or I). Due to the amine groups in P20, the cloud points can be changed by altering the pH as well.107 Alternatively, cationic sulfonium polypeptides P21 undergo UCST-type phase transitions in methanol or ethanol when different alkyl substituents are present. The TcU can be tuned by changing the counterions.107
A few cationic poly(L-cysteines) have been reported to be thermoresponsive in aqueous solutions in the presence of hydrophobic counterions (I−, SCN−, BF4− and ClO4−).108 Imidazolium-based poly(L-cysteine) P22 with a BF4− counterion has a TcU in aqueous solutions similar to that of polyglutamic P15. Due to the higher number of intermolecular H-bonds, a higher TcU was observed for P22 than for P15. More recently, Anas et al. investigated the solution properties of triphenyl phosphonium-based poly(L-cysteine)s (P23) in several aqueous salt solutions, see Fig. 9. In the presence of hydrophobic counterions, polymers phase separate with added salt. The minimum amount of anions required to induce UCST-type phase separation decreases in the order BF4− > I− > ClO4− > SCN− as shown in Fig. 9A and B. Next, TcU values were measured upon changing the polymer concentration and keeping the salt concentration constant. The maximum TcU varied from 60 to 79 °C depending on the counterion, see Fig. 9C, D, E, and F.109
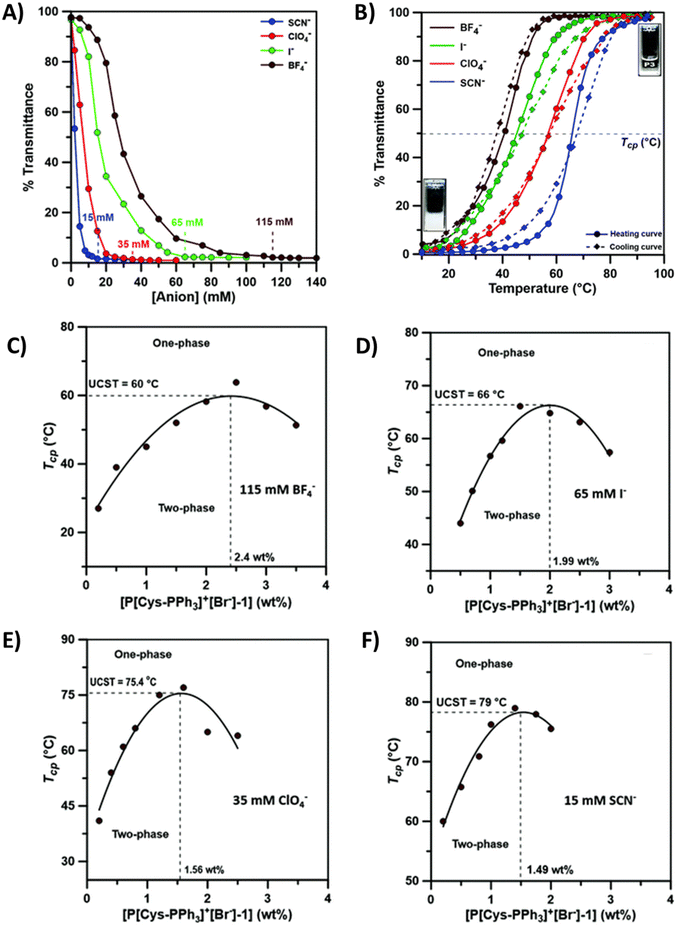 | ||
| Fig. 9 (A) Turbidity curves of 0.5 wt% aqueous P[Cys-PPh3]+[Br−] (P23) solutions with varying concentrations of different chaotropic anions (SCN−, ClO4−, I− and BF4−). (B) Turbidity curves of an aqueous P23 solution (0.5 wt%) in the presence of minimum concentrations of different chaotropic anions (BF4−, I−, ClO4− and SCN−). UCST-type phase diagram of P23 of varying polymer concentrations in the presence of 115 mM of BF4− (C), 65 mM of I− (D), 35 mM of ClO4− (E) and 15 mM of SCN− (F) in water. Reproduced from ref. 108 with permission from the Royal Society of Chemistry, copyright 2021. | ||
A counterion-induced UCST was also observed for water soluble methionine pendant polypetides.109 Polymer P24 phase separates in the presence of Br−, SO42−, I−, ClO4− and SCN− ions. TcU points are very dependent on the type of counterion. TcU values collected for 1 wt% polymer solutions of different ionic strengths were 35 °C (300 mM of Br−), 33 °C (200 mM of I−), 40 °C (100 mM of ClO4−) and 49 °C (50 mM of SCN−).
Polypeptides with 3-methyl-1,2,3-triazolium iodide linkages (P25) exhibit reversible UCST-type phase behaviors in both methanol and ethanol/water binary solvent mixtures.110 The phase transition of polypeptides completely changes when ethylene glycol oligomer side chains are bound to the triazolium cationic units.111 Xu et al. synthesized poly(4-methylbenzyl-L-glutamate) bearing Y-shaped triazolium cationic units with oligoethylene glycol and alkyl (such as 1-butyl, 1-hexyl, and 1-dodecyl) side chains (P26a–c). These triazolium-based polypeptides undergo LCST-type phase transitions in aqueous solutions, similar to their non-charged precursors (i.e., before quaternization) with ethylene glycol oligomer side chains (Fig. 10). The clouding behavior of the polymers is significantly dependent on the DP of the polypeptide chain and the length of the alkyl substituents on the triazolium units (Fig. 10A and B). The cloud point decreases with increasing concentration of the polymer or NaBF4 (Fig. 10C and D).111
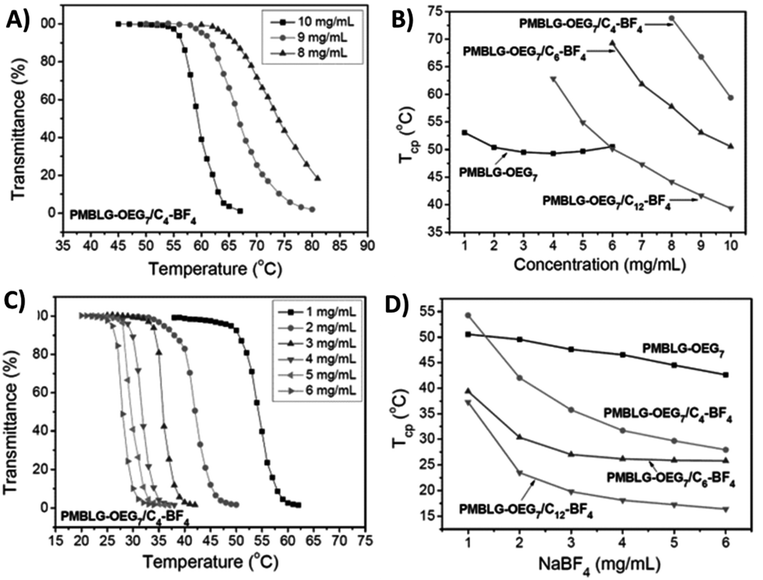 | ||
| Fig. 10 (A) Transmittance curves of PMBLG-OEG7/C4-BF4 (P26a) at different polymer concentrations in DI H2O. (B) Plots of Tcp versus concentrations in DI H2O for P26 with different alkyl substituents. (C) Transmittance curves of PMBLG-OEG7/C4-BF4 (P26a) at different NaBF4 concentrations in aqueous solutions. (D) Plots of Tcp versus NaBF4 in aqueous solutions of P26 with different alkyl substituents. Reproduced from ref. 111 with permission from the Royal Society of Chemistry, copyright 2016. | ||
Water-soluble homopolypeptides with tributylphosphonium cationic units connected via ether linkages (P27) were able to show dual thermoresponsive behavior in aqueous solutions.112 The phase separation of P27a is similar to that of the above discussed styrene-based cationic polymers (P5c, P5d). In the presence of I− counterions, the polymer undergoes stepwise phase segregation (TcL and TcU; TcL < TcU) in aqueous solutions (Fig. 11). The phase behavior is further affected by the molar mass of the polymer and salt concentration. Using more hydrophobic BF4− counterions, the phase behavior changes to only UCST-type transitions or polymers may even become insoluble. Similar polypeptides, containing 1-butylimidazole or 3-methylpyridine cationic pendants, are completely soluble in water or PBS over the temperature range 0–100 °C.112 On the other hand, polymers P27b and P28 with poor water solubility show UCST-type phase transitions in PBS in the presence of I− or BF4− counterions. The TcU can be readily changed from 20 to 90 °C by adjusting the polymer concentration.
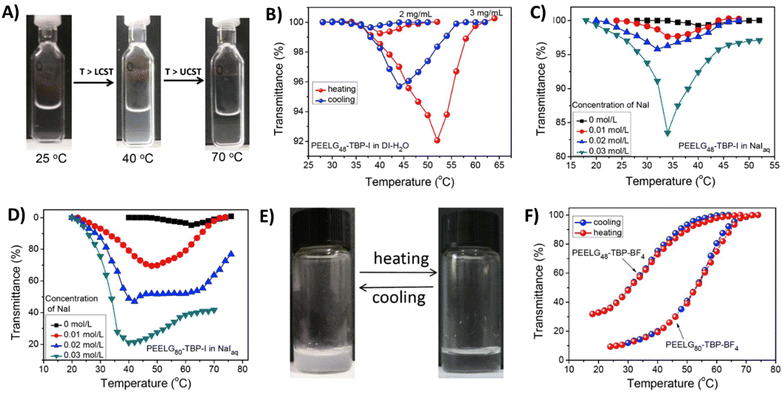 | ||
| Fig. 11 (A) Optical images of P27a (DP = 80, X = I−) in aqueous NaI solutions (0.02 mol L−1) at different temperatures to demonstrate the LCST < UCST behavior. Plots of transmittance at λ = 500 nm versus temperature for (B) P27a (DP = 48, X = I−) in deionized (DI) H2O with different polymer concentrations in heating/cooling cycles. (C) P27a (DP = 48, X = I−) and (D) P27a (DP = 80, X = I−) in DI H2O and NaIaq at different salt concentrations (polymer concentration = 2 mg mL−1). (E) Optical images of P27a (DP = 80, X = BF4−) in DI H2O at different temperatures (polymer concentration = 3 mg mL−1). (F) Plots of transmittance at λ = 500 nm versus temperature for P27a (DP = 48 or 80, X = BF4−) in DI H2O in a cooling/heating cycle (polymer concentration = 3 mg mL−1). Reprinted from ref. 113. Copyright 2017 with permission from Elsevier. | ||
Overall, the hydrophilic–hydrophobic balance of the polymer structures is crucial in designing thermoresponsive polypeptides. The counterion-induced UCST phase separation can be changed to LCST by introducing short hydrophilic ethylene glycol moieties like DEG or OEG. In this case, the cationic polypeptides either undergo a single LCST-type transition or both UCST and LCST transitions, see Table 2.
| Polycation | Compound number | R | XI− | XT− | Type of transition |
|---|---|---|---|---|---|
| XI− = initial counterion from synthesis, XT− = added counterions. | |||||
| PMBLG-Im | P14a | Methyl | Cl− | Cl−, I−, BF4− | LCST or UCST |
| PPLG-Im | P14b | n-Butyl | Cl− | Cl−, I−, BF4− | UCST |
| PMBLG-S-Im | P15 | n-Butyl | Cl− | Cl−, I−, BF4− | UCST |
| PPLG-Py | P16 | n-Butyl | Cl− | Cl−, I−, BF4− | UCST |
| PHLG-Py | P17a | 2-Methyl | Cl− | Cl−, I−, BF4− | UCST |
| PMBLG-Py | P17b | 4-Methyl | Cl− | Cl−, I−, BF4− | UCST |
| PPLG-Im2 | P18 | H, 2-, 3- or 4-methyl | Cl− | BF4− | UCST |
| PPLG-MSEA | P19 | — | Br− | I−, BF4− | UCST |
| PPLG-MS | P20 | — | Cl− | BF4− | UCST |
| PPLC-Im | P21a | Methyl | Br− | I−, BF4− | UCST |
| PCys-PPh3 | P21b | n-Butyl | Br− | Br−, I− | UCST |
| PMetNB | P21c | Propargyl | Br− | Br−, I− | UCST |
| PPLG-TAz | P22 | — | BF4− | Cl−, I−, BF4− | UCST |
| PMBLG-OEG7/Cn | P23 | — | Br− | I−, SCN −, ClO4−,BF4− | UCST |
| PEELG-PBu3 | P24 | — | Br− | Br−, SO4−2 I−, ClO4−, SCN− | UCST |
| PEELG-PPh3 | P25 | — | I− | I− | UCST |
| PPLG-PBu3 | P26a | n-Butyl | Br− | Br−, BF4− | LCST |
| PMBLG-Im | P26b | n-Hexyl | Br− | Br−, BF4− | |
| PPLG-Im | P26c | Dodecyl | Br− | Br−, BF4− | |
| PMBLG-S-Im | P27a | n-Butyl | Cl− | I−, BF4− | LCST and UCST |
| PPLG-Py | P27b | Phenyl | Cl− | I−, BF4− | UCST |
| PHLG-Py | P28 | n-Butyl | Cl− | I−, BF4− | UCST |
4. Thermoresponsive cationic copolymers
4.1. Random copolymers
It has been shown that in nonionic LCST copolymer systems, the cloud point temperatures can be shifted either to lower or higher temperature ranges by adding hydrophobic or hydrophilic repeating units. Correspondingly, in UCST copolymers, the cloud point shifts to a higher temperature when the number of hydrophobic repeating units increases. The thermoresponsive behavior of such copolymers has been thoroughly discussed in the literature.31,40 The phase separation process for copolymers made of opposite thermoresponsive units is often complex. The polymers can either undergo phase separation with dual transitions (TcL and TcU) or become completely soluble with no thermoresponsive behavior.Several copolymers comprising nonionic and cationic repeating units have been reported to show UCST or LCST-type phase transitions in aqueous solutions. The polymers discussed in this section are shown in Scheme 3 and Table 3. Homopolymers of 2-hydroxyethyl methacrylate (PHEMA) undergo LCST-type phase transitions in aqueous solutions.114 However, the solution behavior changes in HEMA copolymers containing either weak cationic DMAEMA units or strong cationic ones such as MOTAC or [3-(methacryloylamido)propyl]trimethylammonium chloride (MAPTAC). Copolymers with strong charges (P29 and 30) undergo both LCST and UCST-type transitions in water. The double phase transitions of these polymers are strongly dependent on the NaCl concentration and also vary with the polymer concentration, copolymer composition, or pH.115 On the other hand, a series of HEMA copolymers P31a–b with phosphonium-based (vinylbenzyl)triphenylphosphonium chloride (VBTPPC) cationic units form turbid dispersions in the presence of Cl− ions. These turbid dispersions undergo UCST-type phase transitions upon heating as the cationic units become soluble in aqueous salt solutions (Fig. 12A). The cloud points shift to higher temperatures with increasing VBTPAC content in the copolymer composition.
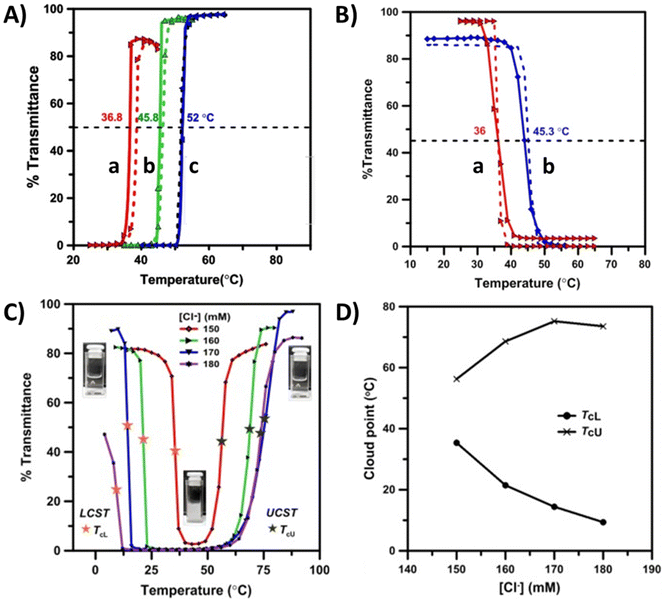 | ||
| Fig. 12 (A) Turbidity curves for aqueous copolymer solutions of P31 (1 wt%) with various Cl− ion contents: (a) P31a, Cl− = 10 mM; (b) P31b, Cl− = 25 mM; (c) P31c, Cl− = 75 mM. (B) Turbidity curves of 1 wt% aqueous solutions of P32a (a) and P32b (b) with various PNIPAM contents. (C) Turbidity curves of aqueous P32c solutions (0.25 wt%) at different Cl− ion concentrations. (D) TcL and TcU points of P32d (0.25 wt%) as a function of [Cl−] ions measured from the heating curves shown in (C). Reproduced from ref. 116. Copyright 2020, with permission from Elsevier. | ||
| Polymer | Compound number | m | n | l | Xl− | Type of transition |
|---|---|---|---|---|---|---|
| m, n, and l indicate the DPs of different repeating units. m refers to the non-ionic monomer, n to the cationic monomer. l is for dodecylmethacrylate (P38). | ||||||
| P(MOETAC-co-HEMA) | P29a | 93 | 7 | — | Cl− | LCST and UCST |
| P29b | 92 | 8 | — | Cl− | ||
| P29c | 86 | 14 | — | Cl− | ||
| P(MPTMAC-co-HEMA) | P30a | 96 | 4 | — | Cl− | LCST and UCST |
| P30b | 93 | 7 | — | Cl− | ||
| P30c | 91 | 9 | — | Cl− | ||
| P(VBTPP-co-HEMA) | P31 | 89.5 | 10.5 | — | Cl− | UCST |
| 80 | 20 | — | Cl− | |||
| 71.4 | 28.6 | — | Cl− | |||
| P(VBTPP-co-NIPAM) | P32a | 98.9 | 1.1 | — | Cl− | LCST |
| P32b | 98 | 2 | — | Cl− | ||
| P32c | 80.7 | 19.3 | — | Cl− | ||
| P32d | 65.5 | 34.5 | — | Cl− | LCST and UCST | |
| P(VBTPP-co-MMA) | P33a | 90 | 10 | — | Cl−, I− | UCST |
| P33b | 82 | 18 | — | Cl−, I− | ||
| P33c | 4 | 96 | — | Cl− | ||
| P(VBTPP-co-St) | P34a | 78 | 22 | — | Cl− | UCST |
| P34b | 35.6 | 64.4 | — | Cl− | ||
| P34c | 9.7 | 90.3 | — | Cl− | ||
| P(VBTPP-co-OEtOxA) | P35a | 25 | 2 | — | Cl− | LCST |
| P35b | 25 | 4 | — | Cl− | ||
| P35c | 25 | 11 | — | Cl− | ||
| P35d | 25 | 25 | — | Cl−, I− | ||
| P35e | 10 | 42 | — | Cl− | LCST and UCST | |
| P35f | 10 | 46 | — | Cl− | UCST | |
| P(VBuIm-co-OEtOxA) | P36a | 20 | 40 | — | Br− | LCST and UCST |
| P36b | 20 | 75 | — | Br− | UCST | |
| P36c | 3 | 60 | — | Br− | ||
| P(VBuIm-co-OEGMA) | P37 | — | — | — | SCN−, BF4−, PF6−, NTf2− | LCST |
| P(PEGMA-co-MOTAI-co-DMA) | P38a | 32 | 99 | 65 | Cl−, I−, NO3−, | LCST |
| P38b | 42 | 92 | 43 | Cl−, I−, NO3−, SO4−2, CH3COO− | ||
| P38c | 59 | 94 | 30 | Cl−, I−, NO3−, | ||
| P38d | 68 | 98 | 21 | Cl−, I−, NO3−, | ||
| P(VEIm-co-NIPAM) | P39 | — | — | — | Br− | LCST |
| P(AMPTAC-co-NIPAM) | P40a | 83 | 7 | — | NTf2− + Cl− | LCST |
| P40b | 71 | 15 | — | NTf2− + Cl− | ||
| P40c | 65 | 23 | — | NTf2− + Cl− | ||
| P40d | 43 | 37 | — | NTf2− + Cl− | LCST and UCST | |
| P40e | 27 | 50 | — | NTf2− + Cl− | UCST | |
When the same VBTPPC cationic units were copolymerized with NIPAM, the polymers (32a and 32b) with low ionic contents underwent LCST-phase transitions (Fig. 12B). This behavior is mainly attributed to NIPAM, and the TcL shifts to higher temperatures with an increase in the cationic units and decreases with an increase in NaCl. Copolymers with a high content of ionic units (19.3 and 34.5%) do not phase separate in pure water. However, copolymer P32c with 19.3% cations exhibits a LCST-type phase transition in the presence of NaCl (69 mM Cl− ions). In the case of copolymer P32d with 34.5% cationic units, the polymers underwent both UCST and LCST-type phase transitions (TcL < TcU) in aqueous solutions in the presence of 160 mM of Cl− ions. The insolubility window can be tuned with varying salt and polymer concentrations (Fig. 12C & D). In addition, the copolymers of VBTPPC and styrene (P33a–c) or methyl methacrylate (P34a–c) undergo UCST phase transitions in aqueous or methanol solutions when external halide ions are introduced.
Jana et al. studied copolymers consisting of oligo(2-ethyl-2-oxazoline)acrylate (OEtOxA) and cationic ionic liquid units VBTPPC or 1-vinyl-3-butylimidazolium bromide (VBuIm-Br).117 The copolymers (P35) with VBTPPC show solution properties and thermoresponsive behavior similar to the NIPAM case and the cloud point can easily be tuned by adjusting the copolymer composition and the ionic strength. The OEtOxA copolymers containing a low amount of cationic segments showed LCST-type phase transitions in aqueous salt solutions. Both LCST and UCST-type transitions occur when the content of ionic repeating units is 73 wt% (P35e, see Fig. 13A).117 The copolymer with much higher ionic PVBTPPC content shows only a UCST-type transition in the presence of 500 mM Cl− ions, as do the PVBTPPC homopolymers. In the copolymers with imidazolium-based cationic units, P36, similar phase behavior was observed in aqueous solutions with I− ions. Polymer P36a with an ionic content of 64 wt% undergoes both LCST and UCST phase separation. TcL decreases with increasing polymer concentration, whereas the TcU shifts to higher temperatures (Fig. 13B). In copolymers with a high content of ionic units, P36b and c, the LCST-type phase transition disappears over a certain I− ion concentration range due to the hydrophobic nature of the cationic moieties.
 | ||
| Fig. 13 (A) Turbidity heating curves for P35e in aqueous solutions at different polymer concentrations in the presence of 500 mM Cl− ions and TcL and TcU points collected from heating curves (right). (B) Turbidity heating curves of aqueous P36a solutions at different polymer concentrations in the presence of 55 mM I− ions and TcL and TcU points collected from heating curves (right). Polymer concentrations are indicated next to the transmittance curves. Adapted from ref. 117. Copyright 2018 American Chemical Society. | ||
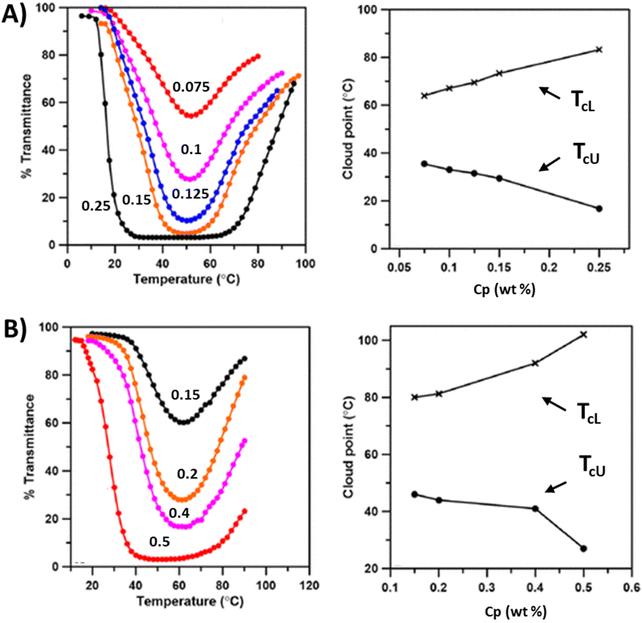
Random copolymer P37 comprising oligo(ethylene glycol) methacrylate (OEGMA) and imidazolium-based cationic units shows a LCST-type phase transition in the presence of SCN−, BF4−, PF6− or NTf2− counterions (Fig. 14A).118 The cloud point temperatures of these copolymers depend on the type of anion. The TcL shifts to high temperatures and stable aggregates form as the hydrophilicity of the anions increases. However, the phase separation process takes place in a single step, as in the case of homopolymers of OEGMA. Recently, amphiphilic random co(ter)polymers P38 bearing poly(ethylene glycol) (PEG), a quaternary ammonium cation and dodecyl groups were reported to exhibit LCST-type phase transitions in aqueous salt solutions.120 The cloud point temperature depended on the ratio of PEG and cation units, salt concentration, and salt species. In solutions of copolymers containing equal amounts of ethylene glycol and cationic units, TcL dramatically changes from 97 °C to 53 °C depending on the salt species in the following order: TcL = 97 °C (CH3COONa), 79 °C (NaCl), 70 °C (NaI), 68 °C (NaNO3), and 53 °C (Na2SO4). Soll et al. prepared thermoresponsive copolymers comprising imidazolium-based 1-vinyl-3-ethylimidazolium bromide (VEIm-Br) cations and NIPAM P39. The thermoresponsiveness of the copolymers is mainly attributed to the NIPAM behavior. The phase transitions change with the polymer compositions and added salts, see Fig. 14B and C.119 Either by increasing the salt concentration or decreasing the ionic content in the copolymers, the cloud point TcL shifts to lower temperatures. At high ionic contents, copolymers form stable aggregates upon phase separation, but it may also be that the thermoresponsiveness totally vanishes. Random copolymers of NIPAM and AMPTMAC, P40, exhibit LCST, UCST, or both phase transitions (TcL < TcU) in the presence of NTf2− ions. These double phase transitions mainly occur when the copolymers consist of equal amounts of NIPAM and AMPTAMC units (P40d). The copolymers experience a LCST-type separation with a lower amount of AMPTMAC (P40a). Only an UCST-type separation was observed for copolymers with a high AMPTMAC content (P40e).121
 | ||
| Fig. 14 (A) Transmittance heating curves collected for P37 in various aqueous ionic solutions (0.1 wt%). Reproduced from ref. 118. Copyright 2017 Royal Society of Chemistry. (B) Turbidity curves of aqueous solutions (10 g L−1) of P39 with 7.6 mol% of VEIm-Br and different concentrations of KBr (from 0.001 to 1 M), CKBr increasing in order from right to left. (C) Turbidity curves of aqueous solutions of P39 with different VEIm-Br contents and a constant concentration of KBr (CKBr = 0.015M), mol% increasing in order from left to right. Adapted from ref. 119. Copyright 2012 American Chemical Society. | ||
4.2. Block copolymers
The thermoresponsive behavior of polymers can be tuned with the copolymer architecture. Block copolymers undergoing phase separation in water when heated have been well reported in the literature. Due to the block architecture, the hydrophilic block copolymers can form self-assembled core–shell nanoparticles when one of the blocks phase separates above TcL or below TcU and the other block remains soluble. In such cases, the copolymers can be used to encapsulate and release poorly water-soluble active substances in a controlled way. Copolymers made of two thermoresponsive blocks are of special interest because, as the temperature changes, the copolymers can build up inverse micelles (LCST > UCST or LCST < UCST). If both blocks lose their solubility upon heating, the phase separation of the polymers may take place stepwise (LCST1 > LCST2 or UCST2 > UCST1). Finally, in the case of amphiphilic block copolymers, phase separation may lead to nanoparticles with a multitude of morphologies, depending on the mutual lengths of the blocks and the TcL of the responsive block.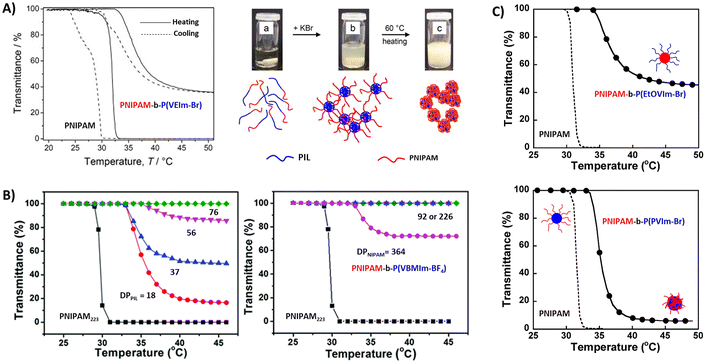 | ||
| Fig. 15 (A) Temperature-dependent transmittance of PNIPAM and block copolymer P42a and photographs of the polymer solutions in pure water at 25 °C (a). (b) and (c) are in the presence of KBr at 25 °C and 60 °C. Reproduced from ref. 126. Copyright 2011 Elsevier. (B) Transmittance curves of aqueous P43 solutions with various DPs of PIL blocks (left) and for copolymers with various DPs of PNIPAM blocks (right). Reproduced from ref. 127 with permission from the Royal Society of Chemistry. (C) Transmittance curves for block copolymers P45 (top) and P46 (bottom) in water. Reproduced from ref. 128. Copyright 2009 American Chemical Society. | ||
| Block copolymer | Compound number | m | n | Xl− | Type of transition |
|---|---|---|---|---|---|
| PNIPAM-b-PEImA | P41 | — | — | Br− | LCST |
| PNIPAM-b-PVEIm | P42a | 60 | 38 | Br− | LCST |
| PNIPAM-b-PVBuIm | P42b | 50 | 17 | Br− | LCST |
| PNIPAM-b-PVBMIm | P43a | 239 | 18 | BF4− | LCST |
| P43b | 231 | 37 | BF4− | ||
| P43c | 221 | 56 | BF4− | ||
| P43d | 226 | 76 | BF4− | ||
| P43e | 92 | 76 | BF4− | ||
| P43f | 364 | 76 | BF4− | ||
| PNIPAM-b-PBuImMA | P44a | 59 | 24 | BF4− | LCST |
| P44b | 88 | 24 | BF4− | ||
| PNIPAM-b-PEtOEVIm | P45 | 27 | 73 | Br− | LCST |
| PNIPAM-b-PPVIm | P46 | 91 | 9 | Br− | LCST |
| [(PNIPAM-b-PVEIm)]4 (star) | P47 | 38 | 62 | Br− | LCST |
| [(PVEIm-b-PNIPAM)]4 (star) | P48 | 64 | 36 | Br− | LCST |
| PEtOx-b-PMetNB | P49 | — | — | I−, ClO4−, SCN − | UCST |
| PEG-b-PVBTMAC | P50a | 93 | 62 | OTf− | UCST and LCST |
| P50b | 93 | 81 | OTf− | UCST and LCST | |
| P50c | 93 | 172 | OTf− | UCST | |
| P50d | 93 | 270 | OTf− | UCST | |
| PS-b-PVBMIm | P51 | — | — | BF4− | UCST |
| PDAAm-b-PVBTMAC | P52 | — | — | OTf− | UCST |
| PIBVE-b-PImVE | P53 | — | — | Cl−, BF4− | UCST |
| PODVE-b-PImVE | P54 | — | — | Cl−, BF4− | UCST |
| PPhOVE-b-PImVE | P55 | — | — | Cl−, BF4− | UCST |
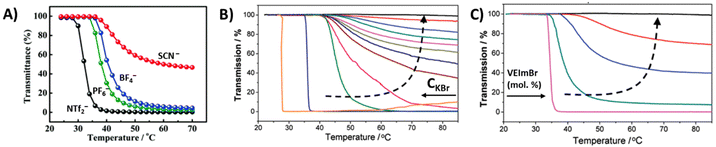
Luo et al. studied a series of block copolymers P43a–f comprised of PNIPAM and styrene-based poly(vinylbenzyl)methylimidazolium (PVBMIm) with BF4− counterions.127 As shown in Fig. 15B, copolymers with long cationic blocks are soluble at all temperatures. However, upon decreasing the DP of PIL or increasing the NIPAM content, the polymer undergoes a LCST-type phase transition and the sizes of the aggregates vary with the block ratios. We investigated a similar system where the imidazolium-based polycation was chain extended with NIPAM with different DPs (P44).129 The copolymer micelles with longer PNIPAM blocks underwent a LCST-type thermal transition and large aggregates were observed by DLS at elevated temperatures. The phase separation behavior of NIPAM block copolymers was also evaluated by changing the substituents on the vinyl imidazolium PIL segments. Block copolymer P45 with hydrophilic 1-(2-ethoxyethyl)-3-vinylimidazolium bromide (EtOEVI-Br) IL segments was soluble in water at low temperature, and formed micelles upon phase separation of PNIPAM blocks above TcL. By changing the IL segments to hydrophobic 1-(3-phenylpropyl)-3-vinylimidazolium bromide (PVI-Br) units, the polymers showed poor solubility in water. In the case of copolymer P46, the block copolymers form micelles with PIL cores at room temperature, which phase separate upon heating as NIPAM chains collapse above the LCST (Fig. 15C).
Mori et al. synthesized linear and star block copolymers containing poly(VEIm-Br) cationic segments and poly(NIPAAm) as a thermoresponsive segment. Similar to the above discussed copolymers, the TcL shifted to higher temperatures in both linear and star block copolymers when compared to homopolymers of NIPAM.125,130 In the case of star polymers, the phase transitions occurred much more sharply in the P47 stars (PNIPAM is the inner block) compared to other ones, P48, with PNIPAM as the outer block. The phase separation was reversible upon cooling and heating. Above TcL, P47 stars phase separated and formed large aggregates (Dh = 570 nm). The aggregates were stable at elevated temperatures of >40 °C. The micelles consisting of a relatively hydrophobic core of PNIPAM and a hydrophilic shell of poly(VEIm-Br) aggregated in solution. On the other hand, a constant increase in the hydrodynamic diameters of the aggregates was observed for the P48 stars from 230 nm at 35 °C to 670 nm at 40 °C. Similar size increments were also observed for linear block copolymers.
Several responsive NIPAM copolymers containing amine based cationic blocks have been reported. In general, these polymers have been employed in studies on polyelectrolyte complexes.131–135
A few diblock copolymers of strong polycations have been found to be thermoresponsive. Polypeptide block copolymers P49 consisting of ethyl-oxazoline and methionine undergo UCST phase separation in aqueous I−, ClO4− or SCN− solutions similar to polymethionine. Polyethyl oxazolines, PEtOX, with DP greater than 100 are known to exhibit a LCST between 0 and 100 °C. However, in the methionine case, the PEtOX block was short and had no influence on the phase separation process. Zhao et al. recently reported self-assembled polypeptide structures of triblock copolymers bearing sulfonium and triphenylphosphonium iodide in aqueous solutions. These single-chain nanoparticles show UCST-type thermoresponsive properties in aqueous or PBS solutions.139
Recently, we studied the phase separation of diblock copolymers P50 comprising PEG and styrene-based polycations with OTf− counterions.140,141 The block copolymers are well soluble in water, but phase separate upon the addition of LiOTf. Interestingly, block copolymers with short cationic blocks, P50a, undergo stepwise phase separation with both UCST and LCST-type transitions (Fig. 16). Upon cooling, the clear polymer solutions become very turbid below TcU. With further cooling, the dispersions slightly clear up below TcL (Fig. 16A). The phase separation process is strongly dependent on the LiOTf concentration and the length of the cationic block. The insolubility window (intermediate phase) becomes wider with increasing the LiOTf concentration up to 400 mM and TcU increases (Fig. 16B) when the cationic block is short (DP 62). At high salt concentrations, TcU decreases and narrow insolubility regions with more than one transmittance minimum are observed. The polymer concentration (inset in Fig. 16B) and end group have no effect on the phase separation process. The phase separation process was different in the block copolymer when the length of the cationic block increased to DP 81 (P50b); both LCST and UCST-type transitions were observed at certain salt concentrations (from 50 to 80 mM). No clearing transition was observed for the polymer solutions with 100 mM or higher LiOTf concentrations.140 Only single UCST-type transitions were observed for block copolymers (P50c and P50d) with long cationic blocks (DP = 172 and 270) at any LiOTf concentration.141
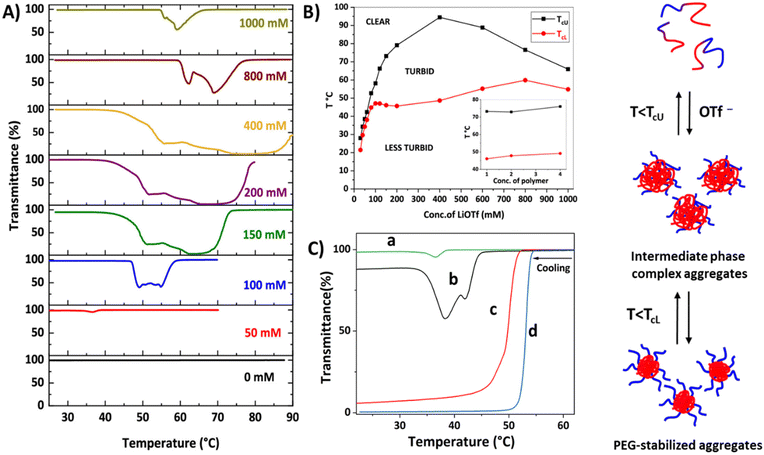 | ||
| Fig. 16 (A) Transmittance cooling curves for P50a solutions (PVBTMAC DP = 62) and (B) TcU and TcL at different LiOTf concentrations (inset, TcU and TcL at different polymer concentrations). (C) Transmittance cooling curves for P50 in aqueous triflate solutions (50 mM LiOTf). PVBTMAC DP (a = 62, b = 81, c = 172 and d = 270). Illustration of PEG-polycation phase separation in aqueous triflate solutions. Reproduced from ref. 141. Copyright 2018 American Chemical Society. | ||
Overall, the length of the polycation block turned out to have a critical effect on the thermal behavior of the polymers, as clearly shown in Fig. 16C. The transmittance curves in this figure were measured in aqueous 50 mM LiOTf. With short cationic blocks, the polymers underwent stepwise phase separation. First, the cationic blocks experienced an UCST-type transition below TcU. Large complex aggregates built up and, therefore, the transmittance dropped. Upon further cooling, PEG-stabilized particles were formed below the clearing temperatures TcL. As the number of cationic units increased, the phase separation of the polymers took place in a single step. 1H NMR spectra suggested that ion-mediated interactions between PEG and cationic blocks led to such complex phase behavior. In the intermediate phase, some of the PEG units were buried in the cationic cores; therefore, no steric stabilization was possible in the high molar mass polymers. The behavior of the long cationic block polymers resembled that of the homopolymers and indicated the formation of unstable aggregates stabilized by electric charges.
Thermoresponsive polycations were also used to construct amphiphilic particles via in situ self-assembly methods. The particles consisted of hydrophobic or solvophobic cores, with the cationic blocks acting as thermoresponsive stabilizer blocks. Zhang et al. prepared diblock copolymer particles (P51) containing VBMIm-BF4 and styrene blocks in methanol/water (85/15 v/v) mixtures.86 The bluish dispersions obtained at 70 °C turned to opalescent gel-like solids upon cooling to room temperature. This is due to the UCST-type behavior of cationic blocks in water/MeOH mixtures. We reported amphiphilic diblock copolymers containing cationic VBTMAC and diacetone acrylamide (DAAM) units. Under dilute conditions, the particles constructed of block copolymers P52 underwent an UCST-type transition in the presence of OTf− ions (Fig. 17A).142 The phase separation was dependent on the ratio of cationic units and OTf ions; the particle phase separation took place in two steps at a certain triflate concentration.
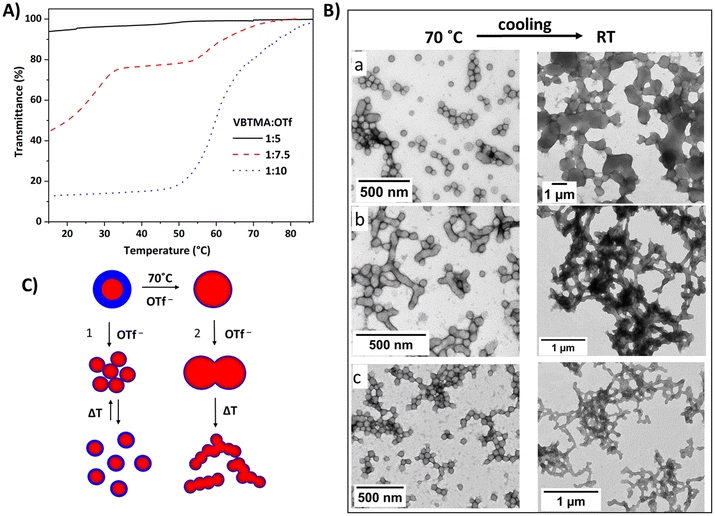 | ||
| Fig. 17 (A) Normalized transmittance cooling curves for spherical nanoparticles of P52 (0.1 w/w%) in aqueous triflate solutions with different cation/anion ratios. Reproduced from ref. 142. Copyright 2021 American Chemical Society. (B) TEM micrographs of P52 particles obtained in aqueous triflate solutions using cationic stabilizers with different block lengths (at 70 °C and RT). DP of PVBTMAC is (a) 24, (b) 50 and (c) 61. Reproduced from ref. 87 Copyright 2022 Royal Society of Chemistry. (C) Schematic view of OTf induced temperature response of particles; case 1: UCST phase separation in dilute conditions; case 2: morphological changes in PISA upon cooling. | ||
Recently, we prepared P52 particles with various cationic block lengths in aqueous triflate solutions (Fig. 17).87 When strong polyelectrolytes are used as particle stabilizers, the morphology of the particles is limited to only spheres. This is due to the strong electrostatic repulsion in the corona, which inhibits fusion of the particles. In the presence of OTf− ions, the diblock copolymers formed fused particles or vesicles due to the strong ionic interactions between the triflate and polycation stabilizers, promoting the high packing efficiency. Particles formed at 70 °C under in situ conditions underwent phase separation and formed gel-like solids upon cooling to room temperature (Fig. 17C). However, a reversible phase transition was not observed upon heating.
Aoshima et al. extensively studied vinyl ether block copolymers that underwent sol–gel transitions upon changing the solution temperature. Recently, they prepared block copolymers P53 consisting of dimethylimidazolium tetrafluoroborate-containing vinyl ether ([Me2Im][BF4]) and isobutyl vinyl ether (IBVE).143 An aqueous solution of this diblock copolymer, P53 (m = 400 units), showed UCST-type thermosensitive physical gelation behavior under dilute conditions. The opaque solutions of the diblock copolymer became viscous gels upon heating to 25 °C (see Fig. 18A). The sol–gel transition is likely due to the tight packing of the micelles at higher temperature and the viscoelastic behavior of the aqueous gels follows typical physical gel behavior. From DLS measurements, larger diameters were recorded above the gelation temperature for P53 aqueous solutions (Fig. 18B). At high temperature, the micelle corona chains are extended because of the electrostatic repulsion and hydration of the [Me2Im][BF4] segments. Therefore, the micelles approach each other with spatial limitation in water, which leads to physical gelation through tight packing of the micelles. Smaller diameters (60 nm at 10 °C) were recorded for the precursor polymer consisting of oxyethylenic moieties, IBVE-b-(2-ethoxyethyl vinyl ether) (IBVE50-EOVE400). An almost horizontal plateau in the storage modulus (G′) over a wide range of frequencies was observed for 1 wt% P53 solution at 55 °C (Fig. 18C). The gelation behavior depends on polymer concentration, counter anions, and the polycation block length (table in Fig. 18). The 0.7 wt% aqueous solution of diblock copolymer P53 (m = 400 units) gelated at 60 °C, while in the 1 wt% solution gelation took place at 25 °C. The 1 wt% solution of copolymer P53 with a long cationic block (m = 800 units) gelates at 0 °C. The gelation concentration of the copolymer with the Cl− counter anion was lower than that with BF4−, while the copolymer with PF6− did not gel at all.
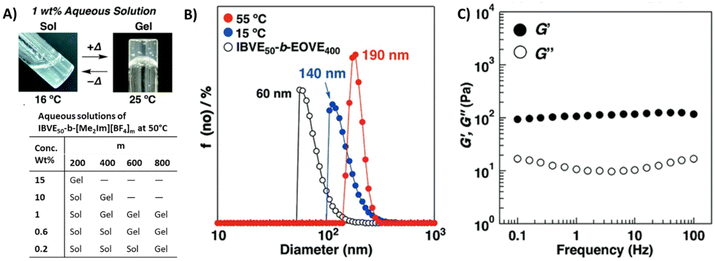 | ||
| Fig. 18 (A) Photographs of thermosensitive physical gelation behavior of P53 (m = 400; sol: 16 °C, gel: 25 °C). (B) DLS size distributions collected for 0.01 wt% aqueous solutions of P53 (m = 400) at 55 °C (red) and 15 °C (blue) and precursor copolymer (IBVE50-EOVE400) of P53 at 10 °C (open symbols). (C) Frequency dependence of dynamic moduli, storage modulus G′ (●) and loss modulus G′′ (○) measured at 55 °C for P53 (m = 400) 1 wt% aqueous solution. Reproduced from ref. 143. Copyright 2018 Royal Society of Chemistry. | ||
Later, solution properties and the sol–gel transition of the block copolymers were investigated by changing the chemical composition of the hydrophobic block and of the alkyl substituents on cationic units, as well as the counterions.144 Gelation was observed only for polymers with Cl− and BF4−, whereas with hydrophobic counterions like SbF6−, PF6−, or NTf2− the polymers were insoluble in water. The solubility and gelation behavior of the amphiphilic diblock copolymers were dependent on the structures of the imidazolium ionic segments. The gelation temperature increased with increasing the lengths of the alkyl substituents. The sol–gel behavior of the diblock copolymers was tuned by the alkyl side chains in the hydrophobic blocks. The block copolymers with hydrophobic segments with octadecyl vinyl ether (P54) or 2-phenoxyethyl vinyl ether (P55) underwent UCST-type thermosensitive sol–gel transitions upon heating, similar to the copolymers with IBVE segments.
5. Summary and future prospects
The review discusses progress made on thermoresponsive polymers, with a special focus on polycations. Solution properties of polycations have been studied for years. However, the importance of counterions for creating stimuli-responsive polymers in aqueous or non-aqueous systems has been recently realized. We briefly discussed thermoresponsive polycations and cationic random and block copolymers, as well as polymer particles.When designing cationic LCST and UCST polymers, one needs to consider (1) the balance between electrostatic and hydrophobic interactions (LCST), and (2) ionic interactions between the polymer chains and counter ions plus the hydrophobicity of the polymer or counter ion (UCST).
Polycations, which are well soluble in water, undergo phase separation at a critical temperature (TcL) upon heating when the alkyl substituents of the charged units are sufficiently hydrophobic. Alternatively, by using hydrophobic counterions, the phase separation process of water-soluble polycations can be reversed. In the presence of hydrophobic counterions, polycations either become totally water insoluble or undergo UCST-type phase transitions. The phase behavior of the polymers can be tuned from single to double phase transitions by changing the alkyl substituents or copolymerizing with nonionic monomers. Compared to nonionic systems, polyelectrolytes that show LCST-type phase transitions are less documented in the literature. However, several cationic copolymers with NIPAM or other thermoresponsive repeating units undergo LCST or UCST-type phase transitions. A few cationic copolymers show both LCST and UCST type transitions.
Besides their interesting tunable solution properties, thermoresponsive polycations may find interesting applications such as in hydrogels taking up/releasing water and in waste water treatment by a forward osmosis process.145,146 In addition, applying the counterion strategies to polyelectrolyte brushes, films, or cationic biopolymers would be of fundamental interest in creating smart polymer systems. To generalize the conclusions drawn so far, it would be beneficial to take a closer look at polyanions. Several biopolymers (such as many polysaccharides and DNA) are anionic and it would certainly be beneficial to better understand the solution properties of such systems.
Author contributions
VB collected the data and wrote the manuscript together with HT.Conflicts of interest
There are no conflicts to declare.Acknowledgements
VB thanks the Magnus Ehrnrooth Foundation for a research grant.References
- A. Katchalsky, Polyelectrolytes, Pure Appl. Chem., 1971, 26(3–4), 327–374, DOI:10.1351/PAC197126030327.
- A. V. Dobrynin, Solutions of Charged Polymers, Polym. Sci., Ser. A, 2012, 1, 81–132, DOI:10.1016/B978-0-444-53349-4.00005-4.
- A. V. Dobrynin, R. H. Colby and M. Rubinstein, Scaling Theory of Polyelectrolyte Solutions, Macromolecules, 1995, 28(6), 1859–1871, DOI:10.1021/MA00110A021.
- A. V. Dobrynin, Polyelectrolytes: On the Doorsteps of the Second Century, Polymer, 2020, 202, 122714, DOI:10.1016/J.POLYMER.2020.122714.
- G. S. Manning and J. Ray, Counterion Condensation Revisited, J. Biomol. Struct. Dyn., 1998, 16(2), 461–476, DOI:10.1080/07391102.1998.10508261.
- M. Muthukumar, 50th Anniversary Perspective: A Perspective on Polyelectrolyte Solutions, Macromolecules, 2017, 50(24), 9528–9560, DOI:10.1021/ACS.MACROMOL.7B01929.
- K. Kaji, H. Urakawa, T. Kanaya and R. Kitamaru, Phase Diagram of Polyelectrolyte Solutions, J. Phys., 1988, 49(6), 993–1000, DOI:10.1051/jphys:01988004906099300.
- P. Lo Nostro and B. W. Ninham, Hofmeister Phenomena: An Update on Ion Specificity in Biology, Chem. Rev., 2012, 2286–2322, DOI:10.1021/cr200271j.
- K. P. Gregory, G. R. Elliott, H. Robertson, A. Kumar, E. J. Wanless, G. B. Webber, V. S. J. Craig, G. G. Andersson and A. J. Page, Understanding Specific Ion Effects and the Hofmeister Series, Phys. Chem. Chem. Phys., 2022, 24(21), 12682–12718, 10.1039/D2CP00847E.
- G. Liu, D. Parsons and V. S. J. Craig, Re-Entrant Swelling and Redissolution of Polyelectrolytes Arises from an Increased Electrostatic Decay Length at High Salt Concentrations, J. Colloid Interface Sci., 2020, 579, 369–378, DOI:10.1016/J.JCIS.2020.06.072.
- R. Kou, J. Zhang, T. Wang and G. Liu, Interactions between Polyelectrolyte Brushes and Hofmeister Ions: Chaotropes versus Kosmotropes, Langmuir, 2015, 31(38), 10461–10468, DOI:10.1021/acs.langmuir.5b02698.
- W. Kunz, J. Henle and B. W. Ninham, ‘Zur Lehre von Der Wirkung Der Salze’ (about the Science of the Effect of Salts): Franz Hofmeister's Historical Papers, Curr. Opin. Colloid Interface Sci., 2004, 9(1–2), 19–37, DOI:10.1016/J.COCIS.2004.05.005.
- A. Kundagrami and M. Muthukumar, Theory of Competitive Counterion Adsorption on Flexible Polyelectrolytes: Divalent Salts, J. Chem. Phys., 2008, 128, 244901, DOI:10.1063/1.2940199.
- G. A. Sorci and W. F. Reed, Effect of Valence and Chemical Species of Added Electrolyte on Polyelectrolyte Conformations and Interactions, Macromolecules, 2004, 37(2), 554–565, DOI:10.1021/MA035551Z.
- W. F. Reed, S. Ghosh, G. Medjahdi and J. François, Dependence of Polyelectrolyte Apparent Persistence Lengths, Viscosity, and Diffusion on Ionic Strength and Linear Charge Density, Macromolecules, 1991, 24(23), 6189–6198, DOI:10.1021/MA00023A021.
- P. Loh, G. Roshan Deen, D. Vollmer, K. Fischer, M. Schmidt, A. Kundagrami and M. Muthukumar, Collapse of Linear Polyelectrolyte Chains in a Poor Solvent: When Does a Collapsing Polyelectrolyte Collect Its Counterions?, Macromolecules, 2008, 41(23), 9352–9358, DOI:10.1021/MA8014239.
- S. Dou and R. H. Colby, Solution Rheology of a Strongly Charged Polyelectrolyte in Good Solvent, Macromolecules, 2008, 41(17), 6505–6510, DOI:10.1021/MA8001438.
- T. Ishikawa, M. Kikuchi, M. Kobayashi, N. Ohta and A. Takahara, Chain Conformation of Poly[2-(Methacryloyloxy)Ethyltrimethylammonium Chloride] in Aqueous Sodium Chloride Solutions, Macromolecules, 2013, 46(10), 4081–4088, DOI:10.1021/ma4001868.
- H. Eisenberg and G. R. Mohan, Aqueous Solutions of Polyvinylsulfonic Acid: Phase Separation and Specific Interaction with Ions, Viscosity, Conductance and Potentiometry, J. Phys. Chem., 1959, 63(5), 671–680, DOI:10.1021/j150575a008.
- S. Förster, M. Schmidt and M. Antonietti, Experimental and Theoretical Investigation of the Electrostatic Persistence Length of Flexible Polyelectrolytes at Various Ionic Strengths, J. Phys. Chem., 1992, 96(10), 4008–4014, DOI:10.1021/J100189A019.
- E. Fouissac, M. Milas, M. Rinaudo and R. Borsali, Influence of the Ionic Strength on the Dimensions of Sodium Hyaluronate, Macromolecules, 1992, 25(21), 5613–5617, DOI:10.1021/MA00047A009.
- M. Beer, M. Schmidt and M. Muthukumar, The Electrostatic Expansion of Linear Polyelectrolytes: Effects of Gegenions, Co-Ions, and Hydrophobicity, Macromolecules, 1997, 30(26), 8375–8385, DOI:10.1021/MA9709821.
- I. Bibi and M. Siddiq, Conformational Transition of Poly (Vinylbenzyltrimethylammonium Chloride) (PVBTMAC) Brush in the Presence of Hofmeister Anions, J. Polym. Res., 2012, 19(9), 1–5, DOI:10.1007/S10965-012-9961-Y/SCHEMES/1.
- T. Itaya, K. Ueda, H. Ochiai and A. Imamura, Binding of Hydrophobic Counterions by Polyelectrolyte and Their Hydrophobic Association around Polyion, Polym. J., 1993, 25(6), 545–552, DOI:10.1295/polymj.25.545.
- O. E. Zimerman, J. J. Cosa and C. M. Previtali, Binding of Ionic Pyrene Derivatives to Polyelectrolytes. A Uv Absorption and Fluorescence Study, J. Macromol. Sci., Part A: Pure Appl.Chem., 1994, 31(7), 859–872, DOI:10.1080/10601329409349763.
- J. Texter, Anion Responsive Imidazolium-Based Polymers, Macromol. Rapid Commun., 2012, 33(23), 1996–2014, DOI:10.1002/MARC.201200525.
- K. Vijayakrishna, S. K. Jewrajka, A. Ruiz, R. Marcilla, J. a. Pomposo, D. Mecerreyes, D. Taton and Y. Gnanou, Synthesis by RAFT and Ionic Responsiveness of Double Hydrophilic Block Copolymers Based on Ionic Liquid Monomer Units, Macromolecules, 2008, 41(17), 6299–6308, DOI:10.1021/ma800677h.
- E. Karjalainen, V. Aseyev and H. Tenhu, Influence of Hydrophobic Anion on Solution Properties of PDMAEMA, Macromolecules, 2014, 47(6), 2103–2111, DOI:10.1021/ma5000706.
- I. Dimitrov, B. Trzebicka, A. H. E. Müller, A. Dworak and C. B. Tsvetanov, Thermosensitive Water-Soluble Copolymers with Doubly Responsive Reversibly Interacting Entities, Prog. Polym. Sci., 2007, 32(11), 1275–1343, DOI:10.1016/J.PROGPOLYMSCI.2007.07.001.
- D. N. Schulz, D. G. Peiffer, P. K. Agarwal, J. Larabee, J. J. Kaladas, L. Soni, B. Handwerker and R. T. Garner, Phase Behaviour and Solution Properties of Sulphobetaine Polymers, Polymer, 1986, 27(11), 1734–1742, DOI:10.1016/0032-3861(86)90269-7.
- J. Seuring and S. Agarwal, Polymers with Upper Critical Solution Temperature in Aqueous Solution, Macromol. Rapid Commun., 2012, 33(22), 1898–1920, DOI:10.1002/marc.201200433.
- H. Uyama and S. Kobayashi, A Novel Thermo-Sensitive Polymer. Poly(2- Iso -Propyl-2-Oxazoline), Chem. Lett., 1992, 21(9), 1643–1646, DOI:10.1246/CL.1992.1643.
- A. Halperin, M. Kröger and F. M. Winnik, Poly(N-Isopropylacrylamide) Phase Diagrams: Fifty Years of Research, Angew. Chem., Int. Ed., 2015, 54(51), 15342–15367, DOI:10.1002/anie.201506663.
- V. Aseyev, H. Tenhu and F. M. Winnik, Non-Ionic Thermoresponsive Polymers in Water, 2010, pp. 29–89. DOI:10.1007/12_2010_57v.
- H. G. Schild and D. A. Tirrell, Microcalorimetric Detection of Lower Critical Solution Temperatures in Aqueous Polymer Solutions, J. Phys. Chem., 1990, 94(10), 4352–4356, DOI:10.1021/J100373A088.
- J. Niskanen and H. Tenhu, How to Manipulate the Upper Critical Solution Temperature (UCST)?, Polym. Chem., 2017, 8(1), 220–232, 10.1039/C6PY01612J.
- M. D. Hossain, C. F. Grandes Reyes, C. Zhang, S. P. R. Chen and M. J. Monteiro, Nonionic Polymer with Flat Upper Critical Solution Temperature Behavior in Water, Biomacromolecules, 2022, 23(1), 174–181, DOI:10.1021/ACS.BIOMAC.1C01198.
- C. Zhao, L. Dolmans and X. X. Zhu, Thermoresponsive Behavior of Poly(Acrylic Acid- Co-Acrylonitrile) with a UCST, Macromolecules, 2019, 52(12), 4441–4446, DOI:10.1021/acs.macromol.9b00794.
- J. Seuring and S. Agarwal, First Example of a Universal and Cost-Effective Approach: Polymers with Tunable Upper Critical Solution Temperature in Water and Electrolyte Solution, Macromolecules, 2012, 45(9), 3910–3918, DOI:10.1021/MA300355K.
- C. Zhao, Z. Ma and X. X. Zhu, Rational Design of Thermoresponsive Polymers in Aqueous Solutions: A Thermodynamics Map, Prog. Polym. Sci., 2019, 90, 269–291, DOI:10.1016/J.PROGPOLYMSCI.2019.01.001.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Thermoresponsive Polyelectrolytes Derived from Ionic Liquids, Polym. Chem., 2015, 6(12), 2163–2178, 10.1039/c4py01665c.
- P. Banerjee, M. Anas, S. Jana and T. K. Mandal, Recent Developments in Stimuli-Responsive Poly(Ionic Liquid)S, J. Polym. Res., 2020, 27(7), 1–23, DOI:10.1007/S10965-020-02091-8.
- Y. Men, H. Schlaad and J. Yuan, Cationic Poly(Ionic Liquid) with Tunable Lower Critical Solution Temperature-Type Phase Transition, ACS Macro Lett., 2013, 2(5), 456–459, DOI:10.1021/MZ400155R.
- E. M. Lewoczko, N. Wang, C. E. Lundberg, M. T. Kelly, E. W. Kent, T. Wu, M. L. Chen, J. H. Wang and B. Zhao, Effects of N-Substituents on the Solution Behavior of Poly(Sulfobetaine Methacrylate)s in Water: Upper and Lower Critical Solution Temperature Transitions, ACS Appl. Polym. Mater., 2021, 3(2), 867–878, DOI:10.1021/ACSAPM.0C01191.
- C. Ge, S. Liu, C. Liang, Y. Ling and H. Tang, Synthesis and UCST-Type Phase Behavior of α-Helical Polypeptides with Y-Shaped and Imidazolium Pendants, Polym. Chem., 2016, 7(38), 5978–5987, 10.1039/C6PY01287F.
- E. Karjalainen, V. Aseyev and H. Tenhu, Counterion-Induced Ucst for Polycations, Macromolecules, 2014, 47(21), 7581–7587, DOI:10.1021/MA501924R.
- A. Laukkanen, L. Valtola, F. M. Winnik and H. Tenhu, Formation of Colloidally Stable Phase Separated Poly(N-Vinylcaprolactam) in Water: A Study by Dynamic Light Scattering, Microcalorimetry, and Pressure Perturbation Calorimetry, Macromolecules, 2004, 37(6), 2268–2274, DOI:10.1021/MA035124L.
- C. Weber, R. Hoogenboom and U. S. Schubert, Temperature Responsive Bio-Compatible Polymers Based on Poly(Ethylene Oxide) and Poly(2-Oxazoline)S, Prog. Polym. Sci., 2012, 37(5), 686–714, DOI:10.1016/J.PROGPOLYMSCI.2011.10.002.
- R. Hoogenboom and H. Schlaad, Thermoresponsive Poly(2-Oxazoline)s, Polypeptoids, and Polypeptides, Polym. Chem., 2016, 8(1), 24–40, 10.1039/C6PY01320A.
- J. Seuring and S. Agarwal, Polymers with Upper Critical Solution Temperature in Aqueous Solution, Macromol. Rapid Commun., 2012, 33(22), 1898–1920, DOI:10.1002/MARC.201200433.
- X. Fu, C. Xing and J. Sun, Tunable LCST/UCST-Type Polypeptoids and Their Structure-Property Relationship, Biomacromolecules, 2020, 21(12), 4980–4988, DOI:10.1021/ACS.BIOMAC.0C01177.
- G. Zhang, Y. Wang and G. Liu, Poly(3-Imidazolyl-2-Hydroxypropyl Methacrylate) – a New Polymer with a Tunable Upper Critical Solution Temperature in Water, Polym. Chem., 2016, 7(43), 6645–6654, 10.1039/C6PY01535B.
- A. Aliakseyeu, R. Hlushko and S. A. Sukhishvili, Nonionic Star Polymers with Upper Critical Solution Temperature in Aqueous Solutions, Polym. Chem., 2022, 13(18), 2637–2650, 10.1039/D2PY00216G.
- T. Aoki, K. Nakamura, K. Sanui, A. Kikuchi, T. Okano, Y. Sakurai and N. Ogata, Adenosine-Induced Changes of the Phase Transition of Poly(6-(Acryloyloxymethyl)Uracil) Aqueous Solution, Polym. J., 1999, 31(11), 1185–1188, DOI:10.1295/polymj.31.1185.
- S. Glatzel, A. Laschewsky and J. F. Lutz, Well-Defined Uncharged Polymers with a Sharp UCST in Water and in Physiological Milieu, Macromolecules, 2011, 44(2), 413–415, DOI:10.1021/MA102677K.
- V. Hildebrand, A. Laschewsky and E. Wischerhoff, Modulating the Solubility of Zwitterionic Poly((3-Methacrylamidopropyl)Ammonioalkane Sulfonate)s in Water and Aqueous Salt Solutions via the Spacer Group Separating the Cationic and the Anionic Moieties, Polym. Chem., 2016, 7(3), 731–740, 10.1039/C5PY01642H.
- J. Niskanen, J. Vapaavuori, C. Pellerin, F. M. Winnik and H. Tenhu, Polysulfobetaine-Surfactant Solutions and Their Use in Stabilizing Hydrophobic Compounds in Saline Solution, Polymer, 2017, 127, 77–87, DOI:10.1016/J.POLYMER.2017.08.057.
- F. Wang, J. Yang and J. Zhao, Understanding Anti-Polyelectrolyte Behavior of a Well-Defined Polyzwitterion at the Single-Chain Level, Polym. Int., 2015, 64(8), 999–1005, DOI:10.1002/PI.4907.
- K. K. Sharker, Y. Shigeta, S. Ozoe, P. Damsongsang, V. P. Hoven and S. I. Yusa, Upper Critical Solution Temperature Behavior of PH-Responsive Amphoteric Statistical Copolymers in Aqueous Solutions, ACS Omega, 2021, 6(13), 9153–9163, DOI:10.1021/ACSOMEGA.1C00351.
- Y. Kawata, S. Kozuka and S. I. Yusa, Thermo-Responsive Behavior of Amphoteric Diblock Copolymers Bearing Sulfonate and Quaternary Amino Pendant Groups, Langmuir, 2019, 35(5), 1458–1464, DOI:10.1021/acs.langmuir.8b01684.
- S. Maji, V. V. Jerca and R. Hoogenboom, Dual PH and Thermoresponsive Alternating Polyampholytes in Alcohol/Water Solvent Mixtures, Polym. Chem., 2020, 11(12), 2205–2211, 10.1039/d0py00032a.
- S. Jana, M. Anas, T. Maji, S. Banerjee and T. K. Mandal, Tryptophan-Based Styryl Homopolymer and Polyzwitterions with Solvent-Induced UCST, Ion-Induced LCST and PH-Induced UCST, Polym. Chem., 2019, 10(4), 526–538, 10.1039/C8PY01512K.
- V. Hildebrand, A. Laschewsky, M. Päch, P. Müller-Buschbaum and C. M. Papadakis, Effect of the Zwitterion Structure on the Thermo-Responsive Behaviour of Poly(Sulfobetaine Methacrylates), Polym. Chem., 2016, 8(1), 310–322, 10.1039/C6PY01220E.
- N. Wang, B. T. Seymour, E. M. Lewoczko, E. W. Kent, M. L. Chen, J. H. Wang and B. Zhao, Zwitterionic Poly(Sulfobetaine Methacrylate)s in Water: From Upper Critical Solution Temperature (UCST) to Lower Critical Solution Temperature (LCST) with Increasing Length of One Alkyl Substituent on the Nitrogen Atom, Polym. Chem., 2018, 9(43), 5257–5261, 10.1039/C8PY01211C.
- Y. Kohno, H. Ohno, Y. Kohno and H. Ohno, Key Factors to Prepare Polyelectrolytes Showing Temperature-Sensitive Lower Critical Solution Temperature-Type Phase Transitions in Water, Aust. J. Chem., 2011, 65(1), 91–94, DOI:10.1071/CH11378.
- Y. Deguchi, Y. Kohno, H. Ohno, Y. Deguchi, Y. Kohno and H. Ohno, Design of Ionic Liquid-Derived Polyelectrolyte Gels Toward Reversible Water Absorption/Desorption System Driven by Small Temperature Change, Aust. J. Chem., 2014, 67(11), 1666–1670, DOI:10.1071/CH14038.
- Y. Men, X. H. Li, M. Antonietti and J. Yuan, Poly(Tetrabutylphosphonium 4-Styrenesulfonate): A Poly(Ionic Liquid) Stabilizer for Graphene Being Multi-Responsive, Polym. Chem., 2012, 3(4), 871–873, 10.1039/C2PY20011B.
- Y. Deguchi, Y. Kohno and H. Ohno, A Fine Tuning of LCST-Type Phase Transition of Poly(Ionic Liquid)s in Water, Chem. Lett., 2015, 44(3), 238–240, DOI:10.1246/CL.141016.
- G. Wang and P. Wu, In-Depth Study of the Phase Separation Behaviour of a Thermoresponsive Ionic Liquid and a Poly(Ionic Liquid) in Concentrated Aqueous Solution, Soft Matter, 2015, 11(26), 5253–5264, 10.1039/C5SM00603A.
- W. Li and P. Wu, Unusual Thermal Phase Transition Behavior of an Ionic Liquid and Poly(Ionic Liquid) in Water with Significantly Different LCST and Dynamic Mechanism, Polym. Chem., 2014, 5(19), 5578–5590, 10.1039/C4PY00593G.
- Y. Men, H. Schlaad, A. Voelkel and J. Yuan, Thermoresponsive Polymerized Gemini Dicationic Ionic Liquid, Polym. Chem., 2014, 5(11), 3719–3724, 10.1039/c3py01790g.
- Y. Zhang, H. Tang and P. Wu, Insights into the Thermal Phase Transition Behavior of a Gemini Dicationic Polyelectrolyte in Aqueous Solution, Soft Matter, 2018, 14(21), 4380–4387, 10.1039/C8SM00598B.
- B. Ziółkowski and D. Diamond, Thermoresponsive Poly(Ionic Liquid) Hydrogels, Chem. Commun., 2013, 49(87), 10308–10310, 10.1039/C3CC45862H.
- G. Beaudoin, A. Lasri, C. Zhao, B. Liberelle, G. De Crescenzo and X. X. Zhu, Making Hydrophilic Polymers Thermoresponsive: The Upper Critical Solution Temperature of Copolymers of Acrylamide and Acrylic Acid, Macromolecules, 2021, 54(17), 7963–7969, DOI:10.1021/ACS.MACROMOL.1C00952.
- J. Emoto, Y. Kitayama and A. Harada, Thermoresponsiveness of Carboxylated Polyallylamines Induced by Divalent Counterions as Ionic Effectors, Macromolecules, 2022, 55(16), 7204–7211, DOI:10.1021/ACS.MACROMOL.2C00795.
- J. Gao, C. Wang, D. W. Han and D. M. Shin, Single-Ion Conducting Polymer Electrolytes as a Key Jigsaw Piece for next-Generation Battery Applications, Chem. Sci., 2021, 12(40), 13248–13272, 10.1039/D1SC04023E.
- V. O. Aseyev, H. Tenhu and S. I. Klenin, Collapse of Poly(Methacryloylethyl Trimethylammonium Methylsulfate) on Addition of Acetone into an Aqueous Solution, Polymer, 1999, 40(5), 1173–1180, DOI:10.1016/S0032-3861(98)00270-5.
- V. O. Aseyev, H. Tenhu and S. I. Klenin, Contraction of a Polyelectrolyte upon Dilution. Light-Scattering Studies on a Polycation in Saltless Water−Acetone Mixtures, Macromolecules, 1998, 31(22), 7717–7722, DOI:10.1021/MA980552Q.
- F. A. Plamper, A. Schmalz, M. Ballauff and A. H. E. Müller, Tuning the Thermoresponsiveness of Weak Polyelectrolytes by PH and Light: Lower and Upper Critical-Solution Temperature of Poly(N,N-Dimethylaminoethyl Methacrylate), J. Am. Chem. Soc., 2007, 129(47), 14538–14539, DOI:10.1021/JA074720I.
- Q. Zhang, F. Tosi, S. Üʇdüler, S. Maji and R. Hoogenboom, Tuning the LCST and UCST Thermoresponsive Behavior of Poly(N,N-Dimethylaminoethyl Methacrylate) by Electrostatic Interactions with Trivalent Metal Hexacyano Anions and Copolymerization, Macromol. Rapid Commun., 2015, 36(7), 633–639, DOI:10.1002/MARC.201400550.
- E. Karjalainen, V. Aseyev and H. Tenhu, Influence of Hydrophobic Anion on Solution Properties of PDMAEMA, Macromolecules, 2014, 47(6), 2103–2111, DOI:10.1021/MA5000706.
- B. Pang, Y. Yu and W. Zhang, Thermoresponsive Polymers Based on Tertiary Amine Moieties, Macromol. Rapid Commun., 2021, 42, 2100504, DOI:10.1002/MARC.202100504.
- M. Noh, S. Kang, Y. Mok, S. J. Choi, J. Park, J. Kingma, J. H. Seo and Y. Lee, Upper Critical Solution Temperature (UCST) Phase Transition of Halide Salts of Branched Polyethylenimine and Methylated Branched Polyethylenimine in Aqueous Solutions, Chem. Commun., 2015, 52(3), 509–512, 10.1039/C5CC08005C.
- X. Cao, Z. An, X. Cao and Z. An, RAFT Synthesis in Water of Cationic Polyelectrolytes with Tunable UCST, Macromol. Rapid Commun., 2015, 36(23), 2107–2110, DOI:10.1002/MARC.201500440.
- E. Karjalainen, V. Aseyev and H. Tenhu, Counterion-Induced UCST for Polycations, Macromolecules, 2014, 47(21), 7581–7587, DOI:10.1021/ma501924r.
- C. Liu, S. Wang, H. Zhou, C. Gao and W. Zhang, Thermoresponsive Poly(Ionic Liquid): Controllable RAFT Synthesis, Thermoresponse, and Application in Dispersion RAFT Polymerization, J. Polym. Sci., Part A: Polym. Chem., 2016, 54(7), 945–954, DOI:10.1002/pola.27929.
- V. Baddam, L. Välinen, L. Kuckling and H. Tenhu, Morphological Transitions of Cationic PISA Particles by Salt, Triflate Ions and Temperature; Comparison of Three Polycations, Polym. Chem., 2022, 13(25), 3790–3799, 10.1039/d2py00301e.
- E. Karjalainen, N. Suvarli and H. Tenhu, Thermoresponsive Behavior of Poly[Trialkyl-(4-Vinylbenzyl)Ammonium] Based Polyelectrolytes in Aqueous Salt Solutions, Polym. Chem., 2020, 11(36), 5870–5883, 10.1039/d0py00917b.
- J. Huang, X. Chen, H. Qin, H. Liang and J. Lu, A New Thermoresponsive Polymer with Reactive Aldehyde Groups for Postmodification to Tune the Solubility and Phase Transition Temperature, Polymer, 2019, 160, 99–106, DOI:10.1016/J.POLYMER.2018.11.044.
- Y. Biswas, T. Maji, M. Dule and T. K. Mandal, Tunable Doubly Responsive UCST-Type Phosphonium Poly(Ionic Liquid): A Thermosensitive Dispersant for Carbon Nanotubes, Polym. Chem., 2016, 7(4), 867–877, 10.1039/C5PY01574J.
- Y. Biswas, T. Maji, M. Dule and T. K. Mandal, Tunable Doubly Responsive UCST-Type Phosphonium Poly(Ionic Liquid): A Thermosensitive Dispersant for Carbon Nanotubes, Polym. Chem., 2016, 7(4), 867–877, 10.1039/c5py01574j.
- Y. Biswas, M. Dule and T. K. Mandal, Poly(Ionic Liquid)-Promoted Solvent-Borne Efficient Exfoliation of MoS2/MoSe2 Nanosheets for Dual-Responsive Dispersion and Polymer Nanocomposites, J. Phys. Chem. C, 2017, 121(8), 4747–4759, DOI:10.1021/ACS.JPCC.7B00952.
- H. Yoshimitsu, A. Kanazawa, S. Kanaoka and S. Aoshima, Well-Defined Polymeric Ionic Liquids with an Upper Critical Solution Temperature in Water, Macromolecules, 2012, 45(23), 9427–9434, DOI:10.1021/ma301746u.
- K. I. Seno, S. Kanaoka and S. Aoshima, Synthesis and LCST-Type Phase Separation Behavior in Organic Solvents of Poly(Vinyl Ethers) with Pendant Imidazolium or Pyridinium Salts, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(17), 5724–5733, DOI:10.1002/POLA.22881.
- S. Amajjahe and H. Ritter, Supramolecular Controlled Pseudo-LCST Effects of Cyclodextrin-Complexed Poly(Ionic Liquids), Macromolecules, 2008, 41(9), 3250–3253, DOI:10.1021/MA702593S.
- A. Okafuji, Y. Kohno, H. Ohno, A. Okafuji, Y. Kohno and H. Ohno, Thermoresponsive Poly(Ionic Liquid)s in Aqueous Salt Solutions: Salting-Out Effect on Their Phase Behavior and Water Absorption/Desorption Properties, Macromol. Rapid Commun., 2016, 37(14), 1130–1134, DOI:10.1002/MARC.201500752.
- C. Ju, C. Park, T. Kim, S. Kang and H. Kang, Thermo-Responsive Draw Solute for Forward Osmosis Process; Poly(Ionic Liquid) Having Lower Critical Solution Temperature Characteristics, RSC Adv., 2019, 9(51), 29493–29501, 10.1039/C9RA04020J.
- Y. Kohno, Y. Deguchi, N. Inoue and H. Ohno, Temperature-Driven and Reversible Assembly of Homopolyelectrolytes Derived from Suitably Designed Ionic Liquids in Water, Aust. J. Chem., 2013, 66(11), 1393, DOI:10.1071/CH13301.
- C. Ju, C. Park, T. Kim, S. Kang and H. Kang, Thermo-Responsive Draw Solute for Forward Osmosis Process; Poly(Ionic Liquid) Having Lower Critical Solution Temperature Characteristics, RSC Adv., 2019, 9(51), 29493–29501, 10.1039/C9RA04020J.
- K. Grygiel, W. Zhang, C. Detrembleur and J. Yuan, Unexpected LCST-Type Phase Behaviour of a Poly(Vinyl Thiazolium) Polymer in Acetone, RSC Adv., 2016, 6(62), 57117–57121, 10.1039/C6RA09023K.
- T. J. Deming, Synthetic Polypeptides for Biomedical Applications, Prog. Polym. Sci., 2007, 32(8–9), 858–875, DOI:10.1016/J.PROGPOLYMSCI.2007.05.010.
- Y. Deng, Y. Xu, X. Wang, Q. Yuan, Y. Ling, H. Tang, Y. Deng, Y. Xu, X. Wang, Q. Yuan, Y. Ling and H. Tang, Water-Soluble Thermoresponsive α-Helical Polypeptide with an Upper Critical Solution Temperature: Synthesis, Characterization, and Thermoresponsive Phase Transition Behaviors, Macromol. Rapid Commun., 2015, 36(5), 453–458, DOI:10.1002/MARC.201400625.
- J. Xiao, M. Li, W. Liu, Y. Li, Y. Ling and H. Tang, Synthesis and Thermoresponsive Properties of Poly(l-Cysteine)s Bearing Imidazolium Salts, Eur. Polym. J., 2017, 88, 340–348, DOI:10.1016/J.EURPOLYMJ.2017.01.036.
- C. Liang, X. Wang, R. Zhou, H. Shi, S. Yan, Y. Ling, S. Luan and H. Tang, Thermo- and Oxidation-Responsive Homopolypeptide: Synthesis, Stimuli-Responsive Property and Antimicrobial Activity, Polym. Chem., 2019, 10(17), 2190–2202, 10.1039/C8PY01735B.
- Y. Wu, X. Wang, Y. Ling and H. Tang, Preparation and Thermoresponsive Properties of Helical Polypeptides Bearing Pyridinium Salts, RSC Adv., 2015, 5(51), 40772–40778, 10.1039/C5RA04541J.
- S. Liu, C. Ge, Y. Ling and H. Tang, Preparation and UCST-Type Phase Behaviours of Poly(γ-4-Methylbenzyl-l-Glutamate) Pyridinium Tetrafluoroborate Conjugates in Methanol or Water, Aust. J. Chem., 2016, 70(3), 245–251, DOI:10.1071/CH16344.
- C. Ge, L. Zhao, Y. Ling and H. Tang, Thermo and PH Dual Responsive Polypeptides Derived from “Clickable” Poly(γ-3-Methylthiopropyl-L-Glutamate), Polym. Chem., 2017, 8(12), 1895–1905, 10.1039/C7PY00170C.
- M. Anas, P. Dinda, M. Kar and T. K. Mandal, Anion-Induced Thermoresponsiveness in Cationic Polycysteine and DNA Binding, Polym. Chem., 2021, 12(43), 6329–6343, 10.1039/D1PY01187A.
- S. Jana, Y. Biswas and T. K. Mandal, Methionine-Based Cationic Polypeptide/Polypeptide Block Copolymer with Triple-Stimuli Responsiveness: DNA Polyplexation and Phototriggered Release, Polym. Chem., 2018, 9(14), 1869–1884, 10.1039/C8PY00178B.
- M. Li, Y. Xu, T. Liu, Y. Li, Y. Ling, H. Tang, M. Li, Y. Xu, T. Liu, Y. Li, Y. Ling and H. Tang, Preparation and Thermoresponsive Properties of UCST-Type Polypeptide Bearing p-Tolyl Pendants and 3-Methyl-1,2,3-Triazolium Linkages in Methanol or Ethanol/Water Solvent Mixtures, Macromol. Chem. Phys., 2017, 218(10), 1700006, DOI:10.1002/MACP.201700006.
- Y. Xu, M. Zhu, M. Li, Y. Ling and H. Tang, Synthesis and LCST-Type Phase Behavior of Water-Soluble Polypeptide with Y-Shaped and Charged Side-Chains, Polym. Chem., 2016, 7(10), 1922–1930, 10.1039/C5PY01991E.
- M. Li, X. He, Y. Ling and H. Tang, Dual Thermoresponsive Homopolypeptide with LCST-Type Linkages and UCST-Type Pendants: Synthesis, Characterization, and Thermoresponsive Properties, Polymer, 2017, 132, 264–272, DOI:10.1016/J.POLYMER.2017.11.016.
- M. Li, X. He, Y. Ling and H. Tang, Dual Thermoresponsive Homopolypeptide with LCST-Type Linkages and UCST-Type Pendants: Synthesis, Characterization, and Thermoresponsive Properties, Polymer, 2017, 132, 264–272, DOI:10.1016/J.POLYMER.2017.11.016.
- J. V. M. Weaver, I. Bannister, K. L. Robinson, X. Bories-Azeau, S. P. Armes, M. Smallridge and P. McKenna, Stimulus-Responsive Water-Soluble Polymers Based on 2-Hydroxyethyl Methacrylate, Macromolecules, 2004, 37(7), 2395–2403, DOI:10.1021/MA0356358.
- R. Longenecker, T. Mu, M. Hanna, N. A. D. Burke and H. D. H. Stöver, Thermally Responsive 2-Hydroxyethyl Methacrylate Polymers: Soluble-Insoluble and Soluble-Insoluble-Soluble Transitions, Macromolecules, 2011, 44(22), 8962–8971, DOI:10.1021/ma201528r.
- P. Banerjee, S. Jana and T. K. Mandal, Coulomb Interaction-Driven UCST in Poly(Ionic Liquid) Random Copolymers, Eur. Polym. J., 2020, 133, 109747, DOI:10.1016/J.EURPOLYMJ.2020.109747v.
- S. Jana, Y. Biswas, M. Anas, A. Saha and T. K. Mandal, Poly[Oligo(2-Ethyl-2-Oxazoline)Acrylate]-Based Poly(Ionic Liquid) Random Copolymers with Coexistent and Tunable Lower Critical Solution Temperature- and Upper Critical Solution Temperature-Type Phase Transitions, Langmuir, 2018, 34(42), 12653–12663, DOI:10.1021/acs.langmuir.8b03022.
- Y. Zhang, H. Tang and P. Wu, Multiple Interaction Regulated Phase Transition Behavior of Thermo-Responsive Copolymers Containing Cationic Poly(Ionic Liquid)S, Phys. Chem. Chem. Phys., 2017, 19(45), 30804–30813, 10.1039/C7CP05846Bv.
- S. Soll, M. Antonietti and J. Yuan, Double Stimuli-Responsive Copolymer Stabilizers for Multiwalled Carbon Nanotubes, ACS Macro Lett., 2012, 1(1), 84–87, DOI:10.1021/mz200042h.
- R. Kanno, M. Ouchi and T. Terashima, Self-Assembly and Salt-Induced Thermoresponsive Properties of Amphiphilic PEG/Cation Random Terpolymers in Water, Polym. Chem., 2023, 14(15), 1718–1726, 10.1039/D3PY00013C.
- E. Karjalainen, V. Aseyev and H. Tenhu, Upper or Lower Critical Solution Temperature, or Both? Studies on Cationic Copolymers of N-Isopropylacrylamide, Polym. Chem., 2015, 6(16), 3074–3082, 10.1039/c4py01700e.
- R. Yu and K. Tauer, From Particles to Stabilizing Blocks – Polymerized Ionic Liquids in Aqueous Heterophase Polymerization, Polym. Chem., 2014, 5(19), 5644–5655, 10.1039/C4PY00458B.
- Z. Wang, H. Lai and P. Wu, Influence of PIL Segment on Solution Properties of Poly(N-Isopropylacrylamide)-b-Poly(Ionic Liquid) Copolymer: Micelles, Thermal Phase Behavior and Microdynamics, Soft Matter, 2012, 8(46), 11644–11653, 10.1039/C2SM26172C.
- K. Tauer, N. Weber and J. Texter, Core-Shell Particle Interconversion with Di-Stimuli-Responsive Diblock Copolymers, Chem. Commun., 2009, 40, 6065–6067, 10.1039/b912148j.
- H. Mori, M. Yanagi and T. Endo, RAFT Polymerization of N-Vinylimidazolium Salts and Synthesis of Thermoresponsive Ionic Liquid Block Copolymers, Macromolecules, 2009, 42(21), 8082–8092, DOI:10.1021/MA901180J.
- J. Yuan, H. Schlaad, C. Giordano and M. Antonietti, Double Hydrophilic Diblock Copolymers Containing a Poly(Ionic Liquid) Segment: Controlled Synthesis, Solution Property, and Application as Carbon Precursor, Eur. Polym. J., 2011, 47(4), 772–781, DOI:10.1016/j.eurpolymj.2010.09.030.
- G. Luo, Y. Guo, C. Liu, G. Han, X. Ma and W. Zhang, What Will Happen When Thermoresponsive Poly(N-Isopropylacrylamide) Is Tethered on Poly(Ionic Liquid)S?, RSC Adv., 2019, 9(23), 12936–12943, 10.1039/C9RA01849B.
- H. Mori, M. Yahagi and T. Endo, RAFT Polymerization of N -Vinylimidazolium Salts and Synthesis of Thermoresponsive Ionic Liquid Block Copolymers, Macromolecules, 2009, 42(21), 8082–8092, DOI:10.1021/ma901180j.
- E. Karjalainen, N. Chenna, P. Laurinmaeki, S. J. Butcher and H. Tenhu, Diblock Copolymers Consisting of a Polymerized Ionic Liquid and Poly(N-Isopropylacrylamide). Effects of PNIPAM Block Length and Counter Ion on Self-Assembling and Thermal Properties, Polym. Chem., 2013, 4, 1014–1024, 10.1039/c2py20815f.
- H. Mori, Y. Ebina, R. Kambara and K. Nakabayashi, Temperature-Responsive Self-Assembly of Star Block Copolymers with Poly(Ionic Liquid) Segments, Polym. J., 2012, 44(6), 550–560, DOI:10.1038/pj.2012.35.
- E. Read, B. Lonetti, S. Gineste, A. T. Sutton, E. Di Cola, P. Castignolles, M. Gaborieau, A. F. Mingotaud, M. Destarac and J. D. Marty, Mechanistic Insights into the Formation of Polyion Complex Aggregates from Cationic Thermoresponsive Diblock Copolymers, J. Colloid Interface Sci., 2021, 590, 268–276, DOI:10.1016/J.JCIS.2021.01.028.
- Y. Li, B. S. Lokitz and C. L. McCormick, Thermally Responsive Vesicles and Their Structural “Locking” through Polyelectrolyte Complex Formation, Angew. Chem., Int. Ed., 2006, 45(35), 5792–5795, DOI:10.1002/ANIE.200602168.
- D. Giaouzi and S. Pispas, Effects of Chemical Modifications on the Thermoresponsive Behavior of a PDMAEA-b-PNIPAM-b-POEGA Triblock Terpolymer, Polymer, 2020, 12(6), 1382, DOI:10.3390/POLYM12061382.
- D. Giaouzi and S. Pispas, PNIPAM-b-PDMAEA Double Stimuli Responsive Copolymers: Effects of Composition, End Groups and Chemical Modification on Solution Self-Assembly, Eur. Polym. J., 2020, 135, 109867, DOI:10.1016/J.EURPOLYMJ.2020.109867.
- A. M. Cardoso, M. T. Calejo, C. M. Morais, A. L. Cardoso, R. Cruz, K. Zhu, M. C. Pedroso De Lima, A. S. Jurado and B. Nyström, Application of Thermoresponsive PNIPAAM-b-PAMPTMA Diblock Copolymers in SiRNA Delivery, Mol. Pharm., 2014, 11(3), 819–827, DOI:10.1021/MP400510A.
- X. Han, X. Zhang, Q. Yin, J. Hu, H. Liu and Y. Hu, Thermoresponsive Diblock Copolymer with Tunable Soluble-Insoluble and Soluble-Insoluble-Soluble Transitions, Macromol. Rapid Commun., 2013, 34(7), 574–580, DOI:10.1002/marc.201200785.
- B. Kim, M. Kwon, A. K. Mohanty, H. Y. Cho, H.-J. Paik, B. Kim, M. Kwon, A. K. Mohanty, H. Y. Cho and H.-J. Paik, LCST and UCST Transition of Poly(DMAEMA-b-MEO2MA) Copolymer in KHP Buffer, Macromol. Chem. Phys., 2021, 222(2), 2000330, DOI:10.1002/MACP.202000330.
- X. Jia, D. Chen and M. Jiang, Preparation of PEO-b-P2VPH+–S2O82− Micelles in Water and Their Reversible UCST and Redox-Responsive Behavior, Chem. Commun., 2006, 16, 1736–1738, 10.1039/B600859C.
- L. Zhao, X. Wang, L. Sun, R. Zhou, X. Zhang, L. Zhang, Z. Zheng, Y. Ling, S. Luan and H. Tang, Synthesis and UCST-Type Thermoresponsive Properties of Polypeptide Based Single-Chain Nanoparticles, Polym. Chem., 2019, 10(38), 5206–5214, 10.1039/C9PY01040H.
- V. Baddam, V. Aseyev, S. Hietala, E. Karjalainen and H. Tenhu, Polycation−PEG Block Copolymer Undergoes Stepwise Phase Separation in Aqueous Triflate Solution, Macromolecules, 2018, 51(23), 9681–9691, DOI:10.1021/acs.macromol.8b01810.
- V. Baddam, R. Missonen, S. Hietala and H. Tenhu, Molecular Mass Affects the Phase Separation of Aqueous PEG–Polycation Block Copolymer, Macromolecules, 2019, 52(17), 6514–6522, DOI:10.1021/acs.macromol.9b01327.
- V. Baddam, L. Välinen and H. Tenhu, Thermoresponsive Polycation-Stabilized Nanoparticles through PISA. Control of Particle Morphology with a Salt, Macromolecules, 2021, 54(9), 4288–4299, DOI:10.1021/acs.macromol.0c02771.
- D. Yokota, A. Kanazawa and S. Aoshima, Precise Synthesis of UCST-Type Amphiphilic Diblock Copolymers with Pendant Imidazolium Ionic Liquid Segments and Their Thermosensitive Physical Gelation at Extremely Low Concentrations in Water, Polym. Chem., 2018, 9(41), 5080–5085, 10.1039/C8PY01139G.
- D. Yokota, A. Kanazawa and S. Aoshima, Precise Synthesis of Amphiphilic Diblock Copolymers Consisting of Various Ionic Liquid-Type Segments and Their Influence on Physical Gelation Behavior in Water, RSC Adv., 2020, 10(69), 42378–42387, 10.1039/D0RA09163D.
- Y. Deguchi, Y. Kohno and H. Ohno, Reversible Water Uptake/Release by Thermoresponsive Polyelectrolyte Hydrogels Derived from Ionic Liquids, Chem. Commun., 2015, 51(45), 9287–9290, 10.1039/c5cc02189h.
- J. Park, H. Joo, M. Noh, Y. Namkoong, S. Lee, K. H. Jung, H. R. Ahn, S. Kim, J. C. Lee, J. H. Yoon and Y. Lee, Systematic Structure Control of Ammonium Iodide Salts as Feasible UCST-Type Forward Osmosis Draw Solutes for the Treatment of Wastewater, J. Mater. Chem. A, 2018, 6(3), 1255–1265, 10.1039/c7ta09741g.
| This journal is © The Royal Society of Chemistry 2023 |