Seashells by the zinc shore: a meeting report of the International Society for Zinc Biology, Asilomar, CA 2014
Michal
Hershfinkel
*a,
Dianne
Ford
b,
Shannon
Kelleher
c and
Elias
Aizenman
ad
aDepartment of Physiology and Cell Biology and The Zlotowski Center of Neuroscience, Ben-Gurion University of the Negev, Faculty of Health Sciences, POB 653, Beer-Sheva, 84105, Israel. E-mail: hmichal@bgu.ac.il; Fax: +972-8-6477627; Tel: +972-8-6477-318
bInstitute for Cell and Molecular Biosciences and Human Nutrition Research Centre, Newcastle University, Newcastle upon Tyne, UK
cDepts. of Cellular and Molecular Physiology, Pharmacology and Surgery, Penn State Hershey College of Medicine, Hershey, PA 17033, USA
dDepartment of Neurobiology and Pittsburgh Institute for Neurodegenerative Disorders, University of Pittsburgh School of Medicine, Pittsburgh, PA 15261, USA
Introduction
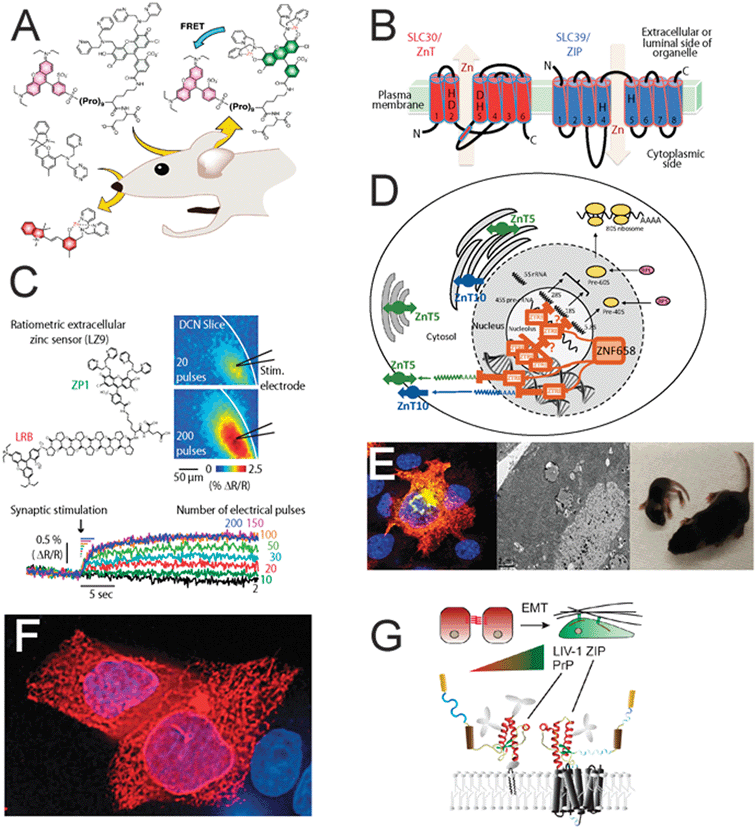
The International Society for Zinc Biology, ISZB (http://www.ISZB.org), held its fourth biennial meeting at the Asilomar Conference Grounds, Pacific Grove, CA in September 2014. The meeting was attended by nearly 175 scientists representing 16 countries from the Americas, Europe, the Middle East, East Asia and Australia. Topics covered in the meeting included the genetics and structure/function relationships of zinc transporters and binding proteins, the physiological functions of zinc in yeast, bacteria, nematodes and mammalian models, as well as the nutritional, pharmacological and toxicological aspects of zinc in humans. Finally, bioinorganic chemistry of novel tools aimed at revealing the biology of zinc was also discussed.Zinc is an essential metal ion in all living organisms, serving not only as a catalytic and structural cofactor, but also as a first and second messenger in a large number of cellular signaling processes (Fukada et al. 2014, ref. 1). The complexity of cellular zinc regulation is indeed reflected by the presence of nearly 3000 zinc-interacting proteins, including 24 identified zinc membrane transporters. This meeting highlighted recent advances in mechanisms underlying zinc transport and signaling, which will pave the way for a better understanding of the physiological roles of this metal and the requirements for zinc as a micronutrient. Moreover, innovative analytical and chemical tools for determining cellular zinc levels, a highly debated topic in the field, were presented at this meeting. The many high impact and exciting new developments in the field of zinc biology (Fig. 1), presented at this vibrant meeting are summarized briefly in this report.
Keynote lectures
In 1993, Walter Schaffner and colleagues isolated the zinc sensitive transcription factor MTF-1, which induces the expression of metallothionein (MT), a cytoplasmic zinc binding protein. In a very stimulating plenary talk, Schaffner described the evolution of this zinc-finger transcription factor and its role in zinc as well as copper metabolism. The performance of this transcription factor in gene induction was correlated to the length of life of individual species, strongly suggesting that longevity and survival depend on proper metal homeostasis.Our knowledge of cellular zinc signaling and homeostasis has lagged behind that of other metal ions due to the previous lack of zinc-specific tools, and the inability to differentiate between calcium and zinc signals within cells. Stephen J. Lippard, who has developed multiple chemical tools for tracking and controlling cellular zinc ions, elegantly described how the field of metal-binding fluorescent tools has evolved, focusing on several novel fluorescent dyes that permit discrimination of minute changes in intracellular free zinc with a high signal to noise ratio. Moreover, Lippard described the use of a new generation of ultra-fast and highly specific zinc chelators as powerful tools to understand the functions of synaptically released zinc in the auditory and olfactory systems.
Measuring zinc status in humans remains a major challenge in the field and is particularly important for studies addressing the essentiality of the metal as a nutrient. In the third Plenary Lecture, Janet C. King suggested that in the absence of a reliable biomarker, dietary zinc intake and organismal physiological measurements such as growth and immunomodulation could be used to determine adequate zinc status. King noted, however, that zinc intake recommendations are confounded by the need to account for effects of factors that inhibit its absorption, such as dietary levels of plant-derived phytates. Multidisciplinary approaches are required to advance the development of reliable assessment tools and to address the consequences of deficient or excess zinc intake on health and disease.
Finally, pioneers of zinc biology gave highly educative historical reviews of the field and challenged the audience with further questions that remain to be addressed in order to move forward in the future. Ananda S. Prasad and Harold H. Sandstead were among the first to identify the critical role of dietary zinc in growth, development and immune system function. They presented the enormous progress made in the field of zinc nutrition observed during their professional lifetime, yet highlighted the continuing need for advancing the knowledge of the impact of proper zinc status on human health. Dennis Choi, an early zinc neurobiologist who investigated the role of this metal in neurodegenerative disorders, highlighted the urgent need for accelerating translation of basic science observations into clinical practice and encouraged the audience, especially the younger participants, to ardently continue to tackle difficult problems with their research.
All keynote lectures showcased the rapid growth of the field and how mechanistic details are being elucidated to give a sound understanding of the role of zinc in biological systems. Although a consensus emerged that we are now seeing beyond the tip of the biological zinc iceberg, we have yet to uncover many pathways that will lead to understanding the role of the metal in living organisms.
The ISZB awarded the Frederickson Prize for seminal studies in the field of zinc biology to John H. Weiss. In his prize acceptance presentation, Weiss described major advances that lead to elucidating the role zinc plays in the central nervous system. The initial description of a releasable pool of zinc ions in synaptic vesicles by Gorm Danscher and Christopher J. Frederickson was the trigger that instigated his own studies on this topic. Weiss described his work that identified a role for calcium-permeable AMPA channels as a major permeation pathway for entry of synaptically released zinc into post-synaptic neurons. He then presented his studies on the intracellular release of zinc from mitochondria, accompanied by the generation of reactive oxygen species and increased neuronal cell death during ischemic injury.
Zinc transporters, providing zinc in the right place at the right time
Fascinating sessions on zinc trafficking via Zn2+ transporters in various genetic models showed how conserved processes point to the essentiality of Zn2+ for all biological systems. There are at least 24 described Zn2+ transporters that belong to either the ZIP (SLC39A) or ZnT (SLC30A) families. These transporters are expressed in a cell-specific manner on different organelles and are responsible for maintaining correct Zn2+ concentrations within the extracellular milieu, cytoplasm, and sub-cellular organelles. Although the existence of Zn2+ transporters has been known for many years, knowledge about their mechanism of action is lacking. In recent years, work from the laboratories of Dax Fu and Israel Sekler have identified the structural properties and transport mechanisms of the ZnTs, yet what regulates the activity of these transporters is still poorly understood. At the meeting, Sekler described how ZIP transporters mediate Zn2+ uptake into the cytoplasm and its removal from organelles through a putative Zn2+ ion permeation pathway, and further discussed the similarity of their function to that of the ZnTs. Kathryn M. Taylor presented her work revealing how phosphorylation and proteolytic cleavage regulate the activity of ZIP transporters.The large number of Zn2+ transporters present in the mammalian cell raises numerous questions regarding their precise roles, a subject that was discussed in several sessions throughout the meeting. For example, Johannes Engelken raised a general hypothesis that changes in Zn2+ transporter expression is a driving force underlying carcinogenesis. Yehuda G. Assaraf showed how ZnT transporters form both, homo- and hetero-dimers using a bimolecular fluorescence complementation (BiFC) technique. A physiological role for such dimerization had been previously described by Gloria Salazar, who presented new results on ZnT10 dimerization and its role in protein localization. Taiho Kambe reported a role for the coordinated action of ZnT transporters and metallothioneins (MTs), required for the activation of Zn2+-dependent enzymes. Miki Kawachi, Dax Fu and Robert E. Dempski showed how putative Zn2+ binding sites on various transporters affect the kinetics of ion transport and thereby regulate physiological functions, such as metal tolerance of cells.
Using Drosophila melanogaster, Richard Burke dissected the roles of various members of the ZnT family in conditions of either zinc excess or deficiency through their effect on the proper development of the fly eye. Fanis Missirlis expanded the analysis to show how excess zinc is stored to protect either individual cells or the whole organism from zinc toxicity. The same Drosophila model was used by David W. Killilea to identify a role of zinc transporters in urinary stone formation and tubule obstruction. He further showed that this effect was dependent on dietary zinc levels, linking transporter function and excess zinc to the formation of urinary stones. Using Caenorhabditis elegans as a model, S. Kerry Kornfeld has identified novel genes that are involved in zinc metabolism and are induced by excess zinc. Christer Hogstrand used the zebrafish model to elegantly show the importance of ZIP and ZnT transporters in development, in particular ZIP10.
Attesting to their importance, ZIP proteins are widely expressed across species and preserved in evolution. Gerold Schmitt-Ulms described his finding that the prion gene family descended from an ancestral Liv-ZIP gene, and maintained structural similarity with the Zn2+ transporter. His study further revealed a role for ZIP6 and the prion protein in the epithelial/mesenchymal transition. Nigel M. Hooper discussed a different angle of Zn2+-related prion protein physiology by showing how neuronal Zn2+ accumulation is enhanced by prion proteins and linked to amyloid-beta oligomer signaling.
Several studies have described the “hijacking” of zinc transporters by other metal ions such as manganese, iron and cadmium. In this context and using Drosophila melanogaster as a model, Guiran Xiao showed a role for ZIP13 in iron transport and suggested that in Ehlers-Danlos syndrome, in which hZIP13 is defective, iron dyshomeostasis, in addition to zinc, may be responsible for some of the observed pathology. Similarly, Michael D. Knutson described a role for ZIP14 in iron transport in the liver, and suggested that modulation of ZIP14 expression is related to hemochromatosis. Collectively, these investigations implicate a role for zinc in modulating the activity of both environmental metals and other biometals.
Cellular zinc binding proteins
The topic of low cellular Zn2+ concentrations and the binding affinities of intracellular proteins is a complex and major issue in our understanding of signaling roles played by Zn2+ ions. There is a strong call to accept a widely understandable terminology. Wojciech Bal showed a rigorous analysis of Zn2+ binding constants of biomolecules.Jeanne A. Hardy presented a study of the binding of Zn2+ to distinct sites on caspases and the role of this process in caspase-initiated apoptosis. Another example for a role of Zn2+-binding in regulation of physiological function was presented by Deborah B. Zamble, who described a regulatory metal binding site on members of a subfamily of P-loop GTPases. Kengo Homma presented results indicating that Zn2+-deficiency induces conformational changes in the Zn2+ binding protein SOD1 and subsequent ER stress, similar to the effects of SOD1-mutants associated with amyotrophic lateral sclerosis (ALS). In the blood Zn2+ is bound to albumin, Alan Stewart and Claudia A. Blindauer identified the binding site for this ion and showed that this site may be disrupted by other metal ions or fatty acids, an issue that may link the metabolism of these essential nutrients.
Zinc binding by microbes is essential for their survival and function, and thus Zn2+ binding proteins were also discussed in relation to host/pathogen interactions. Calprotectin, a protein with antimicrobial functions, was shown to have Zn2+ binding sites. Elizabeth M. Nolan further described how these sites are regulated by Ca2+, suggesting a tunable affinity for Zn2+. Thus upon its release, calprotectin is thought to compete with pathogens for available Zn2+ ions. Megan B. Brophy then suggested that S100 Ca2+ binding proteins may have a role in Zn2+ sequestration and antimicrobial effects. In the gut, Zn2+ is available for absorption; yet, during inflammatory conditions the levels of this ion are regulated by the release of the Zn2+ binding protein calprotectin. Expression of a high affinity Zn2+ transporter by the food borne pathogen Salmonella enterica serovar Typhimurium was described by Manuela Raffatellu. This transporter enhances the resistance of the bacteria to Zn2+. Raffatellu further discussed how intestinal microbes compete for Zn2+ ions in the inflamed gut and limit zinc availability to pathogens, thereby protecting the intestine. A specific role for macrophages in this process was discussed by Mathew J. Sweet, who showed upregulation of Zn2+ transporters in these cells following exposure to bacteria.
As the tight regulation of cellular Zn2+ levels is essential for all living species, the role of transcription factors and proteins regulated by Zn2+ may also have common features across phylogeny. Dianne Ford presented recent work that supports the theory that Zn2+ initially regulates MT and ZnT1 through MTF1, and that the transcription of secondary processes to maintain Zn2+ levels within cells is activated when this buffering capacity is exceeded. She also presented observations revealing that ZNF658, a newly identified zinc-sensitive transcription factor, plays a role in coordinating zinc homeostasis with ribosome biogenesis. Amanda J. Bird described how a yeast transcription factor, Loz1, is regulated by Zn2+ to control intracellular Zn2+ distribution and protect yeast cells from Zn2+ toxicity. David J. Eide described the role of another transcription factor that is regulated by Zn2+ in yeast, Zap1, and its target gene, Tsa1, in dysregulation of protein folding under zinc deficiency. Wolfgang Wohlleben showed that the bacterial Zur gene is a Zn2+ sensor that regulates the production of the zincophore EDDS (ethylenediaminedisuccinic acid), and knockdown of this gene allowed high yield of EDDS production that was independent of Zn2+. This may have applications in industry by allowing replacement of EDTA, a toxic non-biodegradable pollutant, with EDDS.
Physiological roles of zinc signaling
Although the role of cellular zinc as a structural and catalytic element in proteins has been known for several decades, it is now clear that Zn2+ is also a signaling ion that can trigger multiple physiological signaling cascades. Zn2+-dependent signaling was addressed in several sessions throughout the ISZB meeting, demonstrating the physiological role of zinc signals in numerous biological systems. Toshiyuki Fukada presented findings regarding zinc signaling in lymphocytes, indicating that ZIP10 deficiency results in aberrant antigen response and lymphocyte proliferation. A major role for a zinc transporter variant was previously linked to diabetes when a genome-wide association study identified ZnT8 as a susceptibility gene. A different aspect of this disease was addressed by the work of Belma Turan, who showed a role for zinc dyshomeostasis in cardiomyocytes from diabetic animals. Samantha J. Pitt expanded further on the role of zinc in the heart by showing how zinc modulates cardiac ryanodine receptors and thereby cardiac excitation–contraction coupling. Elisa Bellomo described a general role for Zn2+ in modulating protein tyrosine phosphatase activity at concentrations found in cells under physiological conditions. This action links Zn2+ to cancer, diabetes and obesity. Yoshio Fujitani described roles for the zinc ions secreted from the pancreas as modulators of hepatic insulin clearance. Thomas V. O'Halloran applied high-end imaging tools including STEM-EDS/EELS, X-ray fluorescence, and specific Zn2+ probes to show that “zinc sparks” are required for the meiotic cell cycle in early embryonic development.Epithelial cell physiology is becoming an important field for Zn2+ signaling research. Changes in zinc concentrations or in Zn2+ transporters are particularly apparent in breast cells, during normal hormonal cycles as well as during lactation, involution or even in cancer. Shannon L. Kelleher presented results from a comprehensive study on the role of ZnT2 in lactation and in regulating the involution of the tissue after lactation and the consequences of naturally occurring genetic variants. Absorption of dietary Zn2+ is done by epithelial intestinal cells expressing ZIP4 and ZIP5 proteins, and their expression is highly regulated by this ion. Novel results, however, by Agnes Michalczyk suggest that ZIP1 is expressed on the apical microvilli of the Caco-2 colonocyte cells, and is also involved in regulation of Zn2+ accumulation. Intestinal epithelial cells express a metabotropic Zn2+-sensing receptor, ZnR/GPR39, which triggers Zn2+-dependent signaling. Michal Hershfinkel described a role for the ZnR/GPR39 in modulating ion transport function as well as the formation of the tight junction barriers in the colon epithelial cells. These results further suggest that ZnR/GPR39 may be important in inflammatory bowel disease as well as diarrhea. A role for Zn2+ released from mast cells was presented by Keigo Nishida who linked Zn2+-dependent signaling to release of cytokines and wound healing.
Zinc in neurobiology
The neuroscience frontier, where Zn2+ was originally most extensively studied, continued to be a major theme at the conference. An electrophysiological study by Charles T. Anderson and Thanos Tzounopoulos on the role of vesicular Zn2+ in the dorsal cochlear nucleus revealed Zn2+-dependent regulation of extrasynaptic NMDA receptors, found outside the postsynaptic density and activated by glutamate spillover, using Lippard's high affinity, fast zinc chelator. This work also utilized high resolution Zn2+ imaging to directly show the activity-dependent release of Zn2+ from synaptic terminals. Effects of Zn2+ release in the hippocampus and retina were also discussed. Indeed, a novel pathway affecting neuronal survival and regeneration was linked to zinc accumulation in retinal ganglion cells following optic nerve crush by Larry I. Benowitz and Paul A. Rosenberg. These investigators showed that changes in Zn2+ levels dramatically affect neuroprotection and regeneration. Jung-Jin Hwang identified novel neuroprotective compounds attenuating Zn2+-induced cell death via inhibition of PARP and decreasing ERK1/2 phosphorylation. Frederick P. Bellinger showed that selenoprotein P is a selenium-dependent Zn2+ chelator that affects neuronal Zn2+ accumulation and release.Specific physiological roles for vesicular Zn2+ have been a major issue of debate in the field for several years. A particular issue is centered on the fact that ZnT knockout mouse models result in a limited phenotype. One such example is the ZnT3 knockout mice, devoid of vesicular zinc, which appear to have normal neuronal function. Changing this notion, Richard H. Dyck showed that activity-dependent hippocampal neurogenesis is impaired in the ZnT3 deficient mice. This was further related to impairment of hippocampal-dependent behavioral tasks in the ZnT3 deficient animals. A role for Zn2+ in neurogenesis following traumatic brain injury was described by Cathy W. Levenson who identified transcription factors regulating this process in neuronal stem cells. Further supporting a role for synaptic Zn2+ in learning and memory function, Haruna Tamano, Atsushi Takeda and Yang Li presented results from electrophysiological studies of long term potentiation, LTP, linking synaptic Zn2+ release to the regulation of neuronal activity in the hippocampus. Richard L. Chappell suggested that synaptic Zn2+ released from neurons has a feedback role in regulating neuronal response to a very wide range of stimuli in the retina. Using a multiple sclerosis model, Sang Won Suh revealed changes in ZnT3 expression upon induction of the demyelination process. His results thus link ZnT3 and neuronal vesicular zinc to this serious human disease. Paul T. Francis, using tissue from human patients, showed that loss of the same zinc transporter is associated with Lewy body dementia.
Another interesting aspect of neuronal Zn2+ is the association with neurological disorders. For example, Shank scaffold proteins localized to postsynaptic sites seem to be correlated with overall zinc levels in patients with autism, as demonstrated by Andreas M. Grabrucker. Another psychiatric disease sometimes linked to zinc deficiency is schizophrenia, and Elizabeth Scarr showed changes in ZIP12 mRNA levels in patients with this serious neurobehavioral disorder. This protein was also shown by Winyoo Chowanadisai to regulate neurite outgrowth and neuronal differentiation via a CREB-dependent pathway. A mutation in PARK9 ATPase causes Parkinsonism, and was shown by Taiji Tsunemi to render the cells susceptible to Zn2+ toxicity, suggesting that lowering Zn2+ may be beneficial to patients with this disease. Recent developments concerning the importance of zinc in Alzheimer's disease, from the formation of Aβ oligomers to Tau phosphorylation, which has been an important topic at previous ISZB meetings, were presented. Jae-Young Koh suggested a role for Zn2+-dependent lysosomal permeabilization in this disease and Bing Zhou suggested a role for Zn2+ in Tau phosphorylation. Ashley I. Bush described the complexity of applying the tools developed through basic research to therapeutics, via the example of the Zn2+ ionophore, PBT2. This compound was tested on animal models and shown to decrease Aβ load, restore zinc homeostasis and improve cognitive function.
Nutritional zinc and inflammation
Following the comprehensive plenary talks on zinc in nutrition by King, Prasad and Sandstead, methods for monitoring overall zinc levels in humans were discussed. Ruth A. Valentine presented a novel approach for measuring zinc distribution in teeth as a marker for early life exposure to zinc. John H. Beattie then described a systems biology approach to analyzing zinc deficiency, as it results in regulation and modulation of multiple genes and proteins. He applied a large human biomarker screen and identified proteins that could serve as markers for zinc deficiency when used together with a prediction model he went on to describe. M. Leigh Ackland described how nutritional zinc deficiency affects the health of newborns. Her group set out to monitor changes in zinc transporters in zinc deficient patients and showed that epigenetic modifications in ZnT5 may be associated with inadequate levels of zinc in breast milk. As the immune system is a major and early target of nutritional zinc deficiency, Lothar Rink expanded on the role of nutritional zinc deficiency on the immune function of T-cells, which is largely mediated by the Foxp3 transcription factor. Jorg J. Goronzy further showed that overexpression of ZIP8 or MTs allows for Zn2+ accumulation in T-cells, thereby regulating their activity. Charlie J. Pyle demonstrated that ZIP8 expression is modulated by bacterial lipopolysaccharide (LPS) in monocytes, and during their differentiation into macrophages. Activity of macrophages against the Histoplasma capsulatum fungus is also dependent on Zn2+ levels, and George S. Deepe showed that macrophage phagocytosis is triggered when cytokines induce Zn2+ release from the Golgi and that the levels of Zn2+ depend on MT expression.Zinc imaging tools
No zinc meeting would be complete without a session on the latest generation of Zn2+ imaging tools. This field is currently rapidly expanding in exciting directions as experts develop new, sensitive and highly specific reagents. Amy S. Palmer described a whole new array of genetically encoded FRET-based Zn2+ sensors. She showed preliminary results on the utilization of these probes in a wide array of cell sorting studies. Maarten Merkx further expanded the optical properties of similar sensors and showed the availability of these tools in a wide range of excitation/emission wavelengths that can be used simultaneously. Guy A. Rutter showed small molecule probes that are excellent Zn2+ sensors and described their use to study the role of Zn2+ in pancreatic islet function. Another novel approach was presented by Richard B. Thompson, who described ratiometric bioluminescent probes that reduce the requirement for optical penetration in vivo. Finally, Christoph J. Fahrni showed the utilization of X-ray fluorescence (SXRF) microscopy and two-photon excitation microscopy (TPEM) techniques with Zn2+ sensitive probes to monitor changes in Zn2+ distribution in whole embryos.Support of young scientists by the community
Opportunities for exceptional students and post-doctoral fellows to showcase their work were also included in this meeting, thanks in part to support from the United States National Institutes of Health. Speakers were selected based on the quality and novelty of their abstracts. These sessions gave trainees a high-profile opportunity to share their work with experts in the field. Changes in zinc transporter gene expression were monitored in high BMI subjects, revealing that changes in expression of ZnT1, 5, 6, and 10; none of which were expressed in an age-related manner, to be most prominent (Rasmus Olesen). A role for dimerization of ZnT transporters was previously described, and a second study presented at the meeting comprised a thorough screen of such interactions using an overexpression model system (Yarden Golan). Altered zinc homeostasis in obese patients was associated with changes in ZIP14 expression in adipose tissue, with weight loss reversing this effect (Trine Maxel). A study regarding the role of zinc transporters in physiology showed that signaling triggered by TNFα induced translocation of ZnT2 to activate lysosomal-mediated cell death, a process that is essential in mammary tissue involution (Stephen Hennigar). Another study from the same group, addressed the role of ZnT2 in the mammary gland using a ZnT2 knockout mouse model to show that this protein is also essential for milk production and secretion (Sooyeon Lee). A role for Zn2+ in regulating the growth factor gastrin in the digestive system was described and linked to changes in the epithelial/mesenchymal transition (Oneel Patel). A role for the huntingtin protein in Zn2+ deficiency, via regulation of ZIP proteins, suggests that Zn2+ dyshomeostasis may be involved in Huntington's disease (Scott Ayton). A study of nutritional zinc deficiency suggested that DNA strand breaks in leukocytes may serve as an accessible marker for zinc deficiency (Swapna Shenvi). Finally, novel probes based on the ZinPyr molecule that will allow photo uncaging and better temporal resolution of Zn2+ dependent events in the cell were described (Jacob Goldberg). Notably, presentations by students and young investigators were numerous throughout the meeting, encompassing more than 65 posters, in which all of the above mentioned topics were further discussed during a lively and highly interactive poster session.Summary
The 2014 ISZB meeting provided an exciting forum for presenting zinc biology-related research across the full spectrum. ISZB meetings are among the few scientific gatherings that are truly interdisciplinary, bringing together scientists from widely diverging fields, all having in common the desire to study the role of zinc in biological systems. Thus membrane transporters and cell signaling are discussed with relevance to structure/function relationships, while physiological and pathophysiological studies are described in many species and across most organ systems. Novel tools and techniques are presented to researchers and potential users to allow dissemination of zinc research-related information for the benefit of a wide scientific community studying various aspects of zinc in biology. This is one meeting that should not be missed by biochemists, cell and molecular biologists, physiologists, or anyone interested in the rich and exciting biology of zinc. We all look forward to our next gathering in Istanbul, Turkey, September 25–29, 2016, http://iszb2016turkey.medicine.ankara.edu.tr.The ISZB would like to thank Qiagen, Pfizer, Teva Pharmaceutical Industries, Novartis Pharma AG, Shino Test Corporation, Strem Chemicals, Genostaff and Hamari Chemicals for their generous donations that helped support the meeting. Book prizes for excellent talks were kindly provided by The Royal Society of Chemistry, Springer and IOS Press. Travel awards were supported in part by NIH grant GM112419. The authors would also like to thank Wolfgang Maret, Daren L. Knoell, Robert A. Colvin and David I. Soybel for their invaluable contributions to the organization of the meeting.
References
- Zinc Signals in Cellular Functions and Disorders, ed. T. Fukada and T. Kambe, Springer, Japan, 2014 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |