Open Access Article
Open Access ArticleZr4+-terephthalate MOFs with 6-connected structures, highly efficient As(III/V) sorption and superhydrophobic properties†
Anastasia D.
Pournara
a,
Sofia
Rizogianni
a,
Dimitrios A.
Evangelou
a,
Evangelos K.
Andreou
b,
Gerasimos S.
Armatas
 b and
Manolis J.
Manos
b and
Manolis J.
Manos
 *ac
*ac
aDepartment of Chemistry, University of Ioannina, GR-45110, Ioannina, Greece. E-mail: emanos@uoi.gr
bDepartment of Materials Science and Technology, University of Crete, GR-71003, Heraklion, Greece
cInstitute of Materials Science and Computing, University Research Center of Ioannina, GR-45110, Ioannina, Greece
First published on 14th July 2022
Abstract
The use of terephthalate ligands with CnH2n+1NH-chains (n ≥ 6) led to the isolation of the first examples of Zr4+-terephthalate MOFs with 6-connected frameworks. The material with hexyl-amino functional groups has been proved to be an exceptional sorbent for the removal of As(III/V) toxic species from aqueous media, whereas MOFs with heptyl to dodecyl-amino moieties are superhydrophobic with promising oil–water separation properties.
Metal Organic Frameworks (MOFs) based on Zr4+ and multi-carboxylate ligands have attracted tremendous interest, as they combine all unique attributes of MOFs with exceptional hydrolytic stability.1 MOFs with terephthalate ligands and derivatives of them, denoted as UiO-66 MOFs, are typical examples with 12-c nets (fcu topology), which however often present structural defects so that their overall net connectivity appears <12.2 Our group has recently reported several Zr4+-terephthalate MOFs, bearing picolyl-2-ammonium or CnH2n+1NH-(n ≤ 4) groups, with decreased number of linkers and 8-c frameworks.3 The stabilization of such nets vs. the 12-c frameworks results from the effect of functional groups causing increased steric interactions between the ligands.3,4 As a result of missing dicarboxylate linkers, the MOFs with the 8-c frameworks include several terminal water/hydroxy ligands.5 The latter can be easily replaced by toxic or radioactive oxoanions and thus, these materials have been proved excellent sorbents for the removal of harmful oxoanionic species from aqueous media.3b,6 Examples of Zr4+-terephthalate MOFs with less than 8 linkers per Zr6 clusters remain undiscovered. The development of synthetic strategies towards such MOFs could be of significant interest for the isolation of materials with enhanced capability towards sorption of toxic species due to the increased number of the easily replaceable terminal ligands (e.g. 12 terminal water/OH− in 6-c nets vs. 8 terminal water/OH− ligands in 8-c frameworks).1d Furthermore, such MOFs would be particularly suitable as precursor compounds for producing new materials with tuned properties through post-synthetic incorporation of functional molecules or organic linkers.7
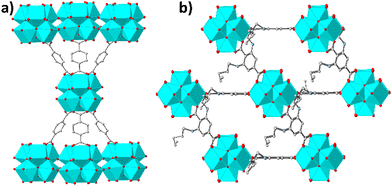
Here we report the initial examples of Zr4+-terephthalate MOFs with 6-c nets, namely MOFs with the general formula H22[Zr6O20(NH2-BDC)x(RNH-BDC)3−x]·solvent (x ≤ 0.18, NH2-BDC2− = 2-amino-terephthalate,RNH-BDC2− = 2-alkyl-amino terephthalate; R = hexyl-, HEX-MOF; R = heptyl-, HEPT-MOF; R = octyl-, OCT-MOF; R = nonyl-, NON-MOF; R = dodecyl-, DODEC-MOF) (Fig. S1 and S2, ESI†). The new compounds are of interest not only due to their structural characteristics, but also because of the promising As(III)/(v) sorption properties of HEX-MOF and the oil–water separation capability of the superhydrophobic (HEPT-, OCT-, NON-, DODEC-MOF) analogues.
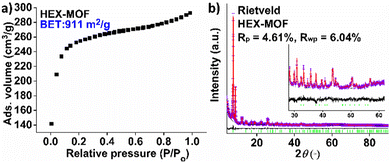
Our initial motivation for the isolation of MOFs with long alkyl chains was the development of new hydrophobic/superhydrophobic materials. At the beginning, we targeted towards a MOF with octyl-amino functional groups, by employing solvothermal reactions in DMF-CH3COOH at 120 °C (ESI†). Surprisingly, the BET surface areas of several samples tested were found 726–733 m2 g−1, which is significantly higher from e.g. the reported MOF with the much shorter n-butyl-amino group (609 m2 g−1).3b Then, we have decided to prepared MOFs with hexyl-, heptyl-, nonyl and dodecyl-amino groups. Again, the surface areas, especially for HEX-MOF (911 m2 g−1) and HEPT-MOF (792 m2 g−1), were much higher than those of known MOFs with shorter alkyl-chains (Fig. 1a, Fig. S3 and Table S1, ESI†). At the same time, TGA data were consistent with the presence of 3 rather than 4 dicarboxylate ligands per Zr6 cluster (Fig. S4–S8 and Table S2, ESI†). These results indicated structures with 6-connected frameworks. We have thus built and optimized (via simulating annealing methods) models of the MOFs with 6-c nets, which were used as starting points for Rietveld refinement (Fig. 1b and Fig. S9–S16 and refinement details in ESI†). The results of the refinements were satisfactory, which is a confirmation of the accuracy of the proposed structural models (Fig. 2 and Fig. S1, ESI†). Analysis of the structural topology of the MOFs indicate the pcu-net for these materials (Fig. S17, ESI†), which has been rarely observed for Zr4+ MOFs.8 Importantly, the calculated (via poreblazer)9 surface areas for the MOFs with 6-c nets are close to the experimental ones (Table S1, ESI†). For comparison, the theoretical BET surface area for e.g. a hypothetical HEX-MOF with 8-c net was found 502 m2 g−1 (i.e. much smaller than the experimental value of 911 m2 g−1) (Fig. 1a). Despite the low net connectivity, HEX-MOF remains highly crystalline after treatment with either strongly acidic (pH∼0) or alkaline (pH∼12) solution for 12 h, as shown by PXRD data and Le Bail analysis (Fig. S18, ESI†). Furthermore, the crystallinity of HEX-MOF is largely retained after treatment of the MOF at 150 and 200 °C under vacuum (Fig. S19 and S20, ESI†).
We should also note that 1H NMR data for the MOFs digested in NaOH/D2O revealed that the linkers largely preserve their integrity in the MOFs’ structures, since the decomposition of alkyl-amino-terephthalate to (non-alkylated) amino-terephthalate ligands was found ≤6% (ESI† and Fig. S21). Additional characterization data (IR, SEM) for the MOFs are provided in ESI† (Fig. S22–S27). We should also mention that the TGA and BET surface area data for the MOF with pentyl-amino functional groups were inconsistent from sample to sample, whereas MOFs with decyl- and undecyl-amino BDC2− linkers showed limited crystallinity. Thus, the above MOFs were not studied in detail.
The reason for the stabilization of 6-c nets for the HEX-, HEPT-, OCT- and DODEC-MOFs is clearly related to the steric interactions between the side alkyl-amino groups that permit the accumulation of limited CnH2n+1NH-BDC2− linkers around the Zr6 clusters. From the previous publication of our group,3b it turns out that the steric interactions between side groups with ethyl to butyl moieties allow the incorporation of 4 linkers per Zr6 unit. However, based on the experimental data presented here, these interactions appear to be significantly stronger for ligands with longer alkyl-amino chains, thus leading to the stabilization of 6-c nets.
HEX-MOF shows a highly porous structure and multiple labile (terminal) water/hydroxy ligands. The latter are known to be easily replaced by anions or neutral molecules.7a Thus, we investigated the capability of HEX-MOF to sorb As(III/V) species, which are well known for their toxic effects, in detail. As(III/V) variable-time sorption studies revealed that both toxic species are removed from aqueous solutions within a few minutes. Kinetic data obeyed to the pseudo-second order model (Fig. S28 and Table S3, ESI†). Such a kinetic model indicates strong interactions between sorbed species and MOF sorbent. Importantly, after the 2nd min of contact, the As content was calculated to be lower than the upper limit of 10 ppb which has been established by WHO and US-EPA for As in drinking water (Fig. 3a). The As(v) sorption isotherm data of HEX-MOF can be fitted very well with the Langmuir model showing maximum sorption capacity as high as 104 ± 8 mg As g−1 of sorbent (Fig. 3c and Table S4, ESI†), close to the calculated value (116 mg g−1) for the incorporation of 3 As per formula unit. In contrary, the As(III) sorption isotherm consists of two components, which can be attributed to a combination of surface binding and pore filling processes.1f The total As sorption capacity was calculated to be 198 ± 5 mg As g−1 (Fig. 3b and Table S4, ESI†), whereas the sorption capacity (98 mg As g−1) observed in the second step is in good agreement with the calculated value for the insertion of 3 As species per Zr6 cluster. The As(III/V)-loaded sorbent can be easily regenerated by treatment with acidic solution, followed by washing with a basic MeOH solution. The regenerated sorbent fully retained the As(III/V) sorption efficiency and the crystallinity of the pristine material even after 4 cycles of sorption (Fig. S29 and S30, ESI†). Further sorption studies, conducted on As-containing samples of a wide pH range from pH∼1 to pH∼12, revealed that HEX-MOF retains its efficiency to remove As(III/V) even under highly acidic or alkaline conditions with sorption percentages higher than 96% (Fig. S31, ESI†). Furthermore, the sorption capacity of HEX-MOF toward As species is exceptional (removal percentages >95%) even in the presence of 1000-fold excess of several anions, including the highly competitive HPO42− species (Fig. S32, ESI†). In addition, HEX-MOF showed excellent performance (removal percentages 98.8–99.8%) for the removal of As(III) and As(v) from genuine water samples (Fig. S32, ESI†) containing a mixture of cations and anions in relatively high concentrations (Table S5, ESI†). Aiming in practical applications, we have also immobilized HEX-MOF in cotton fabric, which was pre-coated with polydopamine (PDA). PDA includes several catecholic units, which can form strong bonds with Zr4+ centers. Thus, PDA-coated substrates are ideal for immobilizing Zr4+ MOFs.2e Indeed, we have been able to fabricate cotton materials loaded with HEX-MOF by applying an in situ coating method (Fig. S33, ESI†). HEX-MOF@cotton fabric was utilized for treatment of natural spring water intentionally contaminated with As(v) (93 ppb) under continuous flow conditions and was found capable of decontaminating more than 3 L of the polluted water sample (Fig. 3d and Fig. S34, ESI†).
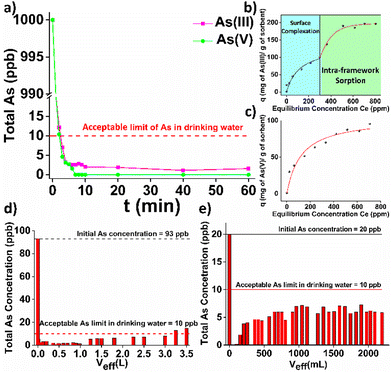 | ||
| Fig. 3 (a) Kinetic study of HEX-MOF towards As(III) (purple line) and As(v) (green line). (Initial As concentration = 1000 ppb), (b) As(III) and (c) As(v) sorption isotherms for HEX-MOF at pH = 7. The solid lines represent the fitting of the data with the isotherms models (ESI†). (d) As(v) sorption under continuous flow conditions for HEX-MOF@cotton fabric (initial As concentration = 93 ppb in artificially contaminated bottled water). (e) As(III) sorption under continuous flow conditions for HEX-MOF/calcium alginate beads (initial As concentration = 20 ppb in artificially contaminated bottled water). The As content, in all above data, represents the total As amount and not that of oxo-arsenic species. | ||
As an alternative approach to use HEX-MOF for removal of toxic species under flow, we have prepared HEX-MOF/calcium alginate beads which have been used as a stationary phase in sorption columns for removal of As(III) from artificially contaminated bottled water solution (Fig. S34 and S35, ESI†). Similarly to the immobilized fabric sorbent, HEX-MOF in the form of beads achieved to reduce the amount of As in the effluent under the acceptable limit in drinking water, even after 2 L have been passed through the column (Fig. 3e).
A detailed comparison of HEX-MOF with other state-of-the art sorbents (Tables S6 and S7, ESI†) revealed that HEX-MOF is among the best As(III/V) sorbents in terms of sorption kinetics, combining also appreciable As(III/V) maximum sorption capacities. Importantly, the As(III/V) sorption of HEX-MOF is retained exceptional even in the presence of tremendous excess (up to 1000-fold) of competitive species and in a wide pH range (1–12), with such performance not shown by the reported sorbents. Not least, HEX-MOF is a rare case of a MOF with demonstrated efficiency for capture of As(III/V) under realistic conditions and continuous flow.
The zeta potential of HEX-MOF was found close to 0 (0.148 mV). Thus, it is unlikely that HEX-MOF will interact with As(v) oxoanions via electrostatic interactions. Furthermore, 1H NMR data on filtrates after sorption studies in D2O (ESI†) revealed no release of organic species and thus, the sorption process does not proceed via exchange of organic ligands by As(v) oxoanions (Fig. S36, ESI†). Considering that (a) HEX-MOF absorbs 3 moles of As(v) per formula unit, (b) As(v) exists as a mixture of H2AsO4− and HAsO42− at pH = 7,10 and (c) the hard Zr4+ will prefer coordination with the divalent HAsO42− rather than the monoanionic H2AsO4−, the As(v) sorption process may be described with the following equation:
| [H22Zr6O20(C6H13NHBDC)3] + 3 HAsO42− → [H16Zr6O14(HAsO4)3(C6H13NHBDC)3] + 6 OH− | (1) |
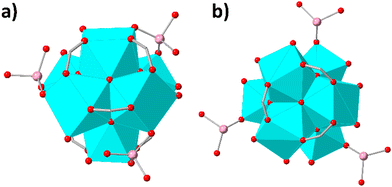
Thus, 3 HAsO42− anions replace 6 terminal hydroxyl ligands and each of the As(v) oxoanions is expected to be inserted as bridging ligand to the Zr6 cluster unit (Fig. 4a). Rietveld refinement confirmed the accuracy of the structure with 3 HAsO42− bridging ligands per Zr6 cluster (Fig. S37, ESI†). Finally, the high preference of HEX-MOF towards As(v) anions, as demonstrated via sorption experiments in the presence of multiple ions in large excess, is attributed to the excellent ligation capability of As(v) species for Zr4+ ions.2e
In addition, taking into account that (a) As(III) is present as the neutral H3AsO3 form at pH = 7,10 and (b) 3 As(III) species (per formula unit) are inserted inside the framework of HEX-MOF, the intra-framework As(III) sorption process can be described via the following equation:
| [H22Zr6O20(C6H13NHBDC)3] + 3 H3AsO3 → [H16Zr6O17(H3AsO3)3(C6H13NHBDC)3] + 3 H2O | (2) |
Therefore, 3 H3AsO3 exchange 3 terminal H2O ligands and each of the inserted As(III) species may be coordinated as monodentate ligand to the Zr4+ ions of the cluster unit (Fig. 4b). Again, Rietveld refinement verified the reliability of the proposed structural model (Fig. S37, ESI†). Further verification of the facile exchange of water by As(III) species was provided via theoretical calculations revealing higher charge of O atoms in H3AsO3vs. that of O in H2O (Fig. S38, ESI†) and thus, a more powerful ligation capability of As(III) towards the hard Zr4+ ions.
In addition, XPS data confirmed the oxidation states (III) and (v) for the As species in As(III) and As(v)-loaded materials respectively (Fig. S39, ESI†). Further characterization of the As(v) and As(III)-loaded materials has been performed via IR, BET surface area measurements and EDS analysis (Fig. S40–S42, ESI†).
The MOFs described here are based on linkers with long alkyl chains and thus, are expected to display hydrophobic or superhydrophobic properties.11 Indeed, the water contact angles for the HEPT-, OCT-, NON- and DODEC-MOFs were determined 150–154 deg implying superhydrophobicity (Fig. S43 and S44, ESI†). In contrast, HEX-MOF is readily wettable by water (contact angles < 10 deg). In addition, the superhydrophobic MOFs, e.g.DODEC-MOF, are easily dispersed in non-polar solvents and are not miscible with water, in contrast to the hydrophilic HEX-MOF forming suspension in aqueous media (Fig. S45, ESI†). We should also mention that HEPT-, OCT-, NON- and DODEC-MOFs retain their superhydrophobic properties in different types of aqueous media (distilled water, lake water, seawater) and in a wide pH range (Fig. S43, ESI†).
Although several hydrophobic/superhydrophobic MOFs have been developed in powder form,11a less efforts have been devoted to the incorporation of such materials into stable substrates, that would be easily recovered after use in e.g. oil/water separation processes.11b Thus, HEPT-, OCT-, NON- and DODEC-MOFs were immobilized in cotton fabrics, with the same method applied for HEX-MOF (see above). The modified fabrics are superhydrophobic with water contact angles of 151–156 deg (Fig. S46 and S47, ESI†). We have then investigated the capability of DODEC-MOF@fabric for removal of crude oil from water under both static and continuous flow conditions. As can be seen in Fig. 5 (Fig. S48 and Video in ESI†), DODEC-MOF@fabric could eliminate rapidly crude oil from the water in either static or continuous flow mode, thus revealing its potential for application in oil spill cleanup and as an oil filter.
In summary, the utilization of terephthalate ligands with long alkyl-amino chains leads to the stabilization of Zr4+ MOFs with unusual 6-c nets. The resulted materials show interesting and diverse properties ranging from sorption of toxic species to oil–water separation capability. The above synthetic approach creates opportunities for the isolation of a series of Zr4+ MOFs with low net-connectivity and multiple terminal water/hydroxyl ligands, by utilizing a variety of multi-carboxylate ligands with elongated substituent groups. Such research efforts are underway in our laboratory.
The research project was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “1st Call for H.F.R.I. Research Projects to support Faculty Members & Researchers and the Procurement of high-cost research equipment grant” (Project Number: 348).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) L. Feng, J. Pang, P. She, J. L. Li, J. S. Qin, D. Y. Du and H. C. Zhou, Adv. Mater., 2020, 32, 2004414 CrossRef PubMed; (b) D. Feng, Z. Y. Gu, J. R. Li, H. L. Jiang, Z. Wei and H. C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed; (c) V. Bon, I. Senkovska, I. A. Baburin and S. Kaskel, Cryst. Growth Des., 2013, 13, 1231–1237 CrossRef CAS; (d) Y. Bai, Y. Dou, L.-H. Xie, W. Rutledge, J.-R. Li and H.-C. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC; (e) H. Wang, X. Dong, J. Lin, S. J. Teat, S. Jensen, J. Cure, E. V. Alexandrov, Q. Xia, K. Tan, Q. Wang, D. H. Olson, D. M. Proserpio, Y. J. Chabal, T. Thonhauser, J. Sun, Y. Han and J. Li, Nat. Commun., 2018, 9, 1745 CrossRef PubMed; (f) Y. Georgiou, S. Rapti, A. Mavrogiorgou, G. Armatas, M. J. Manos, M. Louloudi and Y. Deligiannakis, Sci. Rep., 2020, 10, 9358 CrossRef CAS PubMed.
- (a) M. Perfecto-Irigaray, G. Beobide, O. Castillo, I. Da Silva, D. García-Lojo, A. Luque, A. Mendia and S. Pérez-Yáñez, Chem. Commun., 2019, 55, 5954–5957 RSC; (b) J. Ren, M. Ledwaba, N. M. Musyoka, H. W. Langmi, M. Mathe, S. Liao and W. Pang, Coord. Chem. Rev., 2017, 349, 169–197 CrossRef CAS; (c) G. C. Shearer, S. Chavan, S. Bordiga, S. Svelle, U. Olsbye and K. P. Lillerud, Chem. Mater., 2016, 28, 3749–3761 CrossRef CAS; (d) D. Feng, W. C. Chung, Z. Wei, Z. Y. Gu, H. L. Jiang, Y. P. Chen, D. J. Darensbourg and H. C. Zhou, J. Am. Chem. Soc., 2013, 135, 17105–17110 CrossRef CAS PubMed; (e) A. D. Pournara, E. Moisiadis, V. Gouma, M. J. Manos and D. L. Giokas, J. Environ. Chem. Eng., 2022, 10, 107705 CrossRef CAS.
- (a) S. A. Diamantis, A. D. Pournara, E. D. Koutsouroubi, C. Moularas, Y. Deligiannakis, G. S. Armatas, A. G. Hatzidimitriou, M. J. Manos and T. Lazarides, Inorg. Chem., 2022, 61, 7847–7858 CrossRef CAS PubMed; (b) A. D. Pournara, S. Rapti, A. Valmas, I. Margiolaki, E. Andreou, G. S. Armatas, A. C. Tsipis, J. C. Plakatouras, D. L. Giokas and M. J. Manos, J. Mater. Chem. A, 2021, 9, 3379–3387 RSC.
- Z. Chen, S. L. Hanna, L. R. Redfern, D. Alezi, T. Islamoglu and O. K. Farha, Coord. Chem. Rev., 2019, 386, 32–49 CrossRef CAS.
- S. Yuan, W. Lu, Y. P. Chen, Q. Zhang, T. F. Liu, D. Feng, X. Wang, J. Qin and H. C. Zhou, J. Am. Chem. Soc., 2015, 137, 3177–3180 CrossRef CAS PubMed.
- (a) W. Xiang, Y. Zhang, Y. Chen, C. J. Liu and X. Tu, J. Mater. Chem. A, 2020, 8, 21526–21546 RSC; (b) F. Ahmadijokani, H. Molavi, M. Rezakazemi, S. Tajahmadi, A. Bahi, F. Ko, T. M. Aminabhavi, J. R. Li and M. Arjmand, Prog. Mater. Sci., 2022, 125, 100904 CrossRef CAS.
- (a) L. Feng, G. S. Day, K. Y. Wang, S. Yuan and H. C. Zhou, Chemistry, 2020, 6, 2902–2923 CrossRef CAS; (b) H. Lyu, O. I. F. Chen, N. Hanikel, M. I. Hossain, R. W. Flaig, X. Pei, A. Amin, M. D. Doherty, R. K. Impastato, T. G. Glover, D. R. Moore and O. M. Yaghi, J. Am. Chem. Soc., 2022, 144, 2387–2396 CrossRef CAS PubMed.
- L. H. Xie, X. M. Liu, T. He and J. R. Li, Chemistry, 2018, 4, 1911–1927 CrossRef CAS.
- L. Sarkisov and A. Harrison, Mol. Simul., 2011, 37, 1248–1257 CrossRef CAS.
- P. L. Smedley and D. G. Kinniburgh, Appl. Geochem., 2002, 17, 517–568 CrossRef CAS.
- (a) S. Mukherjee, S. Sharma and S. K. Ghosh, APL Mater., 2019, 7, 050701 CrossRef; (b) D. Kim, D. W. Kim, O. Buyukcakir, M.-K. Kim, K. Polychronopoulou and A. Coskun, Adv. Funct. Mater., 2017, 27, 1700706 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures; Rietveld refinement data and other characterization data; Ion sorption data; Video file for oil–water separation. CCDC 2176057–2176063. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc03090j |
| This journal is © The Royal Society of Chemistry 2022 |