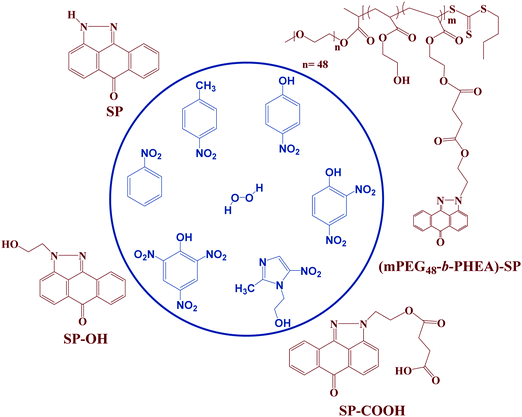
Pyrazoloanthrone-functionalized fluorescent copolymer for the detection and rapid analysis of nitroaromatics†
S.
Saravanan‡
a,
Rafiq
Ahmad‡
a,
S.
Kasthuri
a,
Kunal
Pal
b,
S.
Raviteja
c,
P.
Nagaraaj
 d,
Richard
Hoogenboom
d,
Richard
Hoogenboom
 *e,
Venkatramaiah
Nutalapati
*e,
Venkatramaiah
Nutalapati
 *a and
Samarendra
Maji
*a and
Samarendra
Maji
 *a
*a
aDepartment of Chemistry, Faculty of Engineering and Technology, SRM Institute of Science and Technology (SRMIST), Kattankulathur, Tamil Nadu-603203, India. E-mail: nvenkat83@gmail.com; samarenr@srmist.edu.in
bDepartment of Biotechnology and Medical Engineering, National Institute of Technology, Rourkela-769008, India
cSolid State and Structural Chemistry Unit (SSCU), Indian Institute of Science (IISc), Bangalore 560012, India
dDepartment of Chemistry, College of Engineering Guindy, Anna University, Chennai-600025, India
eSupramolecular Chemistry Group, Centre of Macromolecular Chemistry, Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281 S4, Ghent, Belgium. E-mail: richard.hoogenboom@ugent.be
First published on 30th September 2020
Abstract
The development of sensors for rapid detection of chemical explosives with high sensitivity and selectivity is the focus of many research groups. In this work, we have developed a simple and straightforward synthesis of a block (co)polymer functionalized with pyrazoloanthrone (SP). The block (co)polymer was synthesized via reversible addition–fragmentation chain-transfer (RAFT) polymerization of 2-hydroxyethyl acrylate using a PEG-functionalized RAFT-agent. Subsequently, the SP was coupled to the poly(2-hydroxyethyl acrylate) block through DCC coupling. The structural and physicochemical properties of the (co)polymer were studied to explore its potential towards the detection of nitroaromatics as a model for explosives. A systematic comparison is made on the chemosensing behavior of the (co)polymer and three small molecule pyrazoloanthrone analogues with different functional groups (SP, SP–OH and SP–COOH). Fluorescence studies demonstrated a significant decrease in the fluorescence intensity of the four fluorophores in the presence of different nitroaromatics and showed unprecedented selectivity for 2,4,6 trinitrophenol (TNP). The Stern–Volmer rate constants (Ksv) of the SP-functionalized copolymer (Ksv = 9.74 × 104 M−1) showed an ∼3.7 times higher quenching rate constant than its monomer analog (SP) for TNP with a limit of detection (LOD) of 19 ppm. A static quenching mechanism with photoinduced electron transfer process, intermolecular hydrogen bonding and electrostatic interactions induce turn off fluorescence behavior. The interference studies with other nitroaromatics in an aqueous medium and real-time analysis in the solid-state methods demonstrate the potential of the block (co)polymer towards practical applications.
1. Introduction
Recent escalation in worldwide violence has stimulated the need for inexpensive, sensitive, and remote detection of explosive compounds. Rapid detection of trace amounts of nitroaromatic compounds (NACs) in solid, solution, and vapor phases has attracted much attention owing to its threat towards security for civilians and the military, as well as the environment.1–4 In general, NACs present in the solid-state are sufficiently stable and possess very low vapour pressure at ambient temperature.5–7 These explosive compounds including 2,4,6-trinitrotoluene (TNT), 4-nitrophenol (NP), 2,4-dinitrophenol (DNP), 2,4-dinitrotoluene (DNT) and 2,4,6-trinitrophenol (TNP) are continuously released into the environment from the chemical industry, chemical laboratories, mining industries, military drills and even during space exploration. Excessive exploitation of these perilous chemical compounds leads to accumulation in the environment, which subsequently lead to detrimental effects on living organisms.8–10 Several studies have shown that acute and chronic exposure to NACs can cause the formation of methemeglobin and anemia in addition to other detrimental effects on the liver and bladder.6,7,11–13 Most of the NACs are sparingly soluble in water due to their hydrophobic structure.14,15 However, owing to their high toxicity, they can cause long-term disasters even at low concentrations.16,17 Even though significant advancement was made with different analytical and spectroscopic methods with different materials, the quest for the selective detection of explosive analytes and their rapid analysis for real sample on-field detection remains a challenge.In recent years, unrelenting efforts have been made to develop fluorescent sensor materials due to their high sensitivity and fast response time to develop light-triggered chemical sensors for the NACs with detection limits of attogram level. The sensitivity and binding constants are strongly dependent on the effective π–π interaction between the fluorophore and analyte, intermolecular hydrogen bonding and electrostatic interactions. Kasturi et al. have demonstrated novel amphiphilic Zn(II)phthalocyanines (ZnPcs) peripherally for the detection of NACs in an aqueous medium. ZnPc1 and ZnPc2 showed unprecedented selectivity towards TNP with an LOD of 0.7–1.1 ppm with a Stern–Volmer rate constant (Ksv) of 1.6–2.02 × 105 M−1.18 Rong et al. have developed fluorescent graphitic carbon nitride (g-C3N4) nanosheets which show turn off fluorescence upon contact with TNP owing to π–π, hydrogen bonding and electrostatic interactions with LOD of 8.2 nM.19 Nagarkar et al. developed a 3D fluorescent metal–organic framework (MOF) for the highly selective detection of the TNP in the presence of other nitro compounds, e.g. TNT and RDX in both aqueous and organic solutions. The selectivity is attributed to electron and energy transfer mechanisms, as well as electrostatic interactions between TNP and the fluorophore MOF. The KSV for TNP was found to be 3.5 × 104 M−1.20
In comparison to the small fluorophore molecules, fluorescence polymers have shown higher solubility in common organic solvents and aqueous media. Moreover, higher sensitivity, air and photo stability, and fluorescence quantum yield justify their versatility as a sensor material for explosives detection.21 However, different types of polymers behave differently when NACs interacts with fluorescent polymers. Sohn et al. have developed a photo-luminescent inorganic thin film polysilole for the detection of NACs in seawater. The LOD of TNP in seawater was found to be 6 ppb.22 Several homo and copolymers of metalloles containing tetraphenylsilole or tetraphenyl germole have been developed for the detection of NACs in toluene that exhibited 2 to 5 times better quenching efficiencies than the organic pentiptycene derived polymer.10,21 Using fluorescent quantum dot labeled molecularly imprinted polymer (MIP), an LOD of 0.5–1 ppm for DNT and TNT in water has been achieved.23
Although the effect of side-chain fluorescent polymer based sensors for different analyte detection has been reported, this approach has only sparsely been applied for explosives detection. Due to the presence of side chain fluorescent moieties, a significant transduction signal is anticipated. These fluorescent moieties can transfer their energy to the neighbor quenching sites within the Föster radius.21 Le Barny et al. first demonstrated polystyrene functionalized with a N-substituted-4-amino-1,8-naphthalimide chromophore (PSt-NI) for the detection of DNT in CHCl3 and the vapor phase using spin-coated PSt-NI film.21 Satapathi et al. have reported a fluorescent alanine based dansyl tagged copolymer P(MMA-co-Dansyl-Ala-HEMA) (DCP) synthesized via RAFT polymerization that exhibited high sensitivity and selectivity towards conventional NACs such as DNT, TNT, and TNP both in solution and also with saturated vapor phase of NACs.24 The study demonstrated that a higher KSV value of 1.6 × 104 M−1 was observed for TNP in comparison to TNT (1.3 × 103 M−1) due to the higher LUMO energy levels of DCP compared to the NACs facilitating the electron transfer process. These results indicated that polymer containing side-chain fluorescent moieties can be an alternative and facile design of new fluorescent sensor material for explosive detection. Prasad et al. explored a small molecule fluorescent 1,9-pyrazoloanthrone compound (which has been largely employed as a c-JUN N-terminal kinase (JNK1/2) inhibitor) as a “turn-on” fluorescent probe for the detection of cyanide and fluoride ions.25 The intermolecular hydrogen bonding between 1,9-pyrazoloanthrone (SP) and F− and CN− drives significant colour variation upon interactions. The unsymmetrical nature of the molecular ensemble and presence of imine nitrogen enhance its sensitivity. This report inspired us to perform a systematic study on the chemosensing behavior of SP derivatives with different NACs due to potential intermolecular hydrogen bonding and π–π interactions of SP with NACs. Besides, the development of a (co)polymer with multiple SP derivatives can show enhanced sensitivity and selectivity towards NACs, in comparison to analogous small molecules.
Therefore, in the present investigation, we report the facile synthesis of three low molecular weight fluorophore molecules (SP, SP–OH and SP–COOH) and an SP functionalized with PEGylated block (co)polymer (mPEG48-b-PHEA)-SP synthesized via RAFT polymerization and demonstrate its potential as a chemosensor for the detection of explosive NACs. Their chemical structures are shown in Fig. 1. The design of block copolymer is made to enhance the overall aqueous solubility of the construct by the short PEG-block while the PHEA was chosen to anchor the SP–COOH derivative.
Fluorescence studies in solution phase demonstrated a significant decrease in the emission intensity of all four fluorophore molecules ascribed to photoinduced electron transfer (PET) between electron-rich anthrapyrazolone moieties and the nitroaromatics and showed unprecedented selectivity towards TNP in the presence of other competitive explosive analytes with a limit of detection (LOD) of ∼19 ppm. The mechanistic investigation on the quenching process is studied via optical absorption and Stern–Volmer rate process. Finally, (mPEG48-b-PHEA)-SP based visual identification and contact mode detection of TNP using filter paper has been demonstrated.
2. Materials and methods
All solvents and chemicals used in this work were analytical grade and used without further purification. 2-Hydroxyethylhydrazine, 1-chloroanthraquinone (1-Cl AQ), and 2-hydroxyethylacrylate (HEA) were purchased from Sigma Aldrich. 2-Hydroxyethylacrylate was purified twice by passing through a column filled with basic alumina to remove the inhibitor. NACs that were used in our studies include 2,4,6-trinitrophenol (TNP), 2,4-dinitrophenol (DNP), 4-nitrophenol (NP), 4-nitrobenzene (NB), 4-nitrotoluene (NT) and metronidazole (MNT) and were purchased from Sigma Aldrich. (Caution: nitro aromatic compounds are highly explosive. Hence, extreme measures were taken to handle these while using them and small amounts were taken for carrying out the experiments). Succinic anhydride, N,N′-dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP), and acetonitrile (ACN) were ordered from Sisco Research Laboratories Pvt. Ltd (SRL), India. 2,2′-azobis(isobutyronitrile) (AIBN) was purchased from Lobachemie Pvt. Ltd India. AIBN was recrystallized twice from methanol before use. N,N′-Dimethylformamide (DMF), dichloromethane (DCM), diethyl ether (DEE), ethyl acetate (EtOAc), n-hexane and triethylamine (TEA) were obtained from Finar Chemicals Limited, India. 2-(butylthiocarbonothioylthio)propanoic acid (BTTCP) chain transfer agent (CTA) was prepared as previously reported.262.1. Characterization
Bruker AVANCE (500 and 125 MHz) NMR spectrometers were used for 1H and 13C-NMR studies. CDCl3, DMSO-d6, and D2O were used as solvents for the NMR study. Fourier-transform infrared spectra (FT-IR) were obtained using an IR Tracer-100 Fourier Transform Spectrophotometer SHIMADZU (ATR) at room temperature for the investigation of various functional groups present in the synthesized small molecules and (co)polymer. For the determination of the molecular weights as well as the molecular weight distribution of the synthesized polymer, size exclusion chromatography (SEC) studies were carried out with a Polymer Laboratories PL-GPC-50 integrated SEC instrument interfaced with a Well Chom K-2301 refractive index (RI) detector and a 5 μm PL gel mixed-C column with THF (0.01 mol/LiBr) as the eluent. Gas chromatography (GC) was performed on 7820A from Agilent Technologies with an FID detector. To program the GC oven, temperature ranges from 50 to 250 °C followed by two heating stages: from 50–120 °C with a rate of 20 °C min−1 (up to 0–3.5 min) and 120–300 °C with a rate of 50 °C min−1 (up to 3.5–7.1 min). Mass determination of the synthesized compounds was carried out using an LC–MS 2020 system equipped with an LC10ADVP binary pump (Shimadzu, Japan) device with a Diode Array Detector (DAD) coupled with a Mass (MS) compartment consisting of a single quadrupole mass spectrometer with electrospray ionization ESI (+) source and nitrogen gas was used to assist nebulization with a flow rate of 1.5 mL min−1. Optical absorption studies were carried out in a UV-visible spectrophotometer (Cary 5000 UV-Vis-NIR). Fluorescence studies were carried out on a HORIBA Fluorlog-3 photoluminescence spectrometer. Fluorescence lifetime decay measurements were carried out with the TCSPC method.2.2. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) as eluents. Pure 1,9-pyrazoloanthrone (SP) was obtained after removal of solvents under reduced pressure (Yield: 2.2 g, 32%). IR (ν cm−1): 3146 (NH), 1664 (C
80 v/v) as eluents. Pure 1,9-pyrazoloanthrone (SP) was obtained after removal of solvents under reduced pressure (Yield: 2.2 g, 32%). IR (ν cm−1): 3146 (NH), 1664 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597 (C
O), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).
N).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598 (C
O), 1598 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). ESI-MS: ([C16H12N2O2] + H)+ = 265.0971.
N). ESI-MS: ([C16H12N2O2] + H)+ = 265.0971.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (v/v) as the eluent. The solution was evaporated under vacuum yielding SP–COOH as a yellow solid (yield: 32 mg, 46%). 1H NMR (CDCl3, ppm): δ 8.32 (d, J = 7.9 Hz, 1H), 8.17 (t, J = 8.45 Hz, 2H), 7.97 (d, J = 7.1 Hz, 1H), 7.84–7.77 (m, 2H), 7.63 (t, J = 7.58 Hz, 1H), 4.88 (t, J = 4.9 Hz, 2H), 4.54 (t, J = 5.05 Hz, 2H), 2.37–2.34 (m, 4H).13C NMR (CDCl3, ppm): δ 183.24, 173.99, 172.66, 140.23, 138.87, 134.62, 133.59, 131.63, 129.22, 125.70, 123.37, 120.68, 117.39, 63.44, 48.88, 29.00. IR (ν cm−1): 3437 (OH), 1630 (C
60 (v/v) as the eluent. The solution was evaporated under vacuum yielding SP–COOH as a yellow solid (yield: 32 mg, 46%). 1H NMR (CDCl3, ppm): δ 8.32 (d, J = 7.9 Hz, 1H), 8.17 (t, J = 8.45 Hz, 2H), 7.97 (d, J = 7.1 Hz, 1H), 7.84–7.77 (m, 2H), 7.63 (t, J = 7.58 Hz, 1H), 4.88 (t, J = 4.9 Hz, 2H), 4.54 (t, J = 5.05 Hz, 2H), 2.37–2.34 (m, 4H).13C NMR (CDCl3, ppm): δ 183.24, 173.99, 172.66, 140.23, 138.87, 134.62, 133.59, 131.63, 129.22, 125.70, 123.37, 120.68, 117.39, 63.44, 48.88, 29.00. IR (ν cm−1): 3437 (OH), 1630 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1615 (C
O), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). LC–MS: ([C20H16N2O5]–H)− = 363.05.
N). LC–MS: ([C20H16N2O5]–H)− = 363.05.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.34 (2H, dd, –CH2–CH2–S–), 3.35 (3H, s, CH3–O–), 1.63 (2H, m, –CH2–CH2–CH2–S–), 1.56 (3H, d, CH3–CH), 1.40 (2H, m, CH3–CH2–CH2–), 0.90 (3H, t, CH3–CH2). Mn SEC (THF) = 7100 g mol−1, Đ = 1.11.
CH–), 3.34 (2H, dd, –CH2–CH2–S–), 3.35 (3H, s, CH3–O–), 1.63 (2H, m, –CH2–CH2–CH2–S–), 1.56 (3H, d, CH3–CH), 1.40 (2H, m, CH3–CH2–CH2–), 0.90 (3H, t, CH3–CH2). Mn SEC (THF) = 7100 g mol−1, Đ = 1.11.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1281 (C–O), 1002 (C–O–C). Mn GC = 3300 g mol−1, Mn SEC (THF) = 7000 g mol−1, Đ = 1.78.
O), 1281 (C–O), 1002 (C–O–C). Mn GC = 3300 g mol−1, Mn SEC (THF) = 7000 g mol−1, Đ = 1.78.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1630 (C
O), 1630 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1277 (C–O), 1101 (C–O–C). Mn SEC (THF) = 7800 g mol−1, Đ = 1.55.
N), 1277 (C–O), 1101 (C–O–C). Mn SEC (THF) = 7800 g mol−1, Đ = 1.55.
3. Results and discussion
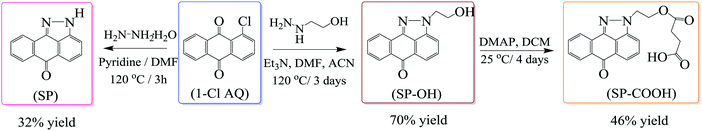
Three pyrazoloanthrone based small molecule fluorophores were synthesized, namely SP (1,9-pyrazoloanthrone), SP–OH, and SP–COOH respectively. At first, 1,9-pyrazoloanthrone (SP) was synthesized by a condensation reaction of 1-chloroanthraquinone (1-Cl AQ) with hydrazine hydrate at 120 °C in the presence of pyridine. SP–OH was prepared by the condensation reaction of 1-chloroanthraquinone with hydrazine ethanol at 120 °C in the presence of triethylamine (Et3N) (Scheme 1). Subsequently, the SP–OH compound was reacted with succinic anhydride to obtain 4-oxo-4-(2-(6-oxodibenzo[cd,g] indazo l-2(6H)-yl)ethoxy)butanoic acid (SP–COOH) in the presence of DMAP. The structures of the compounds were confirmed by NMR (1H and 13C), FT-IR and HR-MS (Fig. S1–S11, ESI†). Furthermore, the structure of SP–COOH was confirmed by single-crystal X-ray analysis.Suitable good quality single crystals were developed by slow solvent evaporation from a solution in a chloroform–methanol solvent mixture at room temperature. Fig. 2a shows the solved single-crystal X-ray structure of SP–COOH. The compound crystallizes in an orthorhombic crystal system with a Pbca space group and having crystal parameters a = 7.7009(7), b = 18.4626(17), c = 24.055(3), α = β = γ = 90, cell volume 3420.11 and Z = 8 (CCDC no: 2002889).† Table S1, ESI,† summarizes the detailed crystallographic information. The supramolecular arrangement in the crystal lattice reveals that in the solid state, the molecules were strongly stabilized by having intermolecular hydrogen bonding interactions between the nitrogen of the pyrazole unit and carboxylic unit attached alkyl chain (N⋯H–O) by 2.745 Å. Due to the strong intermolecular interactions at the peripheral position, the molecules exhibit strong π–π interactions (3.874 Å) between two successive layers.
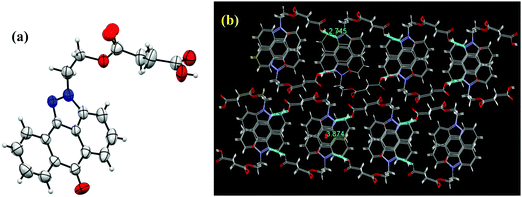 | ||
| Fig. 2 (a) Single-crystal X-ray structure of SP–COOH with 30% probability of thermal ellipsoids. (b) Supra molecular arrangement of SP–COOH in the crystal lattice. | ||
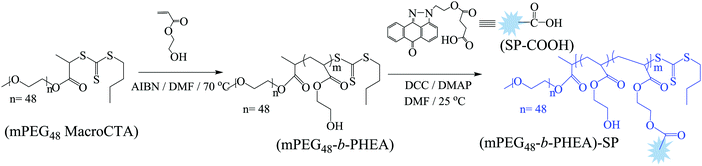
The SP-functionalized PEGylated poly(hydroxyethyl acrylate) block (co)polymer (mPEG48-b-PHEA)-SP was synthesized starting from a PEGylated MacroCTA (Scheme 2), which was synthesized by the DCC coupling reaction of 2-(((butylthio)carbonothioyl)thio)propanoic acid (BTTCP) and mPEG48-OH. RAFT polymerization of the HEA was conducted at 70 °C with AIBN as an initiator and the MacroCTA as a chain transfer agent ([HEA]/[MacroCTA]/[AIBN] = 100/2/0.2) using DMF at 2(M) HEA concentration to obtain the mPEG48-b-PHEA block (co)polymer (Scheme 2). The reaction was stopped after 60 min and the polymer was isolated by precipitation in cold diethylether. The theoretical molecular weight was calculated from the conversion and found to be 3300 g mol−1.
The formation of the block (co)polymer was confirmed by 1H NMR and IR spectroscopy. The 1H NMR spectrum of the block (co)polymer is shown in Fig. S9, ESI.† SEC characterization was used to determine the relative molecular weight of the block (co)polymer. SEC traces of the PEGylated MacroCTA, block (co)polymer mPEG48-b-PHEA, and fluorophore functionalized block (co)polymer (mPEG48-b-PHEA)-SP are shown in Fig. S12, ESI.† The SEC traces indicated the successful formation of the mPEG48-b-PHEA and (mPEG48-b-PHEA)-SP as the SEC trace moves towards lower elution time in comparison to PEGylated MacroCTA. However, the molecular weight results obtained from mPEG48-b-PHEA showed slightly lower value (7000 g mol−1; Đ = 1.78) than the MacroCTA (7100 g mol−1Đ = 1.11), which is attributed to the presence of a low molecular weight polymeric chain in the block (co)polymer which is reflected in the dispersity.
The photophysical properties of the four compounds SP, SP–OH, SP–COOH and (mPEG48-b-PHEA)-SP were investigated via UV-Vis and fluorescence spectroscopy. The UV-Vis absorption spectrum of SP shows two characteristic absorption peaks at 282 ± 2 nm that arise due to the π → π* transition and another transition band at 397 ± 2 nm due to the intramolecular charge-transfer (ICT) process (Fig. 3a). Upon incorporation of different functional units on the pyrazole moiety, the ICT band shows a red shifted absorption at ca. 409 ± 2 nm [for SP–OH, SP–COOH and (mPEG48-b-PHEA)-SP]. The optical band gap of the (co)polymer is found to be ∼2.68 eV, which is lower in comparison to SP (2.85 eV), SP–OH (2.72 eV) and SP–COOH (2.75 eV). Fig. 3b shows the emission spectra of all the compounds upon excitation at λex = 400 nm. All the fluorophore molecules demonstrated a strong blue emission in methanol.
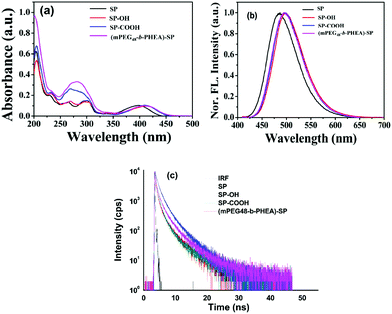 | ||
| Fig. 3 UV-Visible (a), fluorescence emission (b) and lifetime decay (c) of SP, SP–OH, SP–COOH and (mPEG48-b-PHEA)-SP in MeOH solution. | ||
SP shows an emission maximum at λmax = 486 nm with a Stokes shift of ca. 90 ± 2 nm. A slight red shift of ∼11 nm was observed for SP–OH and SP–COOH. The block (co)polymer (mPEG48-b-PHEA)-SP also shows a similar emission spectrum with a Stokes shift of ∼86 nm. The average fluorescence lifetime of all the derivatives is measured to be 3.27, 3.34, 3.29 and 3.49 ns for SP, SP–OH, SP–COOH and (mPEG48-b-PHEA)-SP, respectively (Fig. 3c). It is evident that the intermolecular interactions of the SP-derivative are weaker in the polymer network due to a flexible chain, which limits the effective π–π interactions making it promising as a solid state light emitting material. Geometry optimization studies were carried out to understand the electronic structure, frontier molecular electron densities and energy levels of the different SP derivatives and the SP-functionalized repeating unit of the polymer ((mPEG48-b-PHEA)-SP) in the gas phase at the B3LYP/6-31g* level using the Gaussian 09 programme. The energy levels of the molecules significantly varied with functional units. The HOMO and LUMO energy levels of SP are localized at −5.997 eV and −2.271 eV with a calculated HOMO–LUMO gap of 3.726 eV. N-Alkyl functionalization of the SP derivatives showed a decrease in the HOMO–LUMO energy gap. SP–OH showed similar electronic structures, however, the energy levels of HOMO and LUMO decreased due to N-alkylation. By varying with different N-alkyl chain functional units, the HOMO–LUMO energy gap varied as 3.619, 3.628 and 3.654 eV for SP–OH, SP–COOH and (mPEG48-b-PHEA)-SP respectively (Fig. S13, ESI†). The frontier molecular orbitals of SP showed that the electron density of the HOMO and LUMO was mostly localized on the entire π-conjugated pyrazoloanthrone moiety. For the N-alkylated derivatives, the electron density further extended to the N-alkylated units and the LUMO electron density was only distributed on the central core moiety. A similar result was observed in the optimization of one unit of (mPEG48-b-PHEA)-SP. These results indicate that, in the polymer, the charge transfer process is similar as in the small molecules. However, the low lying LUMO orbitals in the (mPEG48-b-PHEA)-SP unit facilitate a greater and faster charge transfer process with electron deficient compounds. These results are in correlation with the trend observed in the optical and fluorescence properties. The DFT geometry optimization of the SP derivatives and SP-polymer indicate that the molecular backbone exhibits high planarity and the HOMO orbital electron density is localized on the pyrazoloanthrone moiety. The decrease in the HOMO–LUMO energy gap in the polymer and electron richness of the material with high emission inspired us to study the donor–acceptor interactions with high electron deficient nitro explosive compounds, nitro antibiotics and other explosive electron deficient acceptors to ascertain the potential application towards chemosensors for NACs.
To study the chemosensing behaviour of the (mPEG48-b-PHEA)-SP and SP derivatives, fluorescence titration experiments were carried out in methanol solution and solid state methods. In the present study, different nitroanalytes such as 2,4,6-trinitrophenol (TNP), 2,4-dinitrophenol (DNP), 4-nitrotoluene (NT), 4-nitrophenol (NP) and nitrobenzene (NB) along with a few other non-nitroaromatic compounds like H2O2 and nitroantibiotics like metronidazole (MNT) were investigated to ascertain the selectivity of different nitroanalytes. Upon step-wise addition of different amounts of TNP solution to SP (5 × 10−6 M), the initial fluorescence at ∼486 nm gradually decreased. The addition of 80 μM of TNP solution led to a 74% decrease in emission intensity (Fig. 4a). The addition of other nitroanalytes only revealed 8–16% decrease in the emission intensity. This inspired us to systematically establish the applicability of these materials towards the detection of NACs. To gain deeper insights into the quenching rates, the selectivity for NACs and the effect of coupling the small molecule fluorophore to the polymer, fluorescence quenching experiments were carried out. Upon addition of 80 μM of TNP to the solution of SP–OH, the initial emission intensity decreased by 63% (Fig. 4b). Under similar conditions, SP–COOH shows 85% quenching of the initial fluorescence intensity (Fig. 4c). Interestingly, the (mPEG48-b-PHEA)-SP revealed a faster decrease in the emission intensity in comparison to the other SP derivatives.
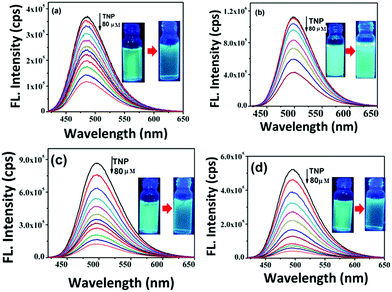 | ||
| Fig. 4 Change in fluorescence emission behavior of (a) SP, (b) SP–OH, (c) SP–COOH and (d) (mPEG48-b-PHEA)-SP in methanol (5 × 10−6 M) upon addition of different concentrations of TNP. | ||
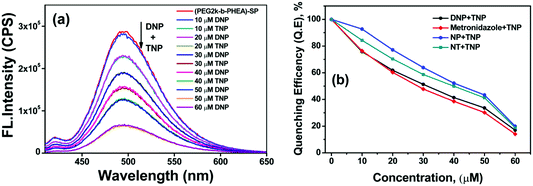
For addition of low concentrations of TNP, the emission intensity dramatically decreased and reached a plateau at higher concentration. Addition of 7.5 μM of TNP solution to (mPEG48-b-PHEA)-SP resulted in 26% quenching of fluorescence emission while 83% quenching was observed upon addition of 69 μM of TNP solution. Further increase in the amount of added TNP solution to 80 μM gave a 95% decrease in the emission intensity (Fig. 4d). The inset in Fig. 4 shows dramatic change in the fluorescence colour of the solutions before and after addition of TNP. These findings clearly demonstrate that (mPEG48-b-PHEA)-SP exhibits superior sensing behaviour to those of other SP derivatives following the order (mPEG48-b-PHEA)-SP > SP–COOH > SP > SP–OH. Besides, the fluorescence titration experiments were carried out with other NACs. The variations in the emission intensity for different concentrations of analytes are summarized in Fig. S14–S20, ESI.† The fast decrease in the emission intensity with increase in the concentration of TNP suggests efficient photo-induced electron transfer (PET) from the pyrazoloanthrone units on the (mPEG48-b-PHEA) (co)polymer to the electron deficient TNP. Fig. 5 shows the variation in the quenching behaviour upon addition of different concentrations of nitroanalytes. The highest selectivity was observed for the TNP with (mPEG48-b-PHEA)-SP and showed high quenching efficiency.
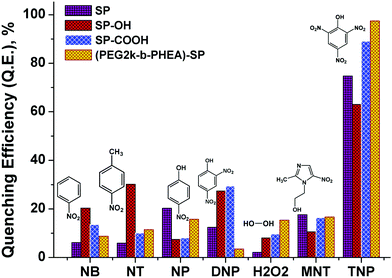 | ||
| Fig. 5 Quenching efficiency of SP, SP–OH, SP–COOH, and (mPEG48-b-PHEA)-SP (5 × 10−6 M) treated with different nitroanalytes and H2O2 (80 μM). | ||
The sensitivity of fluorescence quenching of SP derivatives and block (co)polymer was quantified by the Stern–Volmer equation as
| I0/I = 1 + Ksv [Q] |
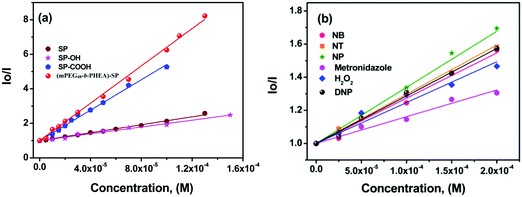 | ||
| Fig. 6 (a) Variation in the Stern–Volmer (SV) plot of SP derivatives treated with different concentrations of TNP. (b) SV plot of (mPEG48-b-PHEA)-SP upon addition of different nitroanalytes. | ||
The limit of detection (LOD) of (mPEG48-b-PHEA)-SP was determined by measuring the emission intensities plotted against the concentration of TNP (Fig. S21, ESI†). The final LODs were measured in terms of the signal to noise ratio using the formula LOD = 3.3 × σ/m, where σ is standard deviation and m is the slope. For (mPEG48-b-PHEA)-SP, we have obtained an LOD of 19 ± 0.1 ppm towards TNP while SP shows a LOD for TNP of 39 ± 0.1 ppm.
Selective detection of TNP among other analytes provoked us to test the selectivity in the presence of other analytes in the aqueous medium. Such study provides a unique significance to a sensor system in measuring real or on-site field samples. To achieve this competitive fluorescence quenching, the emission spectra of the (mPEG48-b-PHEA)-SP (3 μM) was recorded initially. To this solution, 10 μM of DNP solution was added and allowed to equilibrate for 5 min to have an effective interaction. The emission maximum of the solution was not altered significantly upon addition of DNP. To the resulting solution, TNP (10 μM) was added and the emission spectrum was collected, which resulted in a significant fluorescence quenching. The process of adding equal amounts of DNP and TNP was repeated for several cycles and the emission data were collected. The quenching behaviour was observed upon the addition TNP in all the cycles while DNP did not lead to significant emission quenching as illustrated in Fig. 7a. Similar results were also observed when other NACs were used instead of DNP. Fig. 7b shows the variation in the quenching efficiency of the polymer upon addition of sequential amounts of TNP in the presence of other nitroanalytes. The study shows that a stepwise decrease in the quenching efficiency demonstrates the high selectivity of (mPEG48-b-PHEA)-SP towards TNP even in the presence of competing NACs in water.
To understand the high quenching rate constants and selectivity for TNP among the other nitroanalytes, we considered the plausible potential interactions. TNP is a strong acid compared to the other NACs with a pKa value of ∼0.38. The number of electron-deficient nitro groups available on the phenolic derivatives enhances the acidity. The phenolic –OH groups that are acidic in nature can readily undergo deprotonation in protic solvents due to the presence of more electron-withdrawing groups. This facilitates the enhanced electrostatic interactions between TNP and SP derivatives. The SP derivatives contain imine and tertiary amine in the pyrazoloanthrone unit, which can also interact with nitrophenol and lead to the formation of nitrophenolates. This will accelerate the formation of electrostatic interactions with the fluorophore molecules containing lone pair nitrogen atoms (basic functional groups) and leads to an efficient photoinduced electron transfer process and further leads to efficient fluorescence quenching. Interestingly, the co-polymer (mPEG48-b-PHEA)-SP has multiple SP units per polymer chain allowing more efficient, multivalent interactions with TNP. The formation of molecular ion pairs and strong π–π interaction between pyrazoloanthrone units (donor) and TNP (acceptor) also facilitates the effective quenching process. The formation of molecular ion pairs was further confirmed by UV-Visible titration study. Fig. S22a, ESI,† shows the variation in the absorption spectra of (mPEG48-b-PHEA)-SP upon addition of different concentrations of TNP. The absorption coefficient at 430 nm and 353 nm is gradually increased with an increase in the concentration indicating the formation of a charge transfer ion–pair complex between (mPEG48-b-PHEA)-SP and TNP.
To attain more insight into the quenching process, we have correlated the absorption spectra of the NACs (acceptor) and the emission spectrum of (mPEG48-b-PHEA)-SP (donor). Fig. S22b, ESI,† shows that there is no significant spectral overlap between the acceptor absorption and emission spectra of the donor unit revealing that the electron transfer process is the predominant phenomenon for the quenching process. It is worth noting that we have also checked the effect of protonation on the absorption and fluorescence behaviour with different acids (TFA, H2SO4 and HCl) of varied strength of acidity. Upon addition of different acids (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 equiv.), the emission intensity of (mPEG48-b-PHEA)-SP was not altered significantly. The absorption spectrum shows an increase in the ICT band intensity and showed a blue shift (Fig. S23, ESI†). This experimental evidence clearly justifies that the observed high quenching rate of (mPEG48-b-PHEA)-SP with TNP arises from the formation of a stronger ground state charge transfer complex through ion pair electrostatic interactions in combination with a predominant photo induced electron transfer process with a static quenching pathway. To validate this process, we have carried out the fluorescence lifetime measurements for (mPEG48-b-PHEA)-SP, before and after addition of different concentrations of TNP. The fluorescence lifetime decay pattern and lifetime values are not varied significantly. This suggests that the quenching process occurs due to a static quenching phenomenon (Fig. S24, ESI†). Fig. 8 shows the schematic representation of the plausible mode of binding interaction between (mPEG48-b-PHEA)-SP and TNP.
15 equiv.), the emission intensity of (mPEG48-b-PHEA)-SP was not altered significantly. The absorption spectrum shows an increase in the ICT band intensity and showed a blue shift (Fig. S23, ESI†). This experimental evidence clearly justifies that the observed high quenching rate of (mPEG48-b-PHEA)-SP with TNP arises from the formation of a stronger ground state charge transfer complex through ion pair electrostatic interactions in combination with a predominant photo induced electron transfer process with a static quenching pathway. To validate this process, we have carried out the fluorescence lifetime measurements for (mPEG48-b-PHEA)-SP, before and after addition of different concentrations of TNP. The fluorescence lifetime decay pattern and lifetime values are not varied significantly. This suggests that the quenching process occurs due to a static quenching phenomenon (Fig. S24, ESI†). Fig. 8 shows the schematic representation of the plausible mode of binding interaction between (mPEG48-b-PHEA)-SP and TNP.
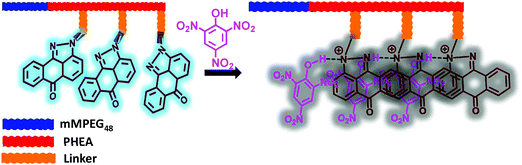 | ||
| Fig. 8 Schematic representation of the plausible mode of interaction between (mPEG48-b-PHEA)-SP and TNP. | ||
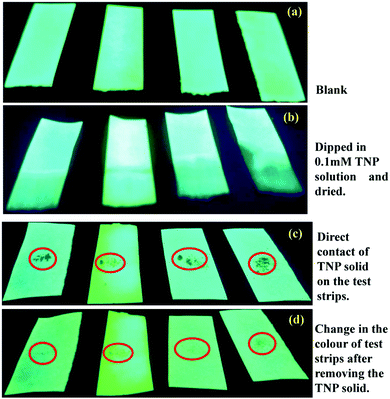
The explosive nitroaromatic compounds, especially TNP, can contaminate the human body and textiles during the preparation and packing of fireworks, explosives and in rocket fuel. The biologically transformed compounds and degradation compounds exhibit high toxicity to biota and lead to chronic diseases. Thus, visual identification and contact mode detection would be an ideal method for a practical screening process for contact with TNP. Towards this goal, we have dissolved the SP derivatives and (mPEG48-b-PHEA)-SP in methanol solution (1 mM) and Whatman 42 filter papers were dipped in the solution for 5 min. The filter papers were removed from the solution and subsequently dried under vacuum. The coated test strips were cut into different pieces and used for the analysis. In the first experiment, the four coated test strips were dipped in the 0.1 mM TNP solution for 2 min and dried. The photographs in Fig. 9a and b show a change in the colour of the solution under UV light at 365 nm. The bright blue fluorescence of the test strips converted into dark showing quenching of the fluorescence. Interestingly, the intensity of the darkness varied significantly, and (mPEG48-b-PHEA)-SP showed a stronger quenching than the other SP derivatives. Upon treating the test strips with other NACs, we have observed a little decrease in bright blue emission intensity. In another method as a contact mode approach, TNP crystals were directly placed on the test strips for 2 min. The formation of the dark spots on the test stripes was noted as shown in Fig. 9c and d. These results demonstrate the potential use of (mPEG48-b-PHEA)-SP for the development of test strips towards the selective detection of TNP specimens in real-time screening.
4. Conclusions
We have developed facile methods to prepare different SP derivatives and (co)polymer (mPEG48-b-PHEA)-SP and their application as a fluorescence-based sensor material towards different nitro and other explosive analytes has been successfully demonstrated. Fluorescence studies indicated that among all, these derivatives showed the highest sensitivity and selectivity for TNP towards (mPEG48-b-PHEA)-SP in the presence of other competitive nitroanalytes. The Stern–Volmer rate constant (Ksv) value was found to be highest (9.74 × 104 M−1) for (mPEG48-b-PHEA)-SP towards TNP with an LOD of 19 ppm. The high selectivity was attributed due to the photoinduced electron transfer process, strong intermolecular hydrogen bonding and electrostatic interactions between the copolymer and TNP. Finally, (mPEG48-b-PHEA)-SP based visual identification and contact mode detection (solid phase) of TNP using filter paper have been demonstrated as a proof of concept.Author contributions
The manuscript was written through the contributions of all authors. All authors have given the approval to the final version of the manuscript.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
S. S. thanks SRM Institute of Science and Technology for providing a fellowship to support his PhD program. S. Kasthuri acknowledges SRMIST and CSIR for Senior Research Fellowship (SRF). R. H. thanks FWO and Ghent University for the financial support. N. V. R thanks SERB for funding through a start-up research grant (SRG/2019/001023) and SRMIST seed grant. S. M. acknowledges SRMIST for providing a SRMIST seed grant. N. V. R. and S. M. also acknowledge DST-FIST fund (No. SR/FST/CST-266/2015(c)) for improvement of the S&T infrastructures to the Department of Chemistry, SRMIST. The authors also acknowledge SRMIST for the UV-Visible-NIR and Photoluminescence spectrometer facility.References
- M. B. Pushkarsky, I. G. Dunayevskiy, M. Prasanna, A. G. Tsekoun, R. Go and C. K. N. Patel, High-sensitivity detection of TNT, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 19630–19634 CrossRef CAS
.
- A. Rose, Z. G. Zhu, C. F. Madigan, T. M. Swager and V. Bulovic, Sensitivity gains in chemosensing by lasing action in organic polymers, Nature, 2005, 434, 876–879 CrossRef CAS
.
- C. Wang, H. Huang, B. R. Bunes, N. Wu, M. Xu, X. Yang, L. Yu and L. Zang, Trace detection of RDX, HMX and PETN explosives using a fluorescence spot sensor, Sci. Rep., 2016, 6, 25015 CrossRef CAS
.
- Y. Ma, H. Li, S. Peng and L. Wang, Highly selective and sensitive fluorescent paper sensor for nitroaromatic explosive detection, Anal. Chem., 2012, 84, 8415–8421 CrossRef CAS
.
- D. Furman, R. Kosloff, F. Dubnikova, S. V. Zybin, W. A. Goddard, N. Rom, B. Hirshberg and Y. Zeiri, Decomposition of condensed phase energetic materials: interplay between uni- and bimolecular mechanisms, J. Am. Chem. Soc., 2014, 136, 4192–4200 CrossRef CAS
.
- I. Lee, J. E. Kwon, C. You, Y. Kang and B. G. Kim, Instantaneous detection of explosive and toxic nitroaromatic compounds via donor–acceptor complexation, J. Mater. Chem. C, 2019, 7, 9257–9262 RSC
.
- K. S. Asha, K. Bhattacharyya and S. Mandal, Discriminative detection of nitro aromatic explosives by a luminescent metal–organic framework, J. Mater. Chem. C, 2014, 2, 10073–10081 RSC
.
- M. Y. Serrano-González, R. Chandra, C. Castillo-Zacarias, F. Robledo-Padilla, M. de, J. Rostro-Alanis and R. Parra-Saldiva, Biotransformation and degradation of 2,4,6-trinitrotoluene by microbial metabolism and their interaction, Def. Technol., 2018, 14, 151–164 CrossRef
.
- E. J. Johnston, E. L. Beynon, A. Lorenz, V. Chechik and N. C. Bruce, Monodehydroascorbate reductase mediates TNT toxicity in plants, Science, 2015, 349, 1072–1075 CrossRef CAS
.
- H. Sohn, M. J. Sailor, D. Magde and W. C. Trogler, Detection of nitroaromatic explosives based on photoluminescent polymers containing metalloles, J. Am. Chem. Soc., 2003, 125, 3821–3830 CrossRef CAS
.
- Y. Zhou, X. Liu, W. Jiang, Y. Shu and G. Xu, A theoretical insight into the reaction mechanisms of a 2,4,6-trinitrotoluene nitroso metabolite with thiols for toxic effects, Toxicol. Res., 2019, 8, 270–276 CrossRef CAS
.
- T. M. Mallon, J. M. Ortiz, W. H. Candler, G. Rogers and R. Hillburn, Investigation of an outbreak of anemia cases at an army trinitrotoluene munitions production plant from 2004 to 2005 and subsequent surveillance 2005–2013, Mil. Med., 2014, 179, 1374–1383 CrossRef
.
- P. Kovacic and R. Somanathan, Nitroaromatic compounds: environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism, J. Appl. Toxicol., 2014, 34, 810–824 CrossRef CAS
.
- J. M. Phelan and J. L. Barnett, Solubility of 2,4-dinitrotoluene and 2,4,6-trinitrotoluene in water, J. Chem. Eng. Data, 2001, 46, 375–376 CrossRef CAS
.
- J. C. Lynch, J. M. Brannon and J. J. Delfino, Dissolution rates of three high explosive compounds: TNT, RDX, and HMX, Chemosphere, 2002, 47, 725–734 CrossRef CAS
.
- E. A. Naumenko, B. Ahlemever and E. Baumgart-Vogt, Species-specific differences in peroxisome proliferation, catalase, and SOD2 upregulation as well as toxicity in human, mouse, and rat hepatoma cells induced by the explosive and environmental pollutant 2,4,6-trinitrotoluene, Environ. Toxicol., 2016, 32, 989–1006 CrossRef
.
- E. R. Travis, N. C. Bruce and S. J. Rosser, Microbial and plant ecology of a long-term TNT-contaminated site, Environ. Pollut., 2008, 153, 119–126 CrossRef CAS
.
- S. Kasthuri, P. Gawas, S. Maji, N. Veeraiah and N. Venkatramaiah, Selective detection of trinitrophenol by amphiphilic dimethylaminopyridine-appended Zn(II)phthalocyanines at the near-infrared region, ACS Omega, 2019, 4, 6218–6228 CrossRef CAS
.
- M. Rong, L. Lin, X. Song, T. Zhao, Y. Zhong, J. Yan, Y. Wang and X. Chen, A label-free fluorescence sensing approach for selective and sensitive detection of 2,4,6-trinitrophenol (TNP) in aqueous solution using graphitic carbon nitride nanosheets, Anal. Chem., 2015, 87, 1288–1296 CrossRef CAS
.
- S. S. Nagarkar, B. Joarder, A. K. Chaudhari, S. Mukherjee and S. K. Ghosh, Highly selective detection of nitro explosives by a luminescent metal–organic framework, Angew. Chem., Int. Ed., 2013, 52, 2881–2885 CrossRef CAS
.
- P. L. Le Barny, E. T. Obert, F. Soyer, J. P. Malval, I. Leray, N. Lemaître, R. Pansu, V. Simic, H. Doyle, G. Redmond and B. Loiseaux, Detection of nitroaromatic compounds based on photoluminescent side chain polymers, Proc. SPIE, 2005, 1–8 Search PubMed
5990, 59900S.
- H. Sohn, R. M. Calhoun, M. J. Sailor and W. C. Trogler, Detection of TNT and picric acid on surfaces and in seawater by using photoluminescent polysiloles, Angew. Chem., Int. Ed., 2001, 40, 2104–2105 CrossRef CAS
.
- R. C. Stringer, S. Gangopadhyay and S. A. Grant, Detection of nitroaromatic explosives using a fluorescent-labeled imprinted polymer, Anal. Chem., 2010, 82, 4015–4019 CrossRef CAS
.
- V. Kumar, B. Maiti, M. K. Chini, P. De and S. Satapathi, multimodal fluorescent polymer sensor for highly sensitive detection of nitroaromatics, Sci. Rep., 2019, 9, 7269 CrossRef
.
- K. D. Prasad, N. Venkataramaiah and T. N. Guru, Row, 1,9-Pyrazoloanthrone as a colorimetric and “turn-on” fluorometric chemosensor: structural implications, Cryst. Growth Des., 2014, 14, 2118–2122 CrossRef
.
- C. J. Ferguson, R. J. Hughes, D. Nguyen, B. T. T. Pham, R. G. Gilbert, A. K. Serelis, C. H. Such and B. S. Hawkett,
Ab initio emulsion polymerization by raft-controlled self-assembly, Macromolecules, 2005, 38, 2191–2204 CrossRef CAS
.
- R. Zhang, X. Wu, L. J. Guziec, F. S. Guziec, G. L. Chee, J. C. Yalowich and B. B. Hasinoff, Design, synthesis and biological evaluation of a novel series of anthrapyrazoles linked with netropsin-like oligopyrrole carboxamides as anticancer agents, Bioorg. Med. Chem., 2010, 18, 3974–3984 CrossRef CAS
.
- H. D. H. Showalter, J. L. Johnson, J. M. Hoftiezer, W. R. Turner, L. M. Werbel, W. R. Leopold, J. L. Shillis, R. C. Jackson and E. F. Elslager, Anthrapyrazole anticancer agents. Synthesis and structure-activity relationships against murine leukemias, J. Med. Chem., 1987, 30, 121–131 CrossRef CAS
.
- W. Bradley and W. K. Geddes, J. Chem. Soc., 1952, 1630 RSC
.
- M. K. Kim and D. F. Wiemer, EDC-mediated condensations of 1-chloro-5-hydrazino-9,10-anthracenedione, 1-hydrazino-9,10-anthracenedione, and the corresponding anthrapyrazoles, Tetrahedron Lett., 2004, 45, 4977–4980 CrossRef CAS
.
- S. Maji, G. Vancoillie, L. Voorhaar, Q. Zhang and R. Hoogenboom, RAFT polymerization of 4-vinylphenylboronic acid as the basis for micellar sugar sensors, Macromol. Rapid Commun., 2014, 35, 214–220 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2002889. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0qm00563k |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2021 |