Open Access Article
Open Access ArticleSelection of diverse polymorphic structures from a small dynamic molecular network controlled by the environment†
Boris
Bartolec
,
Armin
Kiani
,
Meagan A.
Beatty
,
Meniz
Altay
,
Guillermo
Monreal Santiago
and
Sijbren
Otto
 *
*
Centre for Systems Chemistry, Stratingh Institute, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: s.otto@rug.nl
First published on 11th November 2022
Abstract
The complex interplay between systems and their environment plays an important role in processes ranging from self-assembly to evolution. Polymorphism, where, from the same ingredients different products can be formed, is likely to be an important enabler for evolutionary adaptation. Environmental pressures may induce polymorphic behaviour, where different pressures result in different structural organisation. Here we show that by combining covalent and non-covalent bond formation three distinct polymorphs can emerge from the same small dynamic molecular network: vesicular aggregates, self-replicating fibres and nanoribbons, depending on the nature of the solvent environment. Additionally, a particular set of conditions allows the transient co-existence of both vesicles and fibres.
Introduction
Considering the various chemical conditions that prevailed on Earth at the time life originated, the potential role of environmental parameters in both the origin and evolution of life is significant, but also poorly understood.1 Under sudden environmental changes, populations adapt to new conditions or risk extinction.2 This adaption can occur by means of various processes including that of polymorphism.3–4In biology, polymorphism refers to the emergence of multiple specific morphologies from a discrete species as a result of altering the environment.3 Even within a single cell, many biomacromolecules such as proteins,4 carbohydrates5 and lipids6 are able to assemble into different types of supramolecular polymorphs, while maintaining the chemical structures of the assembling molecules.7 This phenomenon suggests a subtle interplay of different self-assembly pathways that can be influenced by changes in non-covalent interactions between molecules. For example, changing pH or ionic strength can lead to different forms in tubular polymers of the tobacco mosaic virus capsid protein.8
Recently, a number of pioneering studies have shown polymorphism in supramolecular structures.9 Numerous examples have been reported of single building blocks systems that form diverse polymorphs by changes in the environment such as co-solvent, temperature and concentration.10 For example, Meijer and co-workers reported a family of carboxylic acid functionalized water-soluble benzene-1,3,5-tricarboxamides (BTAs) that self-assemble in water into either fibres, membranes, or hollow nanotubes depending on slight changes in the temperature.11 In another study, the same group reported three different polymorphs from a tetraamide-substituted biphenyl derivative that emerge from different concentrations of water.12
Whereas the role of the environment on supramolecular polymorphism has been widely studied, the effects of the environment on the emergence of various polymorphs combined with molecular structure transformation remains relatively rare. Allowing for the interplay between molecular structure and self-assembly adds a level of plasticity that should enhance the access to diverse polymorphs. It is perhaps not surprising that many key processes in biology rely on such interplay (e.g. enzyme catalysis, nucleic acid replication).
Dynamic combinatorial chemistry13 is a powerful tool for exploring the role of the environment in the emergence of polymorphs and structural adaption as it allows to amalgamate covalent and non-covalent interactions in a way that allows both processes to influence each other. Dynamic combinatorial libraries (DCLs) are formed from building blocks that react with one another forming reversible covalent bonds. The library members interconvert through exchange of building blocks and the library composition can be influenced by the addition of a template or through stabilization of inter/intramolecular interactions between library members. There are many examples of vesicles, micelles and self-replicating fibrous structures that have emerged from DCLs.14
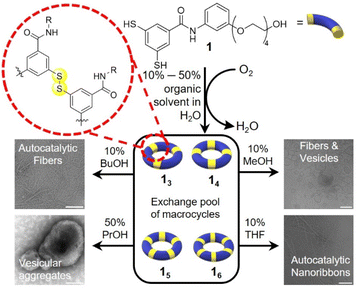
We now show that DCLs made from a single building block in aqueous solution give rise to the spontaneous emergence of various morphologies such as vesicular compartments, self-replicator fibres or both simultaneously. Which type of structures form is critically dependent on the environment and controlled by the nature of the organic co-solvent.
Results and discussion
We previously reported the formation of diverse membranous supramolecular structures in DCLs made from the amphiphilic building block 1.15 Upon air oxidation of 1 the dithiol motifs react with one another to form a library of macrocycles of various sizes linked by disulfide bonds. Upon changing the temperature and agitation, vesicles, nanosheets and sponge-like structures emerged. The fact that this system is sensitive to small perturbations prompted us to explore different environmental conditions by modifying the solvent composition. Co-solvents are well established as agents that break hydrophobic interactions16 and/or swell and fluidise the membrane core of vesicles.17 These effects rely heavily on the intercalation capabilities of solvent molecules within the assemblies. We envisaged that, using solvent mixtures to prepare DCLs, we would be able to further expand the range of morphologies of the self-assembled structures that can be accessed.We now show that simple alcohols and polar aprotic solvents offer a simple way to change the composition of DCLs made from building block 1 from membranous structures to fibres, nanoribbons, and vesicular aggregates and from a collection of large macrocycles to the selective formation of smaller oligomers (Scheme 1).
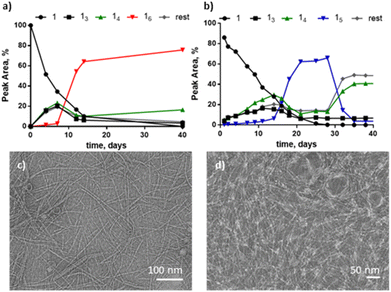
We first explored the DCL made from building block 1 in the presence of 1-propanol. Building block 1 (1.0 mM) was allowed to oxidise in the presence of 10% 1-propanol in B2O3 buffer and the library was monitored by UPLC over 45 days. Macrocycles 15 and 16 emerged together as the two dominant species in the library. Interestingly, different replicate samples yielded different ratios of 14/15/16, indicative of stochastic behaviour, similar to that observed previously with another building block.18 For instance, in the example in Fig. 1a, 16 completely dominates the library while in another sample, shown in Fig. 1b, 15 transiently formed and then gave way to 14 and other products. However, in most other samples either 16 or 15 were the dominant species (Fig. S1†). These results contrast with the library without co-solvent that forms a wide range of supramolecular structures within one library composed mainly of 14 and other large macrocycles.15
Analysis by TEM of samples dominated by 16 or 15 revealed fibres in both cases (Fig. 1c and d). By analogy with the previously observed stochastic systems,18 these results suggest that under these environmental conditions the system is close to the phase boundary between the different assemblies. At higher 1-propanol content (15%) stochastic behaviour was not observed and several repeats all gave 14 as the dominant macrocycle (Fig. S2†). Table S1† gives an overview of the results obtained in these and other solvent compositions.
The observation that fibre formation in the presence of cosolvent requires a minimum ring size is in line with previous observations on peptide-based analogues of 1, where the strength of the interactions between the individual building blocks dictates the minimal ring size for fibre formation.19
The structure of these fibres resembles that of previously reported peptide-based systems which were found to catalyse their own formation, which classifies them as self-replicators.14 In order to determine if our current ethylene oxide fibres behaved similarly, seeding experiments were conducted. The addition of pre-formed 16 fibre seed to a solution made from building block 1 (80% oxidized) revealed a faster growth of 16 comparison to its growth without seed, indicative of self-replication (Fig. S3†). A similar result was observed upon seeding with pre-formed 15 (Fig. S4†). These results indicate that 16 and 15 are self-replicators in this specific solvent environment.
Upon increasing the concentration of 1-propanol (to 50%) fibres no longer formed, but instead vesicular aggregates were obtained. After 1 month a sample made from building block 1 in the presence of 50% 1-propanol in B2O3 buffer yielded 14 as the dominant species (similarly to samples without co-solvent) (Fig. 2a). TEM micrographs revealed large vesicular aggregates with distinct periodic deformities and an average size of 50–100 nm (Fig. 2b and S5†). These unusual structures are very similar to the negatively stained multilamellar liposomes previously reported.20 Interested in the possible self-reproduction of these vesicle-like structures, we conducted similar seeding experiments as before. Pre-formed 14 solution was added to a sample made from 1, however, no autocatalysis in the formation of 14 was observed (Fig. S6†). We speculate that 1-propanol is likely to insert into the assemblies, increasing their hydrophobic volume. In analogy with the packing parameter21 analysis in surfactant chemistry, where increasing the hydrophobic volume of a surfactant causes a transition from (worm-like) micellar to bilayer aggregates, we suspect that such increase in hydrophobic volume might cause a transition from fibrous assemblies to vesicles. This transition is accompanied by a reduction in ring size to yield tetramers, which might pack better in the vesicular aggregates.
 | ||
| Fig. 2 (a) Larger quantities of 1-propanol (50%) promote the formation of 14 and (b) yields vesicular structures as evident from TEM analysis (negative staining). | ||
Using 1-butanol as a co-solvent at low concentrations yielded results that were similar to those obtained with 1-propanol at similar concentrations. Building block 1 (1.0 mM) was stirred (1200 rpm) at room temperature in the presence of 10% 1-butanol in B2O3 buffer (50 mM, pH 8.5) and the sample was monitored by UPLC over the course of 40 days. Initially, 15 dominated the library but after two weeks 15 partially gave way to 16. After 40 days, both macrocycles co-existed (Fig. S7a†) and TEM micrographs revealed the formation of fibrous structures (Fig. S7b†).
Also when using ethanol as the co-solvent the same macrocycles emerged, albeit more slowly. After 4 months, in a sample made from building block 1 in the presence of 10% ethanol, 15 and 16 dominated the library together and TEM micrographs again revealed fibre-like structures (Fig. S8†).
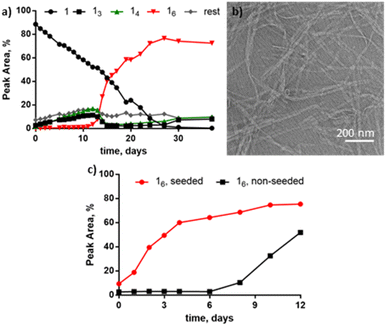
The presence of THF induces a novel nanoribbon structure not yet observed for this system. Building block 1 (1.0 mM) was stirred at room temperature in the presence of 10% THF in B2O3 buffer (50 mM, pH 8) and after 40 days 16 was the major species (Fig. 3a). However, unlike for the alcohols tested before, the TEM micrographs of the THF-containing sample revealed twisted nanoribbons (Fig. 3b). The formation of hexamer nanoribbons occurred in a relatively narrow window of solvent composition as DCLs at 30 and 50% THF were dominated by 14 (Table S1, Fig. S9†). To determine if the ring size or the morphology could be modified by mechanical agitation, two more samples were prepared with 10% THF that were either shaken (1200 rpm) or left non-agitated for 40 days. The shaken sample was dominated by 16 (Fig. S10a†) and resembled the stirred sample while the non-agitated sample contained mainly 13, 14 along with other larger macrocycles (e.g.15–17) and remained clear (Fig. S10b†). In contrast to the agitated samples, visual inspection of the non-agitated samples gave no indication for large-scale assembly formation (although we cannot exclude the presence of small aggregates). Seeding a fresh solution made from 1 with pre-formed nanoribbons from the shaken sample significantly accelerated the growth of 16, in comparison to the non-seeded sample (Fig. 3c), showing that also the nanoribbons form autocatalytically. Thus the hierarchical assembly of fibres into ribbons does not hamper autocatalysis, as was previously reported for the formation of tubular superstructures.22 Attempts to isolate individual macrocycles from the library for further characterization failed due to rapid re-equilibration.
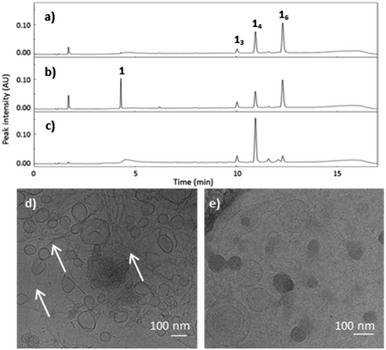
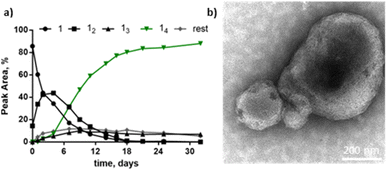
Methanol as the co-solvent promotes the co-existence of two distinct polymorphs. Building block 1 (1.0 mM) first formed 16 as the dominant species in the presence of 10% methanol in B2O3 buffer. After 2 weeks, the library shifted to form more 14 at the expense of 16, to yield a state in which the two macrocycles coexist (Fig. 4a). Cryo-TEM micrographs of the library at 2.5 months reveal both vesicles and fibres co-existing (Fig. 4d). Most vesicles were 50–100 nm in size, and exhibited a more spherical morphology and smooth surface (Fig. S11†) than the vesicular aggregates observed in the samples containing 50% 1-propanol, which featured surfaces with periodic patterns (Fig. S5†). After four months, 16 was completely consumed and 14 (along with smaller rings) became the dominant species. This suggests that 16 produced a metastable phase while 14 assembled into the thermodynamically more stable phase, in this particular solvent environment.
To confirm this difference in stability, the sample was partially reduced and allowed to re-oxidise, to promote thiol-mediated disulfide exchange. The addition of the reducing agent, tris(2-carboxyethyl)phosphine (TCEP) to the mixture containing 16, and 14 (Fig. 4a) did not reveal any differences in stability or reactivity as all molecular species were reduced to approximately the same extent (Fig. 4b). The partially reduced library was left to exchange and oxidise, resulting in a shift in its composition towards 14 after two weeks (Fig. 4c), in agreement with 16 being a metastable species while 14 prevails as the thermodynamic product. Cryo-TEM micrographs of the library containing 14 show the presence of only vesicular structures and the absence of fibres (Fig. 4e). These observations suggest that the two molecular species (14 and 16) form two distinct supramolecular structures with 14 as the main species present in vesicle membranes.
Conclusions
By modulating the environment of DCLs made from a single building block through addition of various co-solvents, we were able to produce four different polymorphic structures: fibres, nanoribbons, and two different vesicle morphologies. Both fibres and nanoribbons were self-replicating supramolecular structures, and in one set of conditions both fibres and vesicles transiently co-existed. Such significant differences in morphologies in a single building block system is important as each structure could in principle impart a unique functionality. For example, we have previously shown that self-replicating fibres derived from a DCL can catalyse catabolic reactions and bind to co-factors that enhance their formation.23Furthermore, compartments are crucial components in the synthesis of life. They protect the system against parasitic molecules and provide an environment for colocalizing and concentrating substrates and allow for excretion of toxic by-products.
Previously, we have shown the emergence and co-existence of two different supramolecular structures (fibres and foldamers) from a two-building block DCL, while in another example another two-building block system yielded four unique supramolecular structures.24 However, the ability to switch between significantly different polymorphs or creating both simultaneously, from a single-building-block DCL, as described herein, has not yet been reported.
The different polymorphs differ in the extent to which they can form through autocatalysis. The fibres and ribbons are formed autocatalytically, while the vesicles are not. We attribute this difference to the presence of catalytic sites at the ends of the fibres or ribbon. Previous work on peptide-based fibres has shown that the rate of replication is proportional to the fibre end concentration, where precursors were found to accumulate.25 Such catalytic sites are likely to be absent in the vesicles, as these are closed structures.
The fact that replication and vesicle-type compartments can form from the same building block and even transiently co-exist is intriguing from the perspective of the emergence of life, which requires replication and compartmentalisation.
From the observation that the switchover from one assembly morphology to another is accompanied by a change in covalent bonds (i.e., a change in the ring size of the macrocycles that assemble) we conclude that the mutual interplay between covalent bond formation and noncovalent interactions promotes polymorphism, and, through that, adaptability. Although the types of building blocks and conditions used in this study are not all prebiotically plausible, this work demonstrates the concept of structural adaption of synthetic systems in a response to a change in environment; one of many conditions needed for evolution. Thus, systems that allow for covalent and non-covalent dynamics appear particularly promising in the context of achieving evolutionary adaptations.
Data availability
The raw data generated and analysed during the current study are available from the authors on reasonable request.Author contributions
BB designed and carried out most of the experiments and wrote the first draft of the manuscript. AK and MAB placed the work in the broader context of polymorphism and co-wrote the manuscript. MA and GMS performed analytic experiments. SO supervised the project and co-wrote the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the ERC (AdG 741774), the NWO (VICI grant 724.012.002), Marie Sklodowska-Curie Grants (642192 and 101028779) and the Dutch Ministry of Education, Culture and Science (Gravitation Program 024.001.035).Notes and references
- I. Budin and J. W. Szostak, Annu. Rev. Biophys., 2010, 39, 245–263 CrossRef CAS PubMed.
- C. K. Ghalambor, J. K. McKay, S. P. Carroll and D. N. Reznick, Funct. Ecol., 2007, 21, 394–407 CrossRef.
- P. M. Sheppard, J. Med. Genet., 1971, 8, 545–546 CrossRef.
- J. Adamcik and R. Mezzenga, Angew. Chem., Int. Ed., 2018, 57, 8370–8382 CrossRef CAS PubMed.
- C. Gao and G. Chen, Acc. Chem. Res., 2020, 53, 740–751 CrossRef CAS PubMed.
- B. d. Kruijff, Curr. Opin. Chem. Biol., 1997, 1, 564–569 CrossRef PubMed.
- K. Ohtsubo and J. D. Marth, Cell, 2006, 126, 855–867 CrossRef CAS PubMed.
- K. Namba and G. Stubbs, Science, 1986, 231, 1401–1406 CrossRef CAS PubMed.
- F. Tantakitti, J. Boekhoven, X. Wang, R. V. Kazantsev, T. Yu, J. Li, E. Zhuang, R. Zandi, J. H. Ortony, C. J. Newcomb, L. C. Palmer, G. S. Shekhawat, M. O. de la Cruz, G. C. Schatz and S. I. Stupp, Nat. Mater., 2016, 15, 469–476 CrossRef CAS PubMed.
- (a) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS PubMed; (b) A. Langenstroer, K. K. Kartha, Y. Dorca, J. Droste, V. Stepanenko, R. Q. Albuquerque, M. R. Hansen, L. Sánchez and G. Fernández, J. Am. Chem. Soc., 2019, 141, 5192–5200 CrossRef CAS PubMed; (c) Y. La, T. H. An, T. J. Shin, C. Park and K. T. Kim, Angew. Chem., Int. Ed., 2015, 54, 10483–10487 CrossRef CAS PubMed; (d) D. Mandal, S. Dinda, P. Choudhury and P. K. Das, Langmuir, 2016, 32, 9780–9789 CrossRef CAS PubMed; (e) Y. Gao, J. Hao, J. Wu, X. Zhang, J. Hu and Y. Ju, Langmuir, 2016, 32, 1685–1692 CrossRef CAS PubMed; (f) P. A. Korevaar, S. J. George, A. J. Markvoort, M. M. J. Smulders, P. A. J. Hilbers, A. P. H. J. Schenning, T. F. A. De Greef and E. W. Meijer, Nature, 2012, 481, 492–496 CrossRef CAS PubMed; (g) B. Kemper, L. Zengerling, D. Spitzer, R. Otter, T. Bauer and P. Besenius, J. Am. Chem. Soc., 2018, 140, 534–537 CrossRef CAS PubMed; (h) A. Aliprandi, M. Mauro and L. De Cola, Nat. Chem., 2016, 8, 10–15 CrossRef CAS PubMed.
- N. M. Matsumoto, R. P. M. Lafleur, X. Lou, K.-C. Shih, S. P. W. Wijnands, C. Guibert, J. W. A. M. van Rosendaal, I. K. Voets, A. R. A. Palmans, Y. Lin and E. W. Meijer, J. Am. Chem. Soc., 2018, 140, 13308–13316 CrossRef CAS PubMed.
- N. J. Van Zee, B. Adelizzi, M. F. J. Mabesoone, X. Meng, A. Aloi, R. H. Zha, M. Lutz, I. A. W. Filot, A. R. A. Palmans and E. W. Meijer, Nature, 2018, 558, 100–103 CrossRef CAS PubMed.
- (a) F. B. L. Cougnon and J. K. M. Sanders, Acc. Chem. Res., 2012, 45, 2211–2221 CrossRef CAS PubMed; (b) E. Moulin, G. Cormos and N. Giuseppone, Chem. Soc. Rev., 2012, 41, 1031–1049 RSC; (c) S. Ladame, Org. Biomol. Chem., 2008, 6, 219–226 RSC; (d) P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652–3711 CrossRef CAS PubMed.
- J. M. A. Carnall, C. A. Waudby, A. M. Belenguer, M. C. A. Stuart, J. J.-P. Peyralans and S. Otto, Science, 2010, 327, 1502–1506 CrossRef CAS PubMed.
- B. Bartolec, G. Leonetti, J. Li, W. Smit, M. Altay, G. Monreal Santiago, Y. Yan and S. Otto, Langmuir, 2019, 35, 5787–5792 CrossRef CAS PubMed.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967–973 CrossRef CAS PubMed.
- (a) J. Boekhoven, A. M. Brizard, P. van Rijn, M. C. A. Stuart, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2011, 50, 12285–12289 CrossRef CAS PubMed; (b) R. P. M. Lafleur, X. Lou, G. M. Pavan, A. R. A. Palmans and E. W. Meijer, Chem. Sci., 2018, 9, 6199–6209 RSC.
- G. Schaeffer, M. J. Eleveld, J. Ottelé, P. C. Kroon, P. W. J. M. Frederix, S. Yang and S. Otto, J. Am. Chem. Soc., 2022, 144, 6291–6297 CrossRef CAS PubMed.
- M. Malakoutikhah, J. J. P. Peyralans, M. Colomb-Delsuc, H. Fanlo-Virgos, M. C. A. Stuart and S. Otto, J. Am. Chem. Soc., 2013, 135, 18406–18417 CrossRef CAS PubMed.
- A. D. Bangham, M. M. Standish and J. C. Watkins, J. Mol. Biol., 1965, 13, 238–252 CrossRef CAS PubMed.
- J. N. Israelachvili, H. E. Mitchel and J. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525 RSC.
- B. Rubinov, N. Wagner, M. Matmor, O. Regev, N. Ashkenasy and G. Ashkenasy, ACS Nano, 2012, 6, 7893–7901 CrossRef CAS PubMed.
- (a) J. Ottelé, A. S. Hussain, C. Mayer and S. Otto, Nat. Catal., 2020, 3, 547–553 CrossRef; (b) G. Monreal Santiago, K. Liu, W. R. Browne and S. Otto, Nat. Chem., 2020, 12, 603–607 CrossRef CAS PubMed.
- (a) B. Liu, M. A. Beatty, C. G. Pappas, K. Liu, J. Ottelé and S. Otto, Angew. Chem., Int. Ed., 2021, 60, 13569–13573 CrossRef CAS PubMed; (b) D. Komáromy, T. Tiemersma-Wegman, J. Kemmink, G. Portale, P. R. Adamski, A. Blokhuis, F. S. Aalbers, I. Marić, G. M. Santiago, J. Ottelé, A. Sood, V. Saggiomo, B. Liu, P. van der Meulen and S. Otto, Chem, 2021, 7, 1933–1951 CrossRef.
- (a) M. Colomb-Delsuc, E. Mattia, J. W. Sadownik and S. Otto, Nat. Commun., 2015, 6, 7427 CrossRef CAS PubMed; (b) S. Maity, J. Ottele, G. M. Santiago, P. Frederix, P. Kroon, O. Markovitch, M. C. A. Stuart, S. J. Marrink, S. Otto and W. H. Roos, J. Am. Chem. Soc., 2020, 142, 13709–13717 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sc03909e |
| This journal is © The Royal Society of Chemistry 2022 |