Open Access Article
Open Access ArticleHighly selective molecular sieving of cis- over trans-1,2-dichloroethene isomers†
Xin
Liu
,
Lukman O.
Alimi
and
Niveen M.
Khashab
 *
*
Smart Hybrid Materials Laboratory (SHMs), Advanced Membranes and Porous Materials Center, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: niveen.khashab@kaust.edu.sa
First published on 27th July 2022
Abstract
An intrinsically porous trianglimine macrocycle 1 is reported to display energy-efficient and cost-effective adsorptive properties by selectively separating cis-1,2-dichloroethene (cis-DCE) from an equimolar cis- and trans-DCE mixture with a purity of over 96%. The selectivity is enhanced by host/guest C–H⋯π intermolecular interactions. Moreover, the macrocycle can be reused many times without any decrease in performance, which further supports the sustainability of using molecular sieves in chemical separation.
Olefins are high-value raw materials in the chemical and petrochemical industries.1,2 The separation of pure olefin derivatives using conventional technology is energy-intensive and time-consuming.3,4 Haloalkenes are typical olefin derivatives, which are mainly produced by direct halogenation of alkenes and obtained as a mixture (cis–trans isomers).5,6 Each isomer has a specific application in the polymer industry and synthetic field.7–9 For instance, trans-1,2-dichloroethene (trans-DCE) is used in the extraction of rubber and as a refrigerant, while cis-1,2-dichloroethene (cis-DCE) is a foam-blowing additive.10,11 Thus, obtaining the pure cis- and trans-DCE isomers is of great industrial and economic importance. However, owing to the proximity of their molecular sizes and boiling points, it is still very challenging to employ fractional distillation with very high towers to separate the isomers with high purity.12,13 Adsorptive separation using porous materials, such as metal organic frameworks, offers an economic and energy-efficient solution but nonthermally-driven adsorptive separation for cis–trans alkene isomers is still relatively unexplored.14–23 Consequently, new adsorbents with good stability and economic feasibility are imperative and desirable towards the practical industrial separation of cis–trans isomers.24
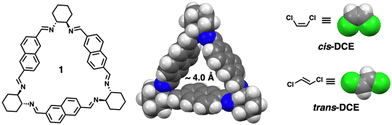
Trianglimine macrocycles are a class of intrinsically porous materials that can be easily prepared and scaled up for selective separations.25–28 In this work, we describe a trianglimine macrocycle 1 that can be used as a molecular sieve for efficient and successful separation of cis-DCE from an equimolar cis/trans-DCE mixture in both liquid and vapor phases (Scheme 1). The naphthalene sidewall increases the rigidity of the macrocycle and guest binding ability.29,30 Meanwhile, the removal of the trapped guests could transform the macrocycle 1 crystals back to the original guest-free form, endowing the macrocycle with excellent recyclability and reversibility (Fig. 1).
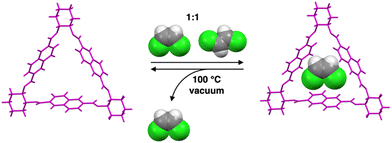 | ||
| Fig. 1 Crystal structure of macrocycle 1 showing the selectivity of cis-DCE over trans in a cis/trans-DCE mixture. | ||
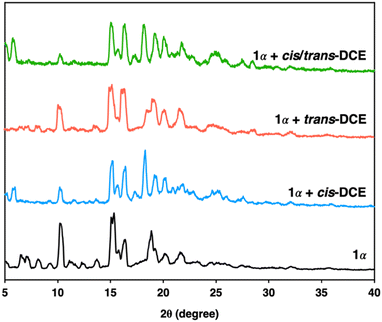
Macrocycle 1 was successfully prepared by an imine condensation reaction (Scheme S1, ESI†), as verified by detailed characterizations (Fig. S1–S3, ESI†). SCXRD analysis of macrocycle 1 in dichloromethane (DCM) (1A) showed a rigid intrinsic cavity of ca. 4.0 Å and demonstrated that macrocycle 1A crystallises in a monoclinic system with a P21 space group and the asymmetric unit contains one unit each of the macrocycle and DCM (Fig. S4 and Table S1, ESI†). The structure analysis also indicated that the macrocycle 1A is packed in a head–tail fashion along the b axis where the DCM occupied the channels (Fig. S5, ESI†). The activated macrocycle (1α) was obtained after activation of 1A at 80 °C for 24 h under a high vacuum. Thermogravimetric analysis (TGA) showed that macrocycle 1A loses DCM to obtain the guest-free material 1α that was used for further adsorption experiments (Fig. S6, ESI†). PXRD analysis revealed that 1α retains its crystallinity even after the removal of solvent molecules from the channels (Fig. 1 and Fig. S7, ESI†). The macrocycle 1α did not adsorb N2 at 77 K and CO2 at 298 K, indicating a nonporous material (Fig. S8, ESI†). However, previous reports based on trianglimine with approximate porosity showed significant molecular recognition features.26,28
To investigate the porosity and selectivity of crystalline 1α towards cis/trans-DCE isomers, single component and equimolar isomer mixture solid–vapor adsorption experiments were conducted at 298 K for commercially pure cis- and trans-DCE. The 1H NMR result revealed the significant uptake of cis-DCE and the negligible uptake of trans-DCE and confirmed the selective adsorption of cis-DCE over trans-DCE from the mixture with a high selectivity of over 92% (Fig. S9–S11, ESI†). PXRD experiments were performed to further support the preference of 1α towards cis-DCE over trans-DCE. PXRD patterns of 1α displayed structural transformation upon exposure to cis-DCE, indicating the adsorption of cis-DCE. However, the PXRD patterns of 1α showed negligible change after being exposed to trans-DCE. Moreover, 1α after exposure to an equimolar mixture of cis/trans-DCE vapors showed preferred adsorption of cis-DCE over trans-DCE (Fig. 2).
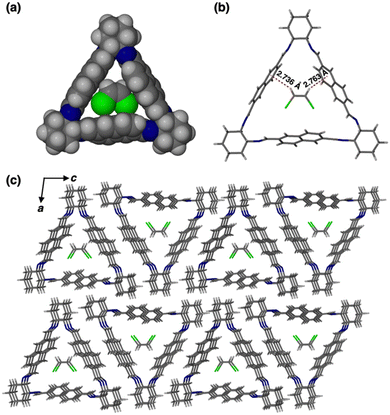
To better understand the host–guest interactions in the guest-induced adsorption process, single crystals of 1 with cis-DCE and trans-DCE were grown by vapor diffusion of methanol into a cis- or trans-DCE solution.26,31 A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex formed for cis-DCE loaded 1 (cis-DCE@1, Fig. 3a). SCXRD analysis indicated that the complex crystallises in a monoclinic crystal system with P21 space group and the asymmetric unit contains one unit of each macrocycle and cis-DCE (Fig. S12 and Table S1, ESI†). The space-filling model revealed that cis-DCE best fits in the intrinsic cavity of 1 (Fig. 3a). Each cis-DCE entrapped in the intrinsic cavity of 1 is stabilized by host–guest C–H⋯π interaction (Fig. 3b and Table S2, ESI†). The macrocycle units are connected to one another by C–H⋯π and C–H⋯N interactions to form a layered structure (Fig. S12e and Table S3, ESI†). The window to window packing mode of macrocycle molecules contributes to the formation of channels that can trap cis-DCE in the internal cavity of the aligned macrocycles along b axis (Fig. 3c). Thermogravimetric analysis (TGA) showed a weight loss of 9.1% up to 100 °C, indicating a 1
1 host–guest complex formed for cis-DCE loaded 1 (cis-DCE@1, Fig. 3a). SCXRD analysis indicated that the complex crystallises in a monoclinic crystal system with P21 space group and the asymmetric unit contains one unit of each macrocycle and cis-DCE (Fig. S12 and Table S1, ESI†). The space-filling model revealed that cis-DCE best fits in the intrinsic cavity of 1 (Fig. 3a). Each cis-DCE entrapped in the intrinsic cavity of 1 is stabilized by host–guest C–H⋯π interaction (Fig. 3b and Table S2, ESI†). The macrocycle units are connected to one another by C–H⋯π and C–H⋯N interactions to form a layered structure (Fig. S12e and Table S3, ESI†). The window to window packing mode of macrocycle molecules contributes to the formation of channels that can trap cis-DCE in the internal cavity of the aligned macrocycles along b axis (Fig. 3c). Thermogravimetric analysis (TGA) showed a weight loss of 9.1% up to 100 °C, indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest ratio of 1 and cis-DCE (Fig. S13, ESI†). However, the single crystal of 1 grown in trans-DCE showed only the macrocycle crystals without the trans-DCE guest molecule in its cavity (Fig. S14, ESI†). This reveals that macrocycle 1 is selective towards the cis-DCE isomer. Furthermore, PXRD patterns of cis- and trans-DCE with 1 match well with the simulated pattern (Fig. S15a and b, ESI†).
1 host–guest ratio of 1 and cis-DCE (Fig. S13, ESI†). However, the single crystal of 1 grown in trans-DCE showed only the macrocycle crystals without the trans-DCE guest molecule in its cavity (Fig. S14, ESI†). This reveals that macrocycle 1 is selective towards the cis-DCE isomer. Furthermore, PXRD patterns of cis- and trans-DCE with 1 match well with the simulated pattern (Fig. S15a and b, ESI†).
This observation prompted us to investigate the competitive crystallisation of 1 with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v mixture of cis- and trans-DCE. The obtained crystals revealed an exact structure of cis-DCE@1 (Fig. S16 and Table S1, ESI†). GC analysis further confirmed that cis-DCE isomer was selectively absorbed and separated with a high purity of over 99% (Fig. S17, ESI†). PXRD patterns and TGA of competitive experiments were further tested for an equimolar mixture of cis- and trans-DCE, which also supported the selectivity towards cis isomer (Fig. S15c and S18, ESI†). It was found that the internal cavity was stabilized by cis-DCE via C–H⋯π interaction in the crystal structure, while trans-DCE is unable to penetrate the cavity due to the size and shape mismatch that was also reflected in the selectivity (Fig. S16, ESI†). In addition to testing the selectivity with equimolar mixtures, we also performed different fractions of cis/trans-DCE mixtures and found that the selectivity toward cis-DCE still can be detected even with 2
1 v/v mixture of cis- and trans-DCE. The obtained crystals revealed an exact structure of cis-DCE@1 (Fig. S16 and Table S1, ESI†). GC analysis further confirmed that cis-DCE isomer was selectively absorbed and separated with a high purity of over 99% (Fig. S17, ESI†). PXRD patterns and TGA of competitive experiments were further tested for an equimolar mixture of cis- and trans-DCE, which also supported the selectivity towards cis isomer (Fig. S15c and S18, ESI†). It was found that the internal cavity was stabilized by cis-DCE via C–H⋯π interaction in the crystal structure, while trans-DCE is unable to penetrate the cavity due to the size and shape mismatch that was also reflected in the selectivity (Fig. S16, ESI†). In addition to testing the selectivity with equimolar mixtures, we also performed different fractions of cis/trans-DCE mixtures and found that the selectivity toward cis-DCE still can be detected even with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 mixtures (Fig. S19, ESI†).
8 mixtures (Fig. S19, ESI†).
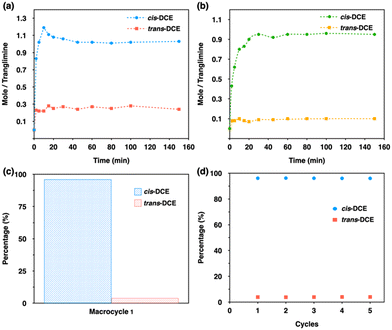
To measure the detailed pathway and uptake rate for the selectivity of cis isomer, time-dependent solid–vapor adsorption studies were performed for single-component and equimolar mixtures. The uptake capacity for single-component increases very fast and reaches saturation within 40 min with ca. 1.0 cis-DCE molecule by one trianglimine. However, it was found that the uptake of trans isomer reaches saturation within 20 min with ca. 0.3 molecule by one macrocycle (Fig. 4a). Then we studied the time-dependent solid–vapor experiment for an equimolar mixture of cis- and trans-DCE using 1α. The result revealed that the uptake of cis-DCE in 1α increased over time and reached saturation within 40 min, while the adsorption of trans isomer was almost negligible throughout the entire period (Fig. 4b). The cis isomer uptake amount was one molecule per macrocycle. GC analysis confirmed the high selectivity towards cis isomer with a purity of 96.6% (Fig. 4c and Fig. S20, ESI†).
As we established that 1α shows high selectivity towards cis-DCE over trans-DCE in both liquid and vapor phases (Fig. S21, ESI†), it is necessary to make it practically useful. In the industrial separation process, an adsorbent must be recyclable with reproducible applicability. In this case, recyclability and stability of crystalline 1A were confirmed accordingly by GC and PXRD over five cycles with no significant loss in selectivity or performance (Fig. 4d and Fig. S22, ESI†).
Finally, we investigated the stability of the macrocycle 1 under possible harsh industrial conditions such as a highly basic, acidic and humid environment using 1H NMR. 1H NMR spectrum confirms that the macrocycle 1 remains stable after being kept in 1 M NaOH aqueous solution for five days. However, the incubation of macrocycle 1 in acidic conditions degrades the macrocycle. Moreover, the stability of the macrocycle 1 was also tested in an aqueous solution where it shows no sign of decomposition or dissolution after several days (Fig. S23 and S24, ESI†).
We have successfully demonstrated the selective properties of an intrinsically porous macrocycle 1 toward cis-and trans-DCE in solution and vapor phases with a high selectivity of over 96% for multiple cycles. Crystal structure analysis shows that the naphthalene side wall plays an important role due to the high rigidity of the macrocycle and increasing guest binding affinity by C–H⋯π interaction. The ease of fabrication, high separation efficiency, and good stability make macrocycle 1 a promising candidate for molecular sieves-based separation of isomers under mild conditions in the future.
Conflicts of interest
There are no conflicts to declare.Notes and references
- D. J. Safarik and R. B. Eldridge, Ind. Eng. Chem. Res., 1998, 37, 2571–2581 CrossRef CAS.
- A. H. Tullo, Chem. Eng. News, 2000, 78, 21–31 Search PubMed.
- E.-L. Dreher, K. K. Beutel, J. D. Myers, T. Lubbe, S. Krieger and L. H. Pottenger, Ullmann's Encyclopedia of Industrical Chemistry, Wiley, 2014 Search PubMed.
- R. B. Eldridge, Ind. Eng. Chem. Res., 1993, 32, 2208–2212 CrossRef.
- Y. Zhou, K. Jie, R. Zhao and F. Huang, J. Am. Chem. Soc., 2019, 141, 11847–11851 CrossRef CAS PubMed.
- J.-R. Wu, B. Li and Y.-W. Yang, Angew. Chem., Int. Ed., 2020, 59, 2251–2255 CrossRef CAS PubMed.
- X. Fang, L. Liu, J. Lei, D. He, S. Zhang, J. Zhou, F. Wang, H. Wu and H. Wang, Nat. Mach. Intell., 2022, 4, 127–134 CrossRef.
- H. Futamata, N. Yoshida, T. Kurogi, S. Kaiya and A. Hirashi, ISME J., 2007, 1, 471–479 CrossRef CAS PubMed.
- Y. Nakayama, A. Ishikawa, R. Sato, K. Uchida and N. Kambe, Polym. J., 2008, 40, 1060–1066 CrossRef CAS.
- Encyclopedia of Toxicology, Philip Wexler, ISBN 9780123864550.
- Y. Wang, K. Xu, B. Li, L. Cui, J. Li, X. Jia, H. Zhao, J. Fang and C. Li, Angew. Chem., Int. Ed., 2019, 58, 10281–10284 CrossRef CAS PubMed.
- K. A. Marshall, Chlorocarbons and Chlorohydrocarbons, Survey, Kirk-Othmer Encyclopedia of Chemical Technology, Wiley-Interscience, New York, 2003 Search PubMed.
- A. E. Wentink, D. Kockmann, N. J. M. Kuipers, A. B. de Haan, J. Scholtz and H. Mulder, Sep. Purif. Technol., 2005, 43, 149–162 CrossRef CAS.
- A. Cadiau, K. Adil, P. M. Bhatt, Y. Belmabkhout and M. Eddaoudi, Science, 2016, 353, 137–140 CrossRef CAS PubMed.
- Y. Wu and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2021, 60, 18930–18949 CrossRef CAS PubMed.
- H. Liu, Y. He, J. Jiao, D. Bai, D. Chen, R. Krishna and B. Chen, Chem. – Eur. J., 2016, 22, 14988–14997 CrossRef CAS PubMed.
- M. Maes, L. Alaerts, F. Vermoortele, R. Ameloot, S. Couck, V. Finsy, J. F. M. Denayer and D. E. De Vos, J. Am. Chem. Soc., 2010, 132, 2284–2292 CrossRef CAS PubMed.
- K. Jie, Y. Zhou, E. Li, R. Zhao, M. Liu and F. Huang, J. Am. Chem. Soc., 2018, 140, 3190–3193 CrossRef CAS PubMed.
- G. Zhang, B. Hua, A. Dey, M. Ghosh, B. A. Moosa and N. M. Khashab, Acc. Chem. Res., 2021, 54, 155–168 CrossRef CAS PubMed.
- J. Pei, K. Shao, L. Zhang, H.-M. Wen, B. Li and G. Dong, Top. Curr. Chem., 2019, 377, 33 CrossRef PubMed.
- P.-Q. Liao, N.-Y. Huang, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Science, 2017, 356, 1193–1196 CrossRef CAS PubMed.
- Z. Zhang, X. Cui, X. Jiang, Q. Ding, J. Cui, Y. Zhang, Y. Belmabkhout, K. Adil, M. Eddaoudi and H. Xing, Engineering, 2021, 11, 80–86 CrossRef.
- Z. Zhang, Q. Yang, X. Cui, L. Yang, Z. Bao, Q. Ren and H. Xing, Angew. Chem., Int. Ed., 2017, 56, 16282–16287 CrossRef CAS PubMed.
- B. Moosa, L. O. Alimi, A. Shkurenko, A. Fakim, P. M. Bhatt, G. Zhang, M. Eddaoudi and N. M. Khashab, Angew. Chem., Int. Ed., 2020, 59, 21367–21371 CrossRef CAS PubMed.
- A. Chaix, G. Mouchaham, A. Shkurenko, P. Hoang, B. Moosa, P. M. Bhatt, K. Adil, K. N. Salama, M. Eddaoudi and N. M. Khashab, J. Am. Chem. Soc., 2018, 140, 12571–12575 CrossRef PubMed.
- Y. Ding, A. Dey, L. O. Alimi, P. M. Bhatt, J. Du, C. Maaliki, M. Eddaoudi, J. Jacquemin and N. M. Khashab, Chem. Mater., 2022, 34, 197–202 CrossRef CAS.
- A. Dey, S. Chand, B. Maity, P. M. Bhatt, M. Ghosh, L. Cavallo, M. Eddaoudi and N. M. Khashab, J. Am. Chem. Soc., 2021, 143, 4090–4094 CrossRef CAS PubMed.
- B. Hua, Y. Ding, L. O. Alimi, B. Moosa, G. Zhang, W. S. Baslyman, J. Sessler and N. M. Khashab, Chem. Sci., 2021, 12, 12286–12291 RSC.
- X. Wang, F. Jia, L.-P. Yang, H. Zhou and W. Jiang, Chem. Soc. Rev., 2020, 49, 4176–4188 RSC.
- X. Huang, X. Wang, M. Quan, H. Yao, H. Ke and W. Jiang, Angew. Chem., Int. Ed., 2021, 60, 1929–1935 CrossRef CAS PubMed.
- J. Cao, Y. Wu, Q. Li, W. Zhu, Z. Wang, Y. Liu, K. Jie, H. Zhu and F. Huang, Chem. Sci., 2022, 13, 7536–7540 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2177715–2177718. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc03574j |
| This journal is © The Royal Society of Chemistry 2022 |