Open Access Article
Open Access ArticleCrystal engineering: from promise to delivery
Dario
Braga

Chemistry Department G. Ciamician, University of Bologna, Via F. Selmi 2, 4016 Bologna, Italy. E-mail: dario.braga@unibo.it
First published on 2nd November 2023
Abstract
Twenty years ago, I wrote a Chem. Commun. feature article entitled “Crystal Engineering: where from? Where to?”: an update is in order. In this Highlight I argue that molecular crystal engineering, one of the areas of fast development of the field, has definitely reached the stage of “delivering the goods”: new functional materials assembled via non-covalent interactions and/or improved properties of existing materials. As a proof of concept, the crystal engineering approach to tackle two contemporary emergencies, namely, urea fertilizer degradation and development of antimicrobial resistance by pathogens, is discussed and application-driven examples are provided.
In the beginning there was no crystal engineering, only crystallization
The products of crystallization were crystals, and determining the “molecular and crystal structure” was the challenge, the beginning and the end of the work of a small-molecule crystallographer.Crystals, often obtained after long and frustrating crystallization reactions, were handed over to the crystallographer with anxiety and expectations by the synthetic chemist. Crystallographers – at least the vast majority of them – were engaged in providing the synthetic chemists the answer they needed most: the structure of their molecule, i.e., the “final word” on the exact nature of the product of their synthetic strategy. Delivery of the results of crystal structure determination, obtained after long days of data collection, was usually met with a happy and grateful smile. It was the end of a long journey or the beginning of a new one. The information about the structure was essential to confirm/contradict working hypotheses, as well as to understand the results of other spectroscopic or analytical experiments or to guide changes in the synthetic strategies.
Under this perspective, it is easy to understand why synthetic chemists and crystallographers, in many areas, were bound by a sort of love–hate relationship. The former depended on things that could not be controlled: availability of the diffractometer, hence time, queue, priorities, stability of the crystal over time or under X-ray, etc., while the latter depended on the preparative chemist to give good crystals to work with, exciting scientific problems, new stuff and challenges.1
Diffusion of automated diffractometers, advancement in direct-methods for structure determination, and less expensive computing facilities concurred to increase exponentially the production of crystal structures, hence the output in crystallographic data. It was thanks to the vision of Olga Kennard, at the University of Cambridge, that in 1965 the Cambridge Crystallographic data Centre2 begun saving from oblivion the plethora of structural data produced daily by the growing community of structural chemists. The CSD contained 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 structures when, in 1983, Allen, Taylor and Kennard wrote “The systematic analysis of large numbers of related structures is a powerful research technique, capable of yielding results that could not be obtained by any other method”.3 Today this figure is ca. 1
000 structures when, in 1983, Allen, Taylor and Kennard wrote “The systematic analysis of large numbers of related structures is a powerful research technique, capable of yielding results that could not be obtained by any other method”.3 Today this figure is ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
At end of the 80s the strong wind of supramolecular chemistry – the “chemistry beyond the molecule, bearing on the organized entities of higher complexity that result from the association of two or more chemical species held together by intermolecular forces” – had begun to blow.4 J. M. Lehn's definition could be easily stretched to encompass molecular crystals, as they are – undeniably – “organized entities of higher complexity held together by intermolecular forces”.
In 1989, G. R. Desiraju reminded us that molecular crystals, as organized entities, are materials with collective properties, and pointed out that these properties could be engineered if only one could learn how to master the supramolecular interactions holding crystal components together. Reading from his book: “Crystal engineering is the understanding of intermolecular interactions in the context of crystal packing and the utilization of such understanding in design of new solids with desired physical and chemical properties”.5 Although the association of the word “crystal” with “engineering” or “design” can be found in earlier papers,6,7 it was this definition that marked the beginning of the crystal engineering era.
The success of crystal engineering is most certainly due to the hybridization of supramolecular chemistry, e.g., the chemistry of intermolecular bonding, with the chemistry of molecular materials, e.g., the utilitarian, application-oriented side of molecular recognition and self-assembly. But there were other reasons for determining the rapid development of crystal engineering as an entirely new area of research. Some of these reasons were certainly technical in nature; as mentioned above, others were a consequence of this progress. In simple terms, life for small-molecule crystallographers had become “too easy”. As crystal structure determination became increasingly more routine, many small molecule crystallographers begun to feel the need for new purposes. Crystal engineering provided the right conceptual frame. Many begun asking themselves the crucial question: “why don’t we start making our own stuff”?
Let's start making our own stuff
What kind of stuff, then? If crystal engineering is at the cross-roads of supramolecular chemistry and materials chemistry, the “stuff”, the chemical products that can be obtained with a crystal engineering approach, must be the result of a supramolecular synthesis, i.e., the result of breaking and forming intermolecular interactions.In this Highlight, the focus is on the engineering of molecular crystals, including coordination complexes and molecular ions as building blocks. However, we must not forget that a similar, perhaps even faster development took place in the area of coordination polymers.8 When researchers moved from the preparation and investigation of (mainly) “0-D” complexes and clusters and began the systematic use of ligands capable of bridging together metal centres or metal clusters, coordination chemistry took up a crystal engineering dimension and 1-D, 2-D and 3-D coordination polymers begun to be systematically investigated.9 The engineering of molecular crystals and that of coordination polymers expanded together and with predictable osmosis. Treating these two subareas of crystal engineering separately can only be a practical choice (as in this article) as there are many examples where there is no net distinction between the two types of crystalline materials. Even a distinction based on topology does not suffice. For example, MOFs10 – metal organic frameworks – have been and are amply investigated for their ability to host/trap/interact with guest molecules in the channels and cavities, generated within the framework via ligand–metal coordination bonds. It has been recently shown that analogous “emptiness” can be obtained in molecular crystals by choosing organic building blocks able to form multiple and “divergent” hydrogen bonds with neighbouring molecules. HOFs11 – hydrogen bonded organic frameworks – are indeed porous molecular crystals with properties analogous to those of coordination polymers.
Indeed, the idea of being able to design crystals with predefined properties was beautifully simple and extremely appealing and was followed by many researchers. The two most important learned chemical Societies, the RSC and the ACS, responded by providing adequate publishing media with CrystEngComm and with Crystal Growth and Design, respectively, to the growing community of crystal engineers.
There is a catch, though. The crystal packing obtained, and the associated patterns and energies of intermolecular interactions, may, or may not, correspond to the free energy global minimum, i.e., to the most stable thermodynamic assembly for that crystal. When it does not, the crystal structure might be thermodynamically stable only within a specific temperature range or be thermodynamically metastable because generated under kinetic control at nucleation/crystallization stage. This is crystal polymorphism of the enantiotropic or of the monotropic type, respectively,12 a phenomenon well known and investigated long before the advent of supramolecular chemistry and crystal engineering.13
Polymorphism, the nemesis?
Clearly, the possibility of multiple crystal forms, not only polymorphs, but also solvates and salts,14 even amorphous phases,15 for the same molecular assembly, and their hardly predictable formation, might seem to undermine the original idea and discourage the crystal engineering practitioner. There can be no engineering if the outcome of the project cannot be truly predicted, or if it is multiple, or changeable. Crucial aspects become particularly worrisome if the crystal of interest is a drug,16 or a high energy material,17 or a pigment,18 or any substance for which persistence of crystal properties over time and/or environmental variables is a must.19 Polymorphism could indeed be seen as a sort of “Damocles’ sword” hanging over the head of the crystal engineer.20Rather than putting people off, the crystal engineering approach became instrumental to tackle crystal polymorphism in a more systematic way. Thanks also to the inspirational work of J. Bernstein,19 the effort to “making crystals by design” was paralleled by a comparable push to gain some degree of control on the outcome of crystallization processes, and on the variables that could lead to different sets of intermolecular interactions, hence to different packings, and to different collective properties. Polymorph and solvate/hydrate screening became not only an indispensable part of any crystal-making process, but also a fundamental step in the development of a new crystalline material, especially in the pharmaceutical field. Several spinoff companies were born and thrived as the industrial world begun to realize that “unattended” crystal polymorphism was a serious threat, indeed a Damocles’ sword.20
Nonetheless, even if we are still far from being able to assert with confidence “this is the most stable crystal form”, the application of systematic screening procedures has minimized the chances of unexpected appearance of a different, often unwanted, crystal form.
The challenge of crystal polymorphism is also being met computationally.21 Crystal structure prediction (CSP) comprises computational methods to search ab initio for the most thermodynamically stable crystal structure of a given molecule by evaluating the crystal energy landscape.22 In this respect, CSP is complementary to experimental screening, and provides information on the existence and relative energies of polymorphs.23 Crystal structure prediction methods are periodically assessed via the Blind Tests organized by the Cambridge Crystallographic Data Centre.24 The Blind Test is carried out on a selection of unpublished crystal structures, which are sent out to scientists developing CSP methods in the form of chemical diagrams. The challenge is to submit predictions of crystal structures by a given deadline. The increasing number of successful “predictions” in the Blind Test indicates that CSP is becoming a useful guide in the experimental quest for “hidden” stable polymorphs.
Co-crystallization: the crystal engineering synthesis
Co-crystals are crystalline materials formed by two or more stable compounds.25 The components that are assembled in co-crystals can be in the neutral state, thus forming molecular co-crystals, or bear charges, such as in ionic co-crystals,25b and can be metal complexes, whether neutral or charged, thus forming hybrid co-crystals.25c The most popular class of co-crystals, namely pharmaceutical co-crystals, generally consist of an active pharmaceutical ingredient, API, and one or more ancillary molecules called ‘‘co-formers’’.26The number of publications on co-crystals is still increasing at a steady pace (see Fig. 1) covering very diverse areas such as pharmaceuticals, nutraceuticals, high energy materials, fertilizers, food, cosmetics, etc.27
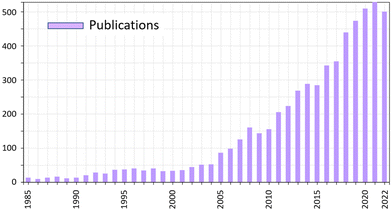 | ||
| Fig. 1 Trend in number of publications containing cocrystal or co-crystal in the title.28 | ||
If preparing new solids with the desired physical and chemical properties by mastering intermolecular interactions is the goal of crystal engineering, the co-crystallization process leading from an initial set of intermolecular interactions in crystals of the reagents to a new set of intermolecular interactions in the crystalline product is a crystal engineering synthesis (see Scheme 1). Intermolecular bond breaking and forming is what happens in the course of a co-crystallization process.25a Obviously, if the co-crystallization process is carried out in solution, intermolecular bond breaking and bond forming with solvent molecules have also to be considered.
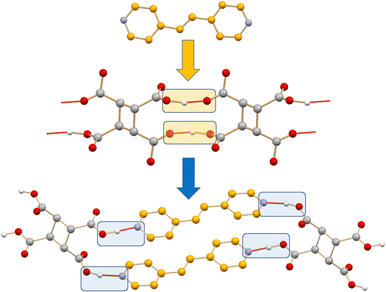 | ||
| Scheme 1 A representation of the intermolecular bond breaking and forming in the formation of a co-crystal. | ||
It is generally assumed that the method of choice to make co-crystals is a crystallization from solution. As a matter of fact, the use of solvents is at the heart of traditional chemistry, and crystallization is the method of choice to purify materials and to grow crystals. However, the problem arising from the difference in solubilities of the crystalline reactants in a same solvent or mixture of solvents, and/or between these and the solubility of the co-crystalline product, often makes solution co-crystallization simply not viable.
The alternative had been available since long. Reactants can be mixed directly without solvent with the aid of mechanical energy.29 Mechanochemistry has a long history, with deep roots in solid-state organic chemistry.29,30
Reactions between crystals can be used to make co-crystals.31 Making co-crystals without dissolving the reactants in a solvent presents the huge advantage of generating crystalline materials regardless of the relative solubilities of the starting components. Moreover, it is cheaper and faster, and may also give access to alternative routes to product formation.32,33
Mechanochemistry: a tool for a sustainable crystal engineering
As a matter of fact, mechanical grinding of molecular materials, with or without the addition of a small quantity of solvent (liquid assisted grinding – LAG),32a has revealed itself to be a greener and cost-effective way not only to prepare molecular and ionic co-crystals,32b,c but also to study the effect of grinding and comminution on polymorphism and/or on the uptake/release of water in the formation of hydrates. Moreover, the course of a reaction can be modulated by changing the milling conditions, such as the amount of solvent, the milling time, the material of the jars in ball milling, and the grinding media (see Fig. 2).33 | ||
| Fig. 2 Porcelain mortar and pestle for manual grinding (left), and ball mill for vibrational grinding (right). | ||
Learning how to “make co-crystals without solution crystallization” has been an important step forward in the development of the field. It became possible to not only combine molecular solids without worrying about differences in solubilities but also create new solid-state reaction pathways. For example, McGillivray and others have shown that mechanochemical methods can be successfully used to combine co-crystallization and photocyclization rections.34
Everything comes with a price
Grinding, ball milling, liquid-assisted grinding (LAG)32 produce polycrystalline powders, no single-crystals, of course. X-Ray powder diffraction then becomes the method of choice to identify and characterize a new product, whether alone or in mixture with unreacted starting materials. Except in those fortunate cases where, by means of seeding or other similar tricks, powders can be recrystallized into crystals of adequate size without changing structure with respect to the original mechanochemical product, it is not possible to enjoy the speed and accuracy of a structure determination by single crystal X-ray methods.Were it not for the progress in methods for structure determination from powder diffraction,35a or from electron diffraction (ED)35b data, as well as in complementary methods based on solid state NMR spectroscopy,36a and for the achievements in in situ methods36b,c to monitor mechanochemical reactions, the development of crystal engineering would have been much slower.
Furthermore, determining the crystal structure of the product is not sufficient. The behaviour with changing temperature, pressure, humidity, mechanical stress, etc. needs also to be assessed for a material to be of any use. The aspiring crystal engineer has to get acquainted with a broad series of complementary techniques for the investigation of solids, such as differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), dynamic vapour sorption (DVS), Fourier transform IR and Raman spectroscopy, and atomic force microscopy (AFM), as well as hot stage microscopy (HSM), scanning tunnelling microscopy (STM), and scanning electron microscopy (SEM). Since for many of these methods and techniques collaborative efforts with experts of diverse fields are indispensable, crystal engineering also generated new collaborations with neighbouring areas towards crystal making and characterization.
Time to deliver the goods
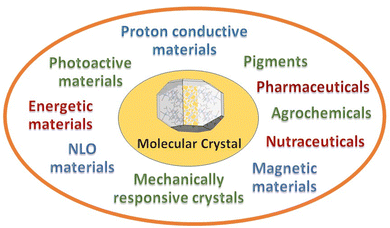
Choose the target, design the strategy to get there, make and characterize the compounds, and evaluate the properties resulting from the convolution of the structural, electronic, magnetic, charge properties of the components with crystal periodicity. At the end of the crystal engineering path there is a functional material. The periodical arrangement of the building blocks will determine colour, crystal habit, surface properties, capacity of vapour uptake, plasticity, mobility, thermodynamic and kinetic stability with varying pressure, temperature, humidity conditions, etc. In multi-component crystals, such as molecular and ionic co-crystals, stoichiometric and non-stoichiometric solvates, coordination polymers, metal organic frameworks, or hydrogen bonded organic frameworks, a virtually unlimited range of potential applications becomes possible. New areas, such as mechanically responsive crystals,37 and proton conducting materials38 are also receiving increasing attention. Fig. 3 shows some of the most popular targets of molecular crystal engineering.There are no means to cover such breath and diversity of applications in this short Highlight. The point that I want to stress is that the attention of researchers is beginning to be even more focused on the evaluation of the performance of the engineered materials with respect to the objective of the crystal engineering strategy. A change of paradigm with respect to the stages of design and synthesis. Indeed, when the target is reached, and a new or modified/improved property is attained, crystal engineering “deliver the goods”.
As this is indeed happening for most of the activity areas designed in Fig. 3, I have chosen, as a proof of concept, to focus on two specific issues of great contemporary relevance. First, the decomposition of the most commonly used fertilizer, urea, by soil enzymes, with consequent loss of fertilizer and air pollution by ammonia and nitrogen oxides, and second, the threat posed by the increase in antimicrobial resistance by pathogens due to antibiotic overuse by humans and in farming and agriculture. The reasons for these choices are evident: everything should be done to guarantee food and health to the ever-increasing human population. Answers can only come from scientific research and from a multidisciplinary approach, hence also from crystal engineering.
Do not waste fertilizer
Urea is the most widely used plant fertilizer, accounting for about 60% of the global nitrogen fertilizer used in the World.39 It is prepared in huge quantities (ca. 210 Mt in 2022) from ammonia (produced from nitrogen and hydrogen via the energy expensive Haber–Bosh process) and from carbon dioxide. However, much of the urea nitrogen is lost through volatilization, denitrification and leaching. Dry urea deposited in the soil is degraded by the nickel-dependent enzyme urease. If the fertilizer is not incorporated with tillage, injected beneath the surface residue and rapidly irrigated,39b urease is able to quickly hydrolyze urea into hydrogen carbonate (HCO3−) and ammonium (NH4+) at a rate 1015 times faster than in the absence of urease.39c Although NH4+ serves as a nutrient to plants, the overall pH increase leads to the formation of gaseous ammonia, which is released in the atmosphere.39b,d Urease is not the only enzyme acting in the soil. The copper-dependent enzyme ammonia monooxygenase (AMO) present in ammonia-oxidizing microorganisms catalyzes the oxidation of NH3 into hydroxylamine (NH2OH),39e,f that is further oxidized to nitrite (NO2−) and nitrate (NO3−), which are precursors of gaseous NO and N2O and contribute to the greenhouse effect. As a consequence of this complex reaction ensemble, a large amount (ca. 50%) of nitrogen fertilizer applied to soils as urea is lost, while the environment is polluted.39bAt present, there are essentially two methods to tackle this huge problem: (1) reduce urea water solubility and dissolution rate and (2) inhibit the urea degradation activity by soil enzymes.
Several research groups are applying co-crystallization strategies to reach these two objectives. As a matter of fact, co-crystallization is not only an efficient way to alter the solubility of an active molecule, in our case urea, but can also provide a means to carry in the soil the enzyme inhibitor together with the fertilizer as a co-crystal coformer.
The recent literature provides many examples of how co-crystallization may help in reducing urea solubility.
Baltrusaitis et al. have shown that, if urea is supplied in the form of mechanochemically prepared CaSO4·4urea ionic co-crystals (see Fig. 4), urea solubility is significantly decreased.40 The reduction of nitrogen loss obtained with this approach has been demonstrated by the observation of an appreciable increase in the productivity of sorghum grains (Sorghum bicolor (L.) Moench).
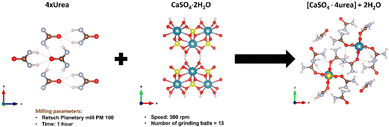 | ||
| Fig. 4 Mechanochemical synthesis of CaSO4·4urea cocrystal. Reproduced under open access creative common from Sustainability, 2023, 15, 8010. | ||
Aakeroy et al. have shown that urea·pimelic acid and urea·4-nitrophenol co-crystals can reduce urea solubility and hygroscopicity.41
More recently, as a follow up of an earlier study of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 urea·adipic acid co-crystals,42a R. Thakuria et al. have reported the mechanochemical synthesis of co-crystals of urea with hydroxybenzoic acid derivatives.42b The release profiles (see Fig. 5) showed that the co-crystals release ∼90% urea in about a month, whereas pure urea reaches a plateau within 8 days with ∼85% urea release.
1 urea·adipic acid co-crystals,42a R. Thakuria et al. have reported the mechanochemical synthesis of co-crystals of urea with hydroxybenzoic acid derivatives.42b The release profiles (see Fig. 5) showed that the co-crystals release ∼90% urea in about a month, whereas pure urea reaches a plateau within 8 days with ∼85% urea release.
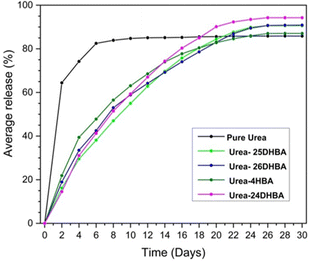 | ||
| Fig. 5 Urea release profile of urea·hydroxybenzoic acid co-crystal compared with commercial urea for a period of 30 days. Reproduced from ref. 42b with permission from the Royal Society of Chemistry. | ||
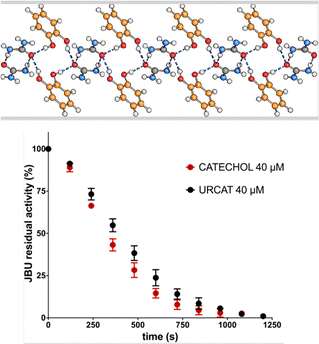
Casali et al.43 have otherwise tackled the problem from the enzyme inhibition side. The mechanochemical reaction of urea and catechol yields quantitatively a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 urea·catechol co-crystal that acts simultaneously as a urease inhibitor (catechol) and as soil fertilizer (urea).43b The activity of the co-crystal has been assessed by evaluating the inhibition of Canavalia ensiformis urease and the effect on water vapor sorption of urea at room temperature (see Fig. 6).
1 urea·catechol co-crystal that acts simultaneously as a urease inhibitor (catechol) and as soil fertilizer (urea).43b The activity of the co-crystal has been assessed by evaluating the inhibition of Canavalia ensiformis urease and the effect on water vapor sorption of urea at room temperature (see Fig. 6).
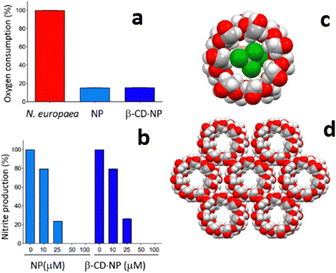
While catechol is a good urease inhibitor, nitrapyrin inhibits the activity of the enzyme ammonia monooxygenase (AMO), also responsible for the urea decomposition cycle. The poor aqueous solubility and high volatility of nitrapyrin was dealt with by mechanochemical preparation of its inclusion complex with β-cyclodextrine (see Fig. 7).43c With respect to nitrapyrin, the complex presents improved solubility and thermal stability, at the same time maintaining an efficient enzymatic inhibition activity.
Respond to antimicrobial resistance
The second emergency that is calling for concerted multidisciplinary research efforts is that of antimicrobial resistance (AMR), the condition in which bacteria, viruses, parasites, and fungi no longer respond to medication. The development of antimicrobial resistance (AMR) in pathogenic microorganisms is caused by the overuse of antimicrobials not only in the treatment of humans and animals but also in agriculture.44 The rates at which clinically and agriculturally relevant pathogens are gaining resistance towards treatment with available antimicrobials are outpacing the rates at which new antimicrobials arrive to the market. WHO now considers AMR the “hidden pandemic”, with a gloomy expectation of millions of human deaths per year by 2050. This huge problem is being addressed in different ways: (1) search for novel therapeutic strategies, (2) strengthening or revitalizing of “exhausted” drugs, and (3) increasing the strength of the biofilm barriers to penetration and spread of pathogens from the environment. Clearly, strategies 2 and 3, and, to some extent also strategy 1, are all very suitable for a crystal engineering approach.Indeed, co-crystallization of known antibiotics with ad hoc coformers takes the lion's share of the research efforts in the field.45 Modifying the properties of “old” drugs, improving solubility and permeability, dissolution rate, etc. or obtaining novel co-drugs by a pharmaceutical synthon approach46 provides a strong motivation for both academic and industrial research. The number of patents filed on pharmaceutical co-crystals, some of which are already on the market, can also be taken as a measure of the impact of crystal engineering in this area.47
One way to increase the efficacy of an “old” drug could be searching for a synergistic interaction with natural antimicrobials. This way was used by Shemchuk et al. with the preparation and antimicrobial assessment of co-crystals of ciprofloxacin, a well-known antibiotic, with thymol and carvacrol, which are natural antimicrobials.48a
The idea was inspired by an earlier study by Bacchi et al. who explored the possibility of using mechanochemically prepared co-crystals of thymol and carvacrol in combination with isonicotinamide, pyrazine, 2,3,5,6-tetramethyl pyrazine, and 2,3-dimethyl quinoxaline to increase their release profiles in the soil in order to make these natural products competitive to conventional pesticides or synthetic food additives.49
The solid-state association with thymol and carvacrol is indeed able to increase the overall antimicrobial activity of ciprofloxacin.48a Since thymol and carvacrol are not effective on E. coli on their own, the increased bacteriostatic activity of the co-crystals, evaluated via measurements of minimal inhibitory concentration (MIC) also with respect to the physical mixtures of the same compounds, was ascribed to the performance of the co-crystals. It is worth mentioning, that the same co-crystallization approach with antibiotics of the cephalosporin (CEPH) class, namely, cephalexin (CPX), cefradine (CFD), and cefaclor (CFC), led to the opposite effect: inhibition of antimicrobial activity.48b The different response of the co-crystals of ciprofloxacin and of the cephalosporines has been ascribed to a significant decrease in solubility of the co-crystals of the latter compounds with respect to the CEPH APIs, due to formation of less soluble hydrates. The results of the two experiments are represented together in Fig. 8.
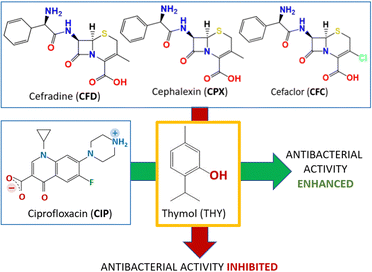 | ||
| Fig. 8 The opposite effects on the antibacterial activity of the co-crystallization with thymol (THY) with ciprofloxacin (CIP)48a and with the cephalosporines cefradine (CFD), cephalexin (CPX) and cefaclor (CFC).48b | ||
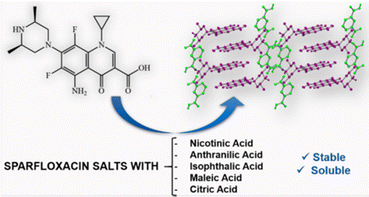
Another example of the application of co-crystallization to modify existing properties is the use of co-crystallization to reduce the intake dosage of the fluoro quinolone antibiotic sparfloxacin, which is widely used mainly in respiratory tract infections. Alternative forms that may allow the intake of lower dosages of sparfloxacin are desirable. André et al.50 have shown that the co-crystals with nicotinic, anthranilic, isophthalic, maleic, and citric acids (see Fig. 9) all showed higher solubility in water than sparfloxacin itself. In particular, the one order higher aqueous solubility of the citrate salt may indeed allow lower dosages of the drug.
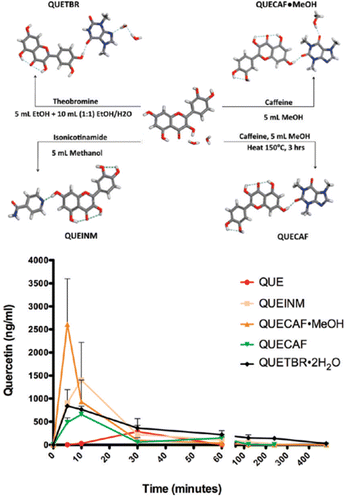
Another significant example involving flavonoids is that of quercetin, which is one of the most abundant flavonoids in natural products. Its use in the pure form is hampered by its low absorption in the gut because of its low solubility. Zaworotko et al. prepared and evaluated co-crystals of quercetin with caffeine, isonicotinamide, and theobromine (see Fig. 10) observing pharmacokinetic properties vastly superior to those of quercetin alone.51 The co-crystals with caffeine showed an increase in solubility of quercetin between 8 and 14 times higher than quercetin dihydrate. This difference in solubility was reflected in an up to nearly 10-fold increase in bioavailability with respect to quercetin dihydrate.
The low solubility and low permeability of the drug naftopidil were also tackled by co-crystallization with mono-, di-, tri-, and tetra-fluorobenzoic acids as coformers (see Fig. 11) by Nangia et al.52 Of all the co-crystals, those with 2,4,5-trifluorobenzoic acid appeared to form the optimal product with faster dissolution and high permeability. Permeability was evaluated in pH 7 phosphate buffered saline medium showing a clear effect of the fluoro-substitution on the passage though the semi-permeable membrane used for testing.52
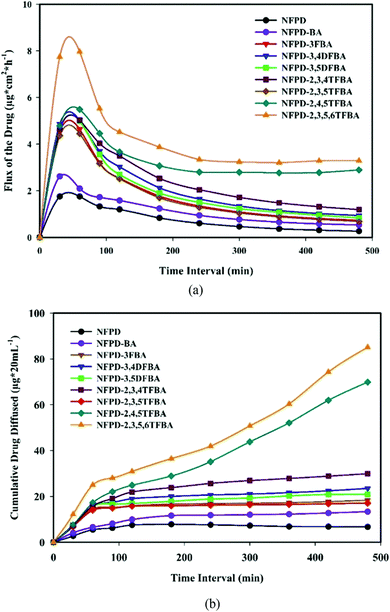 | ||
| Fig. 11 Co-crystals of naftopidil (NFPD) with fluorobenzoic acids (FBA). (a) Drug flux with respect to the duration of the presence of NFPD salts in pH 7 phosphate buffer. (b) Membrane permeability of NFPD–FBA salts in PBS medium. Reproduced from ref. 50 with permission from the Royal Society of Chemistry. | ||
Another area where antimicrobial resistance44 represents a major problem is that of biofilms, which might affect, for example, the sterilization of surfaces and medical devices.
Fiore et al. have applied crystal engineering strategies to prepare co-crystals combining metal complexes and organic molecules that can be used for antimicrobial coating.53 The idea is that of exploiting the antimicrobial properties of metals such as silver, zinc, copper, and others, which have been used for millennia as antimicrobial agents, to enhance antibacterial properties in the association with an active organic molecule in a synergistic way.54
As an example, the mechanochemically prepared co-crystals of the antimicrobial agents proflavine (PF) and methyl viologen (MV) with a silver, copper and zinc complexes have been proven by Lekhan et al. to exert bacteriostatic and antibiofilm activities55 against the pathogen indicator bacteria strains Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli (see Fig. 12 for the proflavine co-crystals). For the biofilm state of growth, the silver proflavine co-crystal appears to have the best antibiofilm activity. However, all other proflavine-metal co-crystals also show activity against E. coli and S. aureus.
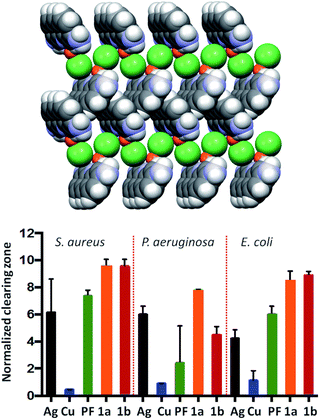 | ||
| Fig. 12 (top) Packing of the co-crystal PF·CuCl (PF = proflavine). (bottom) Efficacy of PF based compounds: normalized zones of growth inhibition with compounds deposited in agar well [1a and 1b are PF·CuCl and PF·AgNO3, respectively]. Reproduced from ref. 56 with permission from the Royal Society of Chemistry. | ||
Extending the co-crystallization approach to metal complexes and active organic molecules is a way to expand the toolbox available to contrast antimicrobial resistance.54
Conclusions
When crystal engineering was born, many small-molecule crystallographers became crystal makers. This evolutionary step had consequences: first, the need to explore viable alternatives to crystallization from solution, for example by mechanically mixing solid reactants; second, the need to strengthen methods for structure determination in the absence of suitable single crystals, hence structure solution from powder and computational simulations; third, the need to expand competence to an ample variety of solid-state techniques complementary to crystallography. Beyond all that, or, perhaps, above all that, the need to minimize the chance of investing efforts and funds on thermodynamically metastable materials was strongly felt, which might change structures and properties as a consequence of “unattended” crystal polymorphism. However, what could be a serious threat to any attempt to control crystal properties by assembling molecules in a designed way became – in the hands of scientists – a challenge to face up. Screening for crystal polymorphs and, of course, also for solvates/hydrate formation, is now an essential step in the investigation of crystal forms of active molecules. Co-crystal screening, especially with GRAS56 (generally recognized as safe) coformers, is also becoming common practice.In view of the enormous implications of the structure–property control in many diverse areas, the prospect of being able to produce new materials or to change the properties of existing ones provide a motivation for scientific innovation in solid state research comparable to that of supramolecular chemistry in solution.
As a “proof of concept”, I have chosen to focus on two huge environmental, economic and health-related problems of our days. I have reported examples of successful applications of co-crystallization techniques to prepare novel materials to inhibit enzyme activity in the soil, to reduce environmental pollution and increase soil productivity, to enhance the activity of antibiotics, and to make available alternative antimicrobials to respond to the antimicrobial resistance threat. It could only be a limited selection from a rapidly expanding literature but should suffice to demonstrate that the promise of crystal engineering is coming true.
This Highlight has been focused on the making and evaluation of crystalline materials formed by molecules or molecular ions, but it is important to underline that the evolutionary sequence design → synthesis → characterization → assessment applies to all areas of crystal engineering.
As a corollary, the examples discussed herein also demonstrate that crystal makers cannot work alone. The assessment of the performance of the engineered materials and the evaluation of the results requires a synergistic interaction not only with scientists operating in neighbouring academic research communities (microbiology, biotechnology, pharmacology, food, nutraceuticals, cosmetics, energetic materials, etc.) but also with researchers operating in the industrial world. New collaborations are established across disciplinary barriers sharing interests, projects and targets.
In summary, crystal engineering has become a “hub” connecting different areas of research from fundamental to applied science. Using a multidisciplinary approach, crystal engineering may indeed contribute to efficaciously address relevant challenges of our time.
Author contributions
The paper has been written by the author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
I am very grateful to all those I have collaborated with through the years, especially Fabrizia Grepioni (Fab), who shares with me much more than I have recounted herein, and Lucia Maini, and Simone d’Agostino who joined the group “en route” and are now leading scientists in their own lines of crystal engineering research. I am also thankful to Stefano Luca Giaffreda and Marco Curzi, who believed in the idea that our experience could be turned into a company in 2005 (PolyCrystalLine S.p.A.). Many of the most significant results of the Bologna's team have been obtained also thanks to the collaboration with Roberto Gobetto and his group of solid-state NMR spectroscopy in Turin. Over the years, I have enjoyed collaborations with many leading scientists: Guy Orpen, who co-chaired Erice 1999 on “Crystal Engineering: from Molecules and Crystals to Materials”; Juan Novoa and Lia Addadi, who co-chaired Erice 2007 on “Engineering of Crystalline Materials Properties: State-of-the-Art, Design and Applications”. I have had the fortune to collaborate with Gautam Desiraju, the founder of crystal engineering, and also with Angelo Gavezzotti, Sally Price and the late Joel Bernstein, and to learn from them. Finally, I stress that the papers of the Bologna's group presented in this Highlight are the result of close synergistic interactions with researchers with indispensable complementary expertise. I gratefully acknowledge the collaboration with the groups of Ray J. Turner (University of Calgary), Stefano Luciano Ciurli (University of Bologna), Vittorio Sambri (University of Bologna), Jonas Baltrusaitis (University of Leigh), and Franziska Emmerling (BAM, Berlin). I also acknowledge financial support from MUR, project “Nature Inspired Crystal Engineering” (PRIN2020), and from the University of Bologna.Notes and references
- D. Braga, Chem. Commun., 2003, 2751–2755 RSC
.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, The Cambridge Structural Database, Acta Cryst., 2016, B72, 171–179, DOI:10.1107/S2052520616003954
.
- F. H. Allen, O. Kennard and R. Taylor, Systematic Analysis of Structural Data as a Research Technique in Organic Chemistry, 1983, 16.
- J. M. Lehn, Angew. Chem., Int. Ed. Engl., 1990, 1304–1319, DOI:10.1002/anie.199013041
.
-
(a)
G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier Amsterdam, 1989 Search PubMed
; (b) G. R. Desiraju, Angew. Chem., Int. Ed., 2007, 8342–8356, DOI:10.1002/anie.200700534
; (c) The Crystal as a Supramolecular Entity, ed. G. R. Desiraju, John Wiley & sons, 1996 Search PubMed
.
-
(a) R. Pepinsky, Phys. Rev., 1955, 100, 971 CAS
; (b) G. M. J. Schmidt, Pure Appl. Chem., 1971, 27, 647–678, DOI:10.1351/pac197127040647
.
- T. W. Panunto, Z. Urbinczyk-lipkowska, R. Johnson and M. C. Etter, J. Am. Chem. Soc., 1987, 109, 7786–7797, DOI:10.1021/ja00259a030
.
-
(a) J. J. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC
; (b) S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Ohrstrom, M. O’Keeffe, M. P. Suh and J. Reedijk, CrystEngComm, 2012, 14, 3001–3004 RSC
.
-
(a) O. M. Yaghi, M. O’Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714, DOI:10.1126/science.1067208
; (b) S. R. Batten and N. R. Champness, Coordination polymers and metal-organic frameworks: Materials by design, Philos. Trans. R. Soc., A, 2017, 375, 2084 CrossRef PubMed
.
-
(a) M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O’Keeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef CAS PubMed
; (b) S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375, DOI:10.1002/anie.200300610
; (c) M. Ding, R. W. Flaig, H. L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC
; (d) K. Suresh and A. J. Matzger, Angew. Chem., Int. Ed., 2019, 58, 16790–16794 CrossRef CAS PubMed
; (e) S. N. Zhao, X. Z. Song, S. Y. Song and H. J. Zhang, Coord. Chem. Rev., 2017, 337, 80–96 CrossRef CAS
.
-
(a) R.-B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC
; (b) P. Li, M. R. Ryder and J. F. Stoddard, Acc. Mater. Res., 2020, 1, 77–87 CrossRef CAS
.
-
J. Bernstein, Polymorphism in Molecular Crystals, Oxford University Press, Oxford, 2002 Search PubMed
.
-
W. C. McCrone, Physics and Chemistry of the Organic Solid State. ed. D. Fox, M. M. Labes and A. Weissenberg, Interscience, New York, 1965, vol. 2, p. 725 Search PubMed
.
-
U. J. Griesser, Importance of Solvates in Polymorphism in the Pharmaceutical Industry, ed. R. Hilfiker, Wiley-VCH, Germany, 2006, pp. 211–234 Search PubMed
.
-
(a) M. Descamps, Adv. Drug Delivery Rev., 2016, 100, 1–2 CrossRef CAS PubMed
; (b) F. Walton, J. Bolling, A. Farrell, J. MacEwen, C. D. Syme, M. González Jiménez, H. M. Senn, C. Wilson, G. Cinque and K. Wynne, J. Am. Chem. Soc., 2020, 142, 7591–7597, DOI:10.1021/jacs.0c01712
.
-
(a)
Polymorphism in the Pharmaceutical Industry: Solid Form and Drug Development, ed. R. Hilfiker, Wiley-VCH, Germany, 2006, pp. 211–234 Search PubMed
; (b) A. J. Cruz-Cabeza and J. Bernstein, Chem. Rev., 2014, 114, 2170–2191 CrossRef CAS PubMed
; (c) S. Cherukuvada and A. Nangia, CrystEngComm, 2014, 50, 906–923 CAS
; (d) T. L. Threlfall, Eur. Pharm. Rev., 2014, 19, 24–29 Search PubMed
.
- G. Liu, R. Gou, H. Li and C. Zhang, Cryst. Growth Des., 2018, 18(7), 4174–4186, DOI:10.1021/acs.cgd.8b00704
.
-
(a) B. A. Nogueira, C. Castiglioni and R. Fausto, Chem. Commun., 2020, 3, 34, DOI:10.1038/s42004-020-0279-0
; (b) J. Bernstein, Polymorphism of pigments and dyes, in Polymorphism in Molecular Crystals, International Union of Crystallography, Oxford Academic, 2nd edn, 2020 DOI:10.1093/oso/9780199655441.003.0008
; (c) D. K. Bučar, S. Filip, M. Arhangelskis, G. O. Lloyd and W. Jones, CrystEngComm, 2013, 15, 6289–6291, 10.1039/C3CE41013G
.
-
(a) D.-K. Bucar, R. W. Lancaster and J. Bernstein, Angew. Chem., 2015, 54, 6972–6993 CrossRef CAS PubMed
; (b) A. J. Cruz-Cabeza, S. M. Reutzel-Edens and J. Bernstein, Chem. Soc. Rev., 2015, 44, 8619–8635, 10.1039/C5CS00227C
.
- D. Braga, L. Casali and G. Grepioni, Int. J. Mol. Sciences, 2022, 23, 9013–9042 CrossRef CAS PubMed
.
- S. L. Price, Chem. Soc. Rev., 2014, 43, 2098–2111, 10.1039/c3cs60279f
.
- S. L. Price, Faraday Discuss., 2018, 211, 9–30, 10.1039/c8fd00121a
.
-
(a) M. Neumann, F. Leusen and J. Kendrick, Angew. Chem., Int. Ed., 2008, 47, 2427–2430, DOI:10.1002/anie.200704247
; (b) I. J. Sugden, D. E. Braun, D. H. Bowskill, C. S. Adjiman and C. C. Pantelides, Cryst. Growth Des., 2022, 22, 4513–4527 CrossRef CAS PubMed
.
- CSP Blind Tests-The Cambridge Crystallographic Data Centre (CCDC), https://www.ccdc.cam.ac.uk/Community/initiatives/cspblindtests/csp-blind-test-7/. https://www.ccdc.cam.ac.uk/discover/news/conclusions-of-the-7th-crystal-structure-prediction-blind-test/.
-
(a) C. B. Aakeröy and D. J. Salmon, CrystEngComm, 2005, 7, 439–448, 10.1039/b505883j
; (b) D. Braga, F. Grepioni, L. Maini, S. Prosperi, R. Gobetto and M. R. Chierotti, Chem. Commun., 2010, 46, 7715–7717 RSC
; (c) F. Grepioni, L. Casali, C. Fiore, L. Mazzei, R. Sun, O. Shemchuk and D. Braga, Dalton Trans., 2022, 51, 7390–7400 RSC
.
- Ö. Almarsson and M. J. Zaworotko, Chem. Commun., 2004, 1889–1896, 10.1039/b402150a
.
-
(a) J. W. Steed, Pharmacol. Sci., 2013, 34, 185–193, DOI:10.1016/J.TIPS.2012.12.003
; (b) M. Karimi-Jafari, L. Padrela, G. M. Walker and D. M. Croker, Cryst. Growth Des., 2018, 18, 6370–6387, DOI:10.1021/ACS.CGD.8B00933
; (c) S. Golob, M. Perry, M. Lusi, M. R. Chierotti, I. Grabnar, L. Lassiani, D. Voinovich and M. J. Zaworotko, J. Pharm. Sci., 2016, 105, 3626–3633, DOI:10.1016/J.XPHS.2016.09.017
; (d) S. S. Meng, Y. M. Yu, F. Z. Bu, C. W. Yan, Z. Y. Wu and Y. T. Li, Cryst. Growth Des., 2022, 22, 6735–6750, DOI:10.1021/ACS.CGD.2C00896
; (e) A. J. Cruz-Cabeza, M. Lusi, H. P. Wheatcroft and D. A. Bond, Faraday Discuss., 2022, 235, 446–466, 10.1039/D1FD00081K
.
- Web of science, https://clarivate.libguides.com/home.
-
(a) K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025–1074, DOI:10.1021/CR940089P
; (b) G. Kaupp, CrystEngComm, 2009, 11, 388–403, 10.1039/b810822f
.
-
(a) M. B. J. Atkinson, D.-K. Bucar, A. N. Sokolov, T. R. Frisčic, C. N. Robinson, M. Y. Bilal, N. G. Sinada, A. Chevannes and L. R. MacGillivray, Chem. Commun., 2008, 5713–5715 RSC
; (b) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Frišcic, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2011, 41, 413–447, 10.1039/C1CS15171A
.
- D. Braga and F. Grepioni, Angew. Chem., 2004, 43, 4002–4011 CrossRef CAS PubMed
.
-
(a) S. Karki, T. Friščić and W. Jones, CrystEngComm, 2009, 11, 470t RSC
; (b) J. L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19, DOI:10.1021/ACSCENTSCI.6B00277
; (c) T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029, DOI:10.1002/ANIE.201906755
.
-
(a) B. P. Hutchings, D. E. Crawford, L. Gao, P. Hu and S. L. James, Angew. Chem., Int. Ed., 2017, 56, 15252–15256, DOI:10.1002/anie.201706723
; (b) F. Cuccu, L. De Luca, F. Delogu, E. Colacino, N. Solin, R. Mocci and A. Porcheddu, ChemSusChem, 2022, 15, 17, DOI:10.1002/CSSC.202200362
.
-
(a) S. P. Yelgaonkar, D. C. Swenson and L. R. MacGillivray, Chem. Sci., 2020, 11, 3569–3573, 10.1039/C9SC05823K
; (b) S. d'Agostino, P. Taddei, E. Boanini, D. Braga and F. Grepioni, Cryst. Growth Des., 2017, 17, 4491–4495, DOI:10.1021/acs.cgd.7b00415
.
-
(a)
K. Shankland, An Overview of Currently Used Structure Determination Methods for Powder Diffraction Data, International Tables for Crystallography, Online MRW, 2019, vol. A–G, pp. 386–394 Search PubMed
; (b) T. Gruene, J. J. Holstein and G. H. Clever, Nat. Rev. Chem., 2021, 5, 660–668, DOI:10.1038/s41570-021-00302-4
.
-
(a) M. R. Chierotti and R. Gobetto, CrystEngComm, 2013, 15, 8599–8612, 10.1039/C3CE41026A
; (b) P. A. Julien and T. Friscic, Cryst. Growth Des., 2022, 22, 5726–5754 CrossRef CAS
; (c) G. I. Lampronti, A. A. L. Michalchuk, P. P. Mazzeo, A. M. Belenguer, J. K. M. Sanders, A. Bacchi and F. Emmerling, Nat. Commun., 2021, 12, 6134, DOI:10.1038/s41467-021-26264
.
-
(a) P. Naumov, S. Chizhik, M. K. Panda, N. K. Nath and E. Boldyrev, Chem. Rev., 2015, 115, 12440 CrossRef CAS PubMed
; (b) E. R. Engel and S. Takamizawa, Angew. Chem., Int. Ed., 2018, 57, 11888 CrossRef CAS PubMed
; (c) S. Saha, M. K. Mishra, C. M. Reddy and G. R. Desiraju, Acc. Chem. Res., 2018, 51, 2957 CrossRef CAS PubMed
.
-
(a) I. Akhmetova, M. Rautenberg, C. Das, B. Bhattacharya and F. Emmerling, ACS Omega, 2023, 8, 16687–16693, DOI:10.1021/acsomega.2c07883
; (b) S. Ocak, F. Poli, D. Braga, T. Salzillo, F. Tarterini, G. Carì, E. Venuti, F. Soavi and S. d’Agostino, Cryst. Growth Des., 2023, 23, 4336–4345, DOI:10.1021/acs.cgd.3c00145
.
-
(a) M. Prud’homme, Global fertilizer supply and trade 2016–2017, https://www.fertilizer.org/ItemDetail?iProductCode=10192Pdf&Category=ECO;
(b) L. B. Fenn and L. R. Hossner, Adv. Soil Sci., 1985, 1, 123–169 CAS
; (c) H. L. Mobley and R. P. Hausinger, Microbiol. Rev., 1989, 53, 85–108 CrossRef CAS PubMed
; (d) B. P. Callahan, Y. Yuan and R. Wolfenden, J. Am. Chem. Soc., 2005, 127, 10828–10829 CrossRef CAS PubMed
; (e) R. P. Hausinger, Microbiol. Rev., 1987, 51, 22–42 CrossRef CAS PubMed
; (f) F. Musiani, V. Broll, E. Evangelisti and S. Ciurli, J. Biol. Inorg. Chem., 2020, 25, 995–1007 CrossRef CAS PubMed
.
-
(a) P. Bista, M. Eisa, D. Ragauskait, S. Sapkota, J. Baltrusaitis and R. Ghimire, Sustainability, 2023, 15, 8010, DOI:10.3390/su15108010
; (b) K. Honer, E. Kalfaoglu, C. Pico, J. McCann and J. Baltrusaitis, ACS Sustainable. Chem. Eng., 2017, 5, 8546–8550 CrossRef CAS
.
- B. Sandhu, A. S. Sinha, J. Desper and C. B. Aakeröy, Chem. Commun., 2018, 54, 4657–4660 RSC
.
-
(a) S. Parakatawella, D. Gogoi, P. Deka, Y. Xu, C. Sandaruwan, A. C. A. Jayasundera, M. Arhangelskis, R. Thakuria and N. M. Adassooriya, ChemSusChem, 2022, 15, e202102445 CrossRef CAS PubMed
; (b) T. Rajbongshi, S. Parakatawella, D. Gogoi, P. Deka, N. M. Adassooriya and R. Thakuria, RSC Sustain., 2023, 1, 1416–1422, 10.1039/D3SU00021D
.
-
(a) L. Casali, L. Mazzei, O. Shemchuk, K. Honer, F. Grepioni, S. Ciurli, D. Braga and J. Baltrusaitis, Chem. Commun., 2018, 54, 7637–7640 RSC
; (b) L. Casali, L. Mazzei, O. Shemchuk, L. Sharma, K. Honer, F. Grepioni, S. Ciurli, D. Braga and J. Baltrusaitis, ACS Sustain. Chem. Eng., 2019, 7, 2852–2859, DOI:10.1021/acssuschemeng.8b06293
; (c) L. Casali, V. Broll, S. Ciurli, F. Emmerling, D. Braga and F. Grepioni, Cryst. Growth Des., 2021, 21, 5792–5799, DOI:10.1021/acs.cgd.1c00681
.
-
(a) Antimicrobial resistance, https://www.who.int/news-room/factsheets/detail/antimicrobial-resistance;
(b) Antimicrobial resistance | European Medicines Agency, https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/antimicrobial-resistance;
(c) C. Ventola, The antibiotic resistance crisis: part 1: causes and threats, PT, 2015, 40(4), 277–283 Search PubMed
.
- G. Bolla, B. Sarma and A. K. Nangia, Chem. Rev., 2022, 122, 11514–11603, DOI:10.1021/acs.chemrev.1c0098756
.
- A. Kumar, S. Kumar and A. Nanda, Adv. Pharm. Bull., 2018, 8, 355–363, DOI:10.15171/apb.2018.042
.
- K. Nangia and G. R. Desiraju, Angew. Chem., Int. Ed., 2022, 61, 748 CrossRef PubMed
.
-
(a) O. Shemchuk, S. d’Agostino, C. Fiore, V. Sambri, S. Zannoli, F. Grepioni and D. Braga, Cryst. Growth Des., 2020, 20, 6796–6803, DOI:10.1021/acs.cgd.0c00900
, ISSN: 1528-7483; (b) C. Fiore, A. Baraghini, O. Shemchuk, V. Sambri, M. Morotti, F. Grepioni and D. Braga, Cryst. Growth Des., 2022, 22, 1467–1475 CrossRef CAS
.
-
(a) P. P. Mazzeo, C. Carraro, A. Monica, D. Capucci, P. Pelagatti, F. Bianchi, S. Agazzi, M. Careri, A. Raio, M. Carta, F. Menicucci, M. Belli, M. Michelozzi and A. Bacchi, ACS Sustain. Chem. Eng., 2019, 7, 17929–17940, DOI:10.1021/acssuschemeng.9b04576
; (b) F. Montisci, P. P. Mazzeo, C. Carraro, M. Prencipe, P. Pelagatti, F. Fornari, F. Bianchi, M. Careri and A. Bacchi, ACS Sustainable Chem. Eng., 2022, 10, 8388–8399, DOI:10.1021/acssuschemeng.2c01257
.
- M. Djaló, A. E. S. Cunha, J. P. Luís, S. Quaresma, A. Fernandes, V. André and M. T. Duarte, Cryst. Growth Des., 2021, 21, 995–1005, DOI:10.1021/acs.cgd.0c01346
.
- A. J. Smith, P. Kavuru, L. Wojtas, M. J. Zaworotko and R. D. Shytle, Mol. Pharmaceutics, 2011, 8, 1867–1876, DOI:10.1021/mp200209j
.
- M. K. C. Mannava, M. K. Bommaka, R. Dandela, K. Solomon and A. K. Nangia, Chem. Commun., 2022, 58, 5582–5585, 10.1039/d1cc07187d
.
-
(a) C. Fiore, O. Shemchuk, F. Grepioni, R. J. Turner and D. Braga, CrystEngComm, 2021, 23, 4494–4499, 10.1039/D1CE00612F
; (b) A. Lekhan, C. Fiore, O. Shemchuk, F. Grepioni, D. Braga and R. J. Turner, ACS Appl. Bio Mater., 2022, 5, 4203–4212 CrossRef CAS PubMed
; (c) M. Guerrini, S. d’Agostino, F. Grepioni, D. Braga, A. Lekhan and R. J. Turner, Sci. Rep., 2022, 12, 1–8 CrossRef PubMed
.
-
(a) R. J. Turner, Microb. Biotechnol., 2017, 10, 1062–1065, DOI:10.1111/1751-7915.12785
; (b) J. A. Lemire, J. J. Harrison and R. J. Turner, Nat. Rev. Microbiol., 2013, 11, 371–384, DOI:10.1038/nrmicro3028
; (c) T. Bjarnsholt, APMIS, 2013, 121, 1–58, DOI:10.1111/APM.12099
.
- O. Shemchuk, D. Braga, F. Grepioni and R. J. Turner, RSC Adv., 2020, 10, 2146–2149, 10.1039/C9RA10353H
.
- Generally Recognized as Safe (GRAS). Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 15 June 2022).
| This journal is © The Royal Society of Chemistry 2023 |