Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Circularly polarised luminescence (CPL) control of oligopeptide–Eu(III) hybridized luminophores by interaction with peptide side chains†
Yuki Mimuraa,
Takuya Satoa,
Yuki Motomuraa,
Hiroki Yoshikawaa,
Motohiro Shizuma b,
Mizuki Kitamatsu*a and
Yoshitane Imai
b,
Mizuki Kitamatsu*a and
Yoshitane Imai *a
*a
aDepartment of Applied Chemistry, Faculty of Science and Engineering, Kindai University, 3-4-1 Kowakae, Higashi-Osaka, Osaka 577-8502, Japan. E-mail: y-imai@apch.kindai.ac.jp; kitamatu@apch.kindai.ac.jp
bDepartment of Biochemistry, Osaka Research Institute of Industrial Science and Technology, 1-6-50 Morinomiya, Joto-ku, Osaka 536-8553, Japan
First published on 14th January 2020
Abstract
Chiral oligopeptide-naphthalene/Eu(III) hybridized luminophores emit strong circularly polarised solution-state luminescence (CPL) from Eu(III) at 592 and 614 nm (|gCPL| ≤ 2.1 × 10−2). Although the peptide ligands have matching absolute configurations, the CPL sign is controllable by varying the number of naphthalene units and peptide/Eu(III) coordination ratio.
Photoluminescent organic and inorganic materials are used in high-performance electroluminescent and optoelectronic devices. Recently, optically active luminophores that emit circularly polarised luminescence (CPL) with high quantum yield (ΦF) have received a great deal of interest.1 When coordinated by chiral organic ligands, f–f transition metal ions, such as Eu(III), create strong CPL-emitting luminophores due to high CPL dissymmetry ratio (gCPL), very narrow full-width at half-maximum (FWHM) bandwidth, and highly tuneable CPL intensity, spanning the visible to near-infrared (NIR) region.2
Generally, two enantiomeric organometallic luminophores bearing opposite chirality are required for the selective emission of left-handed and right-handed CPL; however, such chiral organic ligands are not always synthetically tractable. Therefore, it is highly desirable to develop a novel method for controlling the CPL sign of chiral organometallic luminophores without the need to synthesise their respective enantiomers.
Recently, we reported a series of chiral oligopeptide-pyrene luminophores in which the CPL signs of the bipyrenyl-based excimer were successfully controlled by tuning either the distance between the two pyrenyl fluorophores or the solvent.3 In addition, the CPL signs of binaphthyl-Eu(III) hybridized luminophores were successfully inverted by altering the distance between the axial chiral point and the Eu(III) ion.4 Nevertheless, it remains difficult to control the chiroptical properties of metal-based luminophores coordinated to stereoisomeric ligands of the same absolute configuration owing to the metal's multiple coordination sites and the large distance between the metal and the ligand's chiral centre.
Herein, we report oligopeptide–Eu(III) hybridized luminophores, in which the signs of the Eu(III) CPL signals at the 5D0 → 7F1 and 5D0 → 7F2 transitions were subtly inverted in accordance with the number of naphthalene rings in the peptide and the coordination ratio of Eu(III) to peptide, despite the oligopeptide ligand having the same stereocentres (L or D). Notably, the CPL signals were inverted even though the corresponding chiroptical signs in the circular dichroism (CD) signals of all oligopeptides with L- or D-stereocentres were identical.
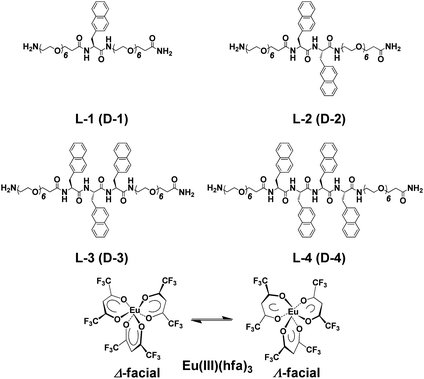
Using conventional solid-phase peptide synthesis, we prepared eight compounds comprised of the L- and D-isomers of four chiral oligopeptide–naphthalene organic ligands having different numbers of aromatic naphthalene groups: H-Sp6-L-Ala(Nap)-Sp6-NH2 (L-1), H-Sp6-L-Ala(Nap)-L-Ala(Nap)-Sp6-NH2 (L-2), H-Sp6-L-Ala(Nap)-L-Ala(Nap)-L-Ala(Nap)-Sp6-NH2 (L-3), and H-Sp6-L-Ala(Nap)-L-Ala(Nap)-L-Ala(Nap)-L-Ala(Nap)-Sp6-NH2 (L-4), and the corresponding D-isomers, D-1–D-4 (Chart 1; ESI, Fig. S1–S4†). Optically inactive Eu(III)(hfa)3(H2O)2 [hfa; 1,1,1,5,5,5-hexafluoropentane-1,4-dione] was prepared as previously described (Chart 1).4
Four hybridized luminophores, L-1/Eu–L-4/Eu (and D-1/Eu–D-4/Eu), were obtained by mixing the corresponding 1–4 with Eu(III)(hfa)3(H2O)2 in the desired molar ratio in chloroform (CHCl3) at room temperature. We assume that, in the ground state, Eu(III)(hfa)3 exists as a mixture of Δ- and Λ-isomers of D3-symmetrical facial geometry.
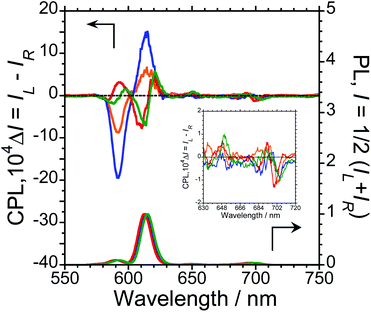
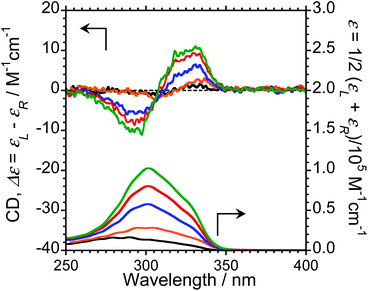
To characterise the photophysical properties of the L-1–L-4/Eu (1/1 molar ratio) luminophores as a function of naphthalene unit number, the steady-state unpolarised photoluminescence (PL) and CPL spectra of dilute L-1–L-4/Eu and L-1–L-4 solutions in CHCl3 were obtained (Fig. 1 and ESI, Fig. S5–S9,† and Table 1). Although aggregation-induced quenching regularly limits the use of luminophores, L-1–L-4/Eu remarkably exhibited Eu(III) PL in CHCl3 (Fig. 1, lower panel). The photoluminescence maxima (λem) for L-1–L-4/Eu are ∼592 nm (5D0 → 7F1), ∼614 nm (5D0 → 7F2), ∼651 nm (5D0 → 7F3), and ∼698 nm (5D0 → 7F4). The values of λem for L-1–L-4/Eu subtly differ from one another by approximately 1−2 nm. The ΦF values for L-1–L-4/Eu, mainly arising from the electric-dipole-allowed 5D0 → 7F2 transitions, are also similar (0.21, 0.27, 0.34 and 0.32, respectively), owing to the ligands' identical peptide backbone unit.
| CPL | ΦF | CD | |||||
|---|---|---|---|---|---|---|---|
| λCPL (nm) | gCPL (×10−2) | λCPL (nm) | gCPL (×10−2) | λCD (nm) | gCD (×10−4) | ||
| L-1/Eu | 592 | −0.93 | 615 | +0.067 | 0.21 | 330 | +3.1 |
| L-2/Eu | 592 | −2.1 | 615 | +0.15 | 0.27 | 329 | +5.3 |
| L-3/Eu | 593 | +0.39 | 611 | −0.083 | 0.34 | 333 | +4.9 |
| L-4/Eu | 597 | +0.24 | 614 | −0.074 | 0.32 | 334 | +0.8 |
Luminophores L-1–L-4/Eu exhibit clear CPL signals characteristic of 5D0 → 7F1 and 5D0 → 7F2 transitions (Fig. 1, upper panel). The chiral oligopeptides are thus powerful ligands for Eu(III)(hfa)3 luminescence, affording CPL at 4f–4f transitions. Interestingly, although L-1–L-4 have the same chirality centre, the signs of their CPL spectra for the 5D0 → 7F1 and 5D0 → 7F2 transitions changed in accordance with the number of naphthalene units in the peptide; specifically, −/+ signs for L-1–L-4/Eu having one or two naphthalene units and −/+/−/+ signs for L-1–L-4/Eu having three or four naphthalene units are clearly observed. As expected for stereoisomers, the CPL spectra of L-1–L-4/Eu and D-1–D-4/Eu almost mirror one another (ESI, Fig. S5b–S8b†). The gCPL values in the photoexcited state allow us to quantitatively describe the degree of CPL inducibility provided by L-1–L-4/Eu, defined as gCPL = (IL – IR)/[1/2 (IL + IR)] where IL and IR denote the intensities of the left- and right-handed CPL, respectively, following excitation with unpolarised light. The gCPL values for L-1/Eu and L-2/Eu are −0.93 × 10−2 at 592 nm and +0.067 × 10−2 at 615 nm, and −2.1 × 10−2 at 592 nm and +0.15 × 10−2 at 615 nm, respectively. The gCPL values for L-3/Eu and L-4/Eu are +0.39 × 10−2 at 593 nm and −0.083 × 10−2 at 611 nm, and +0.24 × 10−2 at 597 nm and −0.074 × 10−2 at 614 nm, respectively. Overall, L-2/Eu delivers the highest |gCPL| values among the luminophores. These results suggest that the chirality, L or D, of the ligand's peptide backbone does not determine the CPL signs of Eu(III) ions at 4f–4f transitions; rather, the CPL signs of peptide ligand-bound Eu(III) are guided by the number of naphthalene units in the peptide.
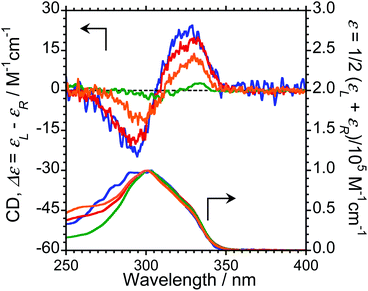
We next measured the CD properties of L-1–L-4/Eu (1/1 molar ratio) and L-1–L-4 in CHCl3 (Fig. 2 and ESI, Fig. S10–S12,† upper panel, and Table 1). In contrast to the variation observed for the CPL signs, L-1–L-4/Eu complexes commonly exhibit negative (−)-CD spectra at 297 nm, characteristic of the π–π* transitions of the chiral naphthalene units, while the positive (+)-CD spectra at 331 nm arise from the π–π* transitions of both the chiral naphthalene units and the hfa ligands. The CD spectra for L-1–L-4/Eu and D-1–D-4/Eu are mirror images of each other (ESI, Fig. S10†). The dimensionless Kuhn's anisotropy factors for the ground state chirality, gCD, for 1–4/Eu were ∼+3.1 × 10−4 at 330 nm (λCD), ∼+5.3 × 10−4 at 329 nm (λCD), ∼+4.9 × 10−4 at 333 nm (λCD), and ∼+0.8 × 10−4 at 334 nm (λCD), respectively.
Upon comparing the CPL and CD signs of 1–4/Eu, we concluded that the S0 ground state chiralities of the luminophores are the same, but the S1 photoexcited state chiralities are opposite. In solution, optically inactive Eu(hfa)3 exists as a mixture of interconvertible D3-symmetric Δ- and Λ-isomers, owing to the low barrier height between the two forms. The CPL sign inversion in 1–4/Eu may therefore result from the dynamic switching between the Δ- and Λ-forms of Eu(III)(hfa)3 owing to a dynamic change in the chiral peptide chain, which occurs only in the photo-excited states in the CHCl3 solution.
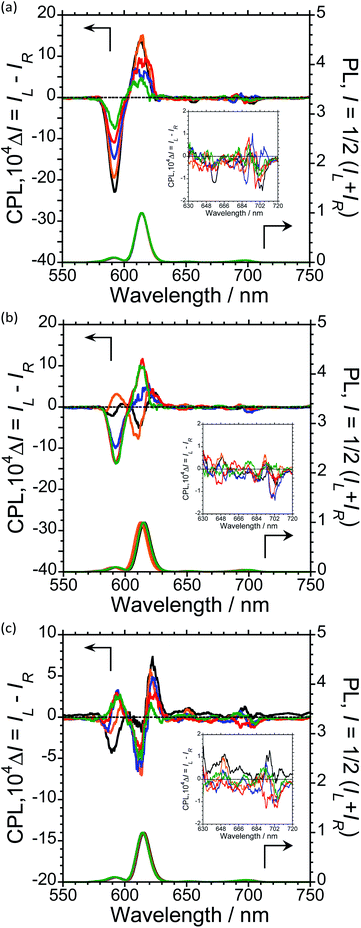
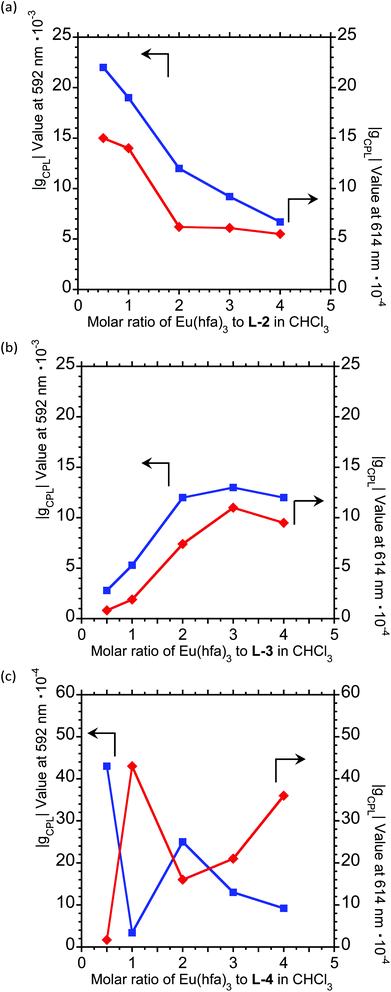
To further expand the CPL control produced by oligopeptide–Eu(III) hybridized luminophores, we sought to modulate the CPL by varying the peptide/Eu(III) molar ratio. Concentration-dependent CPL and PL spectra of L-1–L-4/Eu (1/0.5, 1/1, 1/2, 1/3, and 1/4 molar ratios) were obtained using a fixed concentration of [L-2–4] (1.0 × 10−4 M in CHCl3) (Fig. 3; ESI, Fig. S6–S8†). The CPL intensities at ∼592 nm and ∼614 nm are similar for (L-2/Eu) ratios of 1/0.5 and 1/1. However, when the molar ratio of L-2 to Eu varies from 1/1 to 1/4, these intensities decreased. These results suggest that L-2 coordinates to Eu(III) in a 1/1 ratio in CHCl3. Unfortunately, despite the intensity changes, the CPL sign remained constant.
The CPL spectra of L-3/Eu in CHCl3 differ greatly from those of L-2/Eu (Fig. 3(b), ESI, Fig. S7†). Surprisingly, the CPL sign could be controlled by varying the molar ratio of Eu(III) to L-3. The 5D0 → 7F1 and 5D0 → 7F2 transitions have decisively opposite CPL signs: −/− signs for 1/0.5 (L-3/Eu) molar ratios, +/− signs for 1/1 (L-3/Eu) molar ratios and −/+ signs for 1/2–4 (L-3/Eu) molar ratios. Additionally, 1/2–4 (L-3/Eu) molar ratios display similar CPL intensities, indicating that L-3 coordinates to Eu(III) at a maximum 1/2 (L-3/Eu) ratio in CHCl3. The CPL sign could also be controlled by varying the molar ratio in the L-4/Eu system (Fig. 3(c), ESI, Fig S8†). While the CPL signs for the 5D0 → 7F2 transitions were unchanged, decisively opposite CPL signs for the 5D0 → 7F1 transition were observed: − signs for 1/0.5–1 (L-4/Eu) molar ratios and + signs for 1/2–4 (L-4/Eu) molar ratios. These results are consistent with the idea that the CPL sign can be tailored by tuning both the molar ratio of Eu(III) to peptide and the configurational chirality (L or D) of the oligopeptide.
To investigate the ground-state chirality of different L-3/Eu molar ratios, CD and UV-Vis absorption spectra of 1/0.5–4 L-3/Eu in CHCl3 were obtained (Fig. 4; ESI, Fig S10†). Despite the L-3/Eu molar ratio, several characteristic CD bands arising from the π–π* transitions of the naphthalene units and the hfa ligands were observed. There were no CD signal sign changes observed. These results further confirm that there are no differences between the S0 ground state chiralities of the four luminophores.
For a fixed concentration of L-2–L-4 (1.0 × 10−4 M), the gCPL value at the two CPL bands (592 and 614 nm) in CHCl3 slightly depended on the molar ratio when changed from 1/0.5 to 1/4 (L-2–4/Eu) (Fig. 5; ESI, Fig. S6–S8†). In L-2, although the |gCPL| values decreased as the molar ratio of Eu(III) to L-2 increased from 1/0.5 to 1/4, the slope of the curve drastically changes between L-2/Eu molar ratios of 1/1 and 1/2 (Fig. 5(a); ESI, Fig. S6†). On the other hand, for L-3, the |gCPL| values increased with an increasing L-3/Eu molar ratio from 1/0.5 to 1/2 (Fig. 5(b); ESI, Fig. S7†). A dramatic slope change was observed between 1/2 and 1/3 (L-3/Eu) molar ratios. Complex changes in the |gCPL| values were observed for L-4 (Fig. 5(c); ESI, Fig. S8†). These results further confirm that L-2 and L-3 coordinate to Eu(III) in CHCl3 in 1/1 and 1/2 ratios, respectively.
The coordination capacity of the amide units of 1–4 to Eu(III) was supported by positive mode LCMS-IT-TOF analysis (Table 2 and ESI, Fig. S13 to S18†). This work confirmed that 1–4 coordinated to Eu(III): [Eu(L-1)(hfa)2]+ (m/z 1451.4020) for L-1, [Eu(L-2)(hfa)2]+ (m/z 1648.4832) for L-2, [Eu(L-3)(hfa)3HNa]2+ (m/z 1037.2530) for L-3, and [Eu(L-4)(hfa)2]+ (m/z 2042.6524) for L-4. Although three hfa ligands were observed for L-3/Eu, only two hfa ligands were observed for L-1, L-2, and L-4 complexes. In addition, the molar ratio of Eu(III) complex to peptide ligand for L-3 changed from 1/1 to 1/3. Unfortunately, in the case of 1/2 and 1/3 (L-3/Eu) ratios, only the signal corresponding to a 1/1 L-3/Eu ratio was observed; i.e., [Eu(L-3)(hfa)2]+ (m/z 1845.5684) for L-3/Eu = 1/2 and [Eu(L-3)(hfa)2]+ (m/z 1845.5662) for L-3/Eu = 1/3. The coordination of Eu(III) to O (from the peptide) is rather weak; therefore, the Eu(III)–O bonds of the hybrid luminophores may easily dissociate under laser-assisted ionisation energy.
| m/z | Found Eu complex | |
|---|---|---|
| L-1 | 1451.4020 | [Eu(L-1)(hfa)2]+ |
| L-2 | 1648.4832 | [Eu(L-2)(hfa)2]+ |
| L-3 (L-3/Eu = 1/1) | 1037.2530 | [Eu(L-3)(hfa)3HNa]2+ |
| L-3 (L-3/Eu = 1/2) | 1845.5684 | [Eu(L-3)(hfa)2]+ |
| L-3 (L-3/Eu = 1/3) | 1845.5662 | [Eu(L-3)(hfa)2]+ |
| L-4 | 2042.6524 | [Eu(L-4)(hfa)2]+ |
19F-NMR spectra of Eu(III)(hfa)3 with 1–4 (1/1) were measured in CDCl3 (ESI, Fig. S19–S24†). The 19F-NMR spectra of L-1–L-4/Eu in CDCl3 indicates that L-1–L-4/Eu exhibit strong resonance signals at −75.23 ppm (L-1), −79.53 ppm (L-2), −79.46 ppm (L-3), and −79.59 ppm (L-4), respectively, and weak resonance signals at −78.85 ppm and −79.59 ppm (L-1), −75.14 ppm (L-2), −75.08 ppm (L-3), −75.23 ppm and −78.85 ppm (L-4), respectively. These clear chemical shift changes were attributed to the dynamic coordination of 1–4 to Eu(III) in solution. The eighteen fluorine atoms of Eu(hfa)3 are no longer magnetically equivalent due to a lowering of the molecular symmetry from D3 to C1 upon interaction with L-1–L-4, as the chirality inducing ligands in the ground state and possibly in the photoexcited states. A significant reorganisation of the three strong hfa ligands along with weaker 1–4 ligands to induce chirality towards Eu(III) ions might occur in the photoexcited state.
The reason behind the CPL sign inversion has not yet been elucidated. The rotational freedom of the peptide backbone in ligands 1–4 is likely the key to imparting the CPL sign-swapping capability in the photoexcited state, even though 1–4/Eu have the same CD sign in the ground state. It is possible that the twists of the peptide chain, naphthalene units, and hfa ligands in these luminophores are opposite only in the photo-excited states in chloroform.
Chiral 1–4/Eu luminophores were easily prepared by mixing the oligopeptide ligands with Eu(III)(hfa)3 in CHCl3. By varying the number of naphthalene units in the ligand and the molar ratio of peptide ligands/Eu(III), the CPL signs of the luminophores could be controlled. Our findings offer a novel method to control the CPL sign of CPL-functionalised Eu(III)-based luminophores and have obvious applications to other chiral peptide ligands and, potentially, other lanthanide(III) ions. In addition, these results provide a deeper understanding of how photoexcited, chiral organometallic luminophores can relax to their ground states with minimum to maximum reorganisation, as revealed by the conservation and breaking of signs between the CD and CPL signals.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (no. 18K05094 and 19H04600) from MEXT/Japan Society for the Promotion of Science and the KDDI and Futaba Foundations.Notes and references
- (a) E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem.–Eur. J., 2015, 21, 13448 CrossRef PubMed; (b) G. Longhi, E. Castiglioni, J. Koshoubu, G. Mazzeo and S. Abbate, Chirality, 2016, 28, 696 CrossRef CAS PubMed; (c) J. Roose, B. Z. Tang and K. S. Wong, Small, 2016, 12, 6495 CrossRef CAS PubMed; (d) J. Yan, F. Ota, B. A. San Jose and K. Akagi, Adv. Funct. Mater., 2017, 27, 1604529 CrossRef.
- (a) R. B. Rexwinkel, S. C. J. Meskers, J. P. Riel and H. P. J. M. Dekkers, J. Phys. Chem., 1992, 96, 1112 CrossRef CAS; (b) S. Petoud, G. Muller, E. G. Moore, J. Xu, J. Sokolnicki, J. P. Riehl, U. N. Le, S. M. Cohen and K. N. Raymond, J. Am. Chem. Soc., 2007, 129, 77 CrossRef CAS PubMed; (c) J. L. Lunkley, D. Shirotani, K. Yamanari, S. Kaizaki and G. Muller, J. Am. Chem. Soc., 2008, 130, 13814 CrossRef CAS PubMed; (d) J. W. Walton, R. Carr, N. H. Evans, A. M. Funk, A. M. Kenwright, D. Parker, D. S. Yufit, M. Botta, S. De Pinto and K.-L. Wong, Inorg. Chem., 2012, 51, 8042 CrossRef CAS PubMed; (e) F. Zinna and L. Di Bari, Chirality, 2015, 27, 1 CrossRef CAS PubMed; (f) T.-Y. Li, Y.-M. Jing, X. Liu, Y. Zhao, L. Shi, Z. Tang, Y.-X. Zheng and J.-L. Zuo, Sci. Rep., 2015, 5, 14912 CrossRef CAS PubMed; (g) F. Zinna, U. Giovanella and L. Di Bari, Adv. Mater., 2015, 27, 1791 CrossRef CAS PubMed; (h) K. Binnemans, Coord. Chem. Rev., 2015, 295, 1 CrossRef CAS; (i) T. Wu, J. Prusa, J. Kessler, M. Dracinsky, J. Valenta and P. Bour, Anal. Chem., 2016, 88, 8878 CrossRef CAS PubMed; (j) E. Brichtova, J. Hudecova, N. Vrskova, J. Sebestik, P. Bour and T. Wu, Chem.–Eur. J., 2018, 24, 8664 CrossRef CAS PubMed.
- (a) T. Nishikawa, S. Kitamura, M. Kitamatsu, M. Fujiki and Y. Imai, ChemistrySelect, 2016, 4, 831 CrossRef; (b) Y. Mimura, S. Kitamura, M. Shizuma, M. Kitamatsu, M. Fujiki and Y. Imai, ChemistrySelect, 2017, 2, 7759 CrossRef CAS.
- N. Hara, M. Okazaki, M. Shizuma, S. Marumoto, N. Tajima, M. Fujiki and Y. Imai, ChemistrySelect, 2017, 2, 10317 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09708b |
| This journal is © The Royal Society of Chemistry 2020 |