Themed collection Celebrating the 75th Birthday of Professor Barry Trost

Editorial: In celebration of Professor Barry M. Trost's 75th birthday
This special issue is dedicated to Barry M. Trost, Tamaki Professor of Humanities and Sciences, on the occasion of his 75th birthday.

Org. Chem. Front., 2017,4, 11-13
https://doi.org/10.1039/C6QO90046A
Barry Martin Trost: Educator
Honoring the 75th Birthday of Barry M. Trost.

Org. Chem. Front., 2016,3, 1225-1227
https://doi.org/10.1039/C6QO90033J
Ruthenium-catalysed hydrosilylation of carbon–carbon multiple bonds
Recent advances in ruthenium-catalysed hydrosilylation of C–C multiple bonds and its application to organic synthesis are highlighted.

Org. Chem. Front., 2016,3, 1337-1344
https://doi.org/10.1039/C6QO00261G
Non-stabilized enolates – versatile nucleophiles in transition metal-catalysed allylic alkylations
This review covers new developments in the transition metal-catalyzed allylic alkylations of non-stabilized enolates, preferentially generated from ketone, esters or amides.

Org. Chem. Front., 2016,3, 1541-1560
https://doi.org/10.1039/C6QO00192K
Beyond Fischer and Schrock carbenes: non-heteroatom-stabilized group 6 metal carbene complexes – a general overview
Non-heteroatom-stabilized group 6 metal carbene complexes: versatile reagents or intermediates in stoichiometric or catalytic reactions in organic synthesis.

Org. Chem. Front., 2016,3, 1561-1588
https://doi.org/10.1039/C6QO00206D
Recent progress in rhodium-catalyzed hydroaminomethylation
This review covers the recent studies featuring the development of catalysts for alkene hydroaminomethylation.
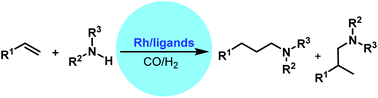
Org. Chem. Front., 2016,3, 1359-1370
https://doi.org/10.1039/C6QO00233A
Learning lessons from directed evolution of stereoselective enzymes
With the advent of directed evolution of stereoselective enzymes almost 20 years ago and the rapid development of this exciting area of research, the traditional limitations of biocatalysts in organic chemistry have been eliminated.

Org. Chem. Front., 2016,3, 1350-1358
https://doi.org/10.1039/C6QO00210B
Synthesis and reactivity of alkoxy-activated cyclobutane-1,1-dicarboxylates
Donor–acceptor (DA) cyclopropanes are extensively studied and well established as building blocks for the synthesis of highly substituted carbo- and heterocyclic compounds. Despite of similar ring strain, the homologous DA cyclobutanes were long ignored. This personal account details our group's research towards the application of DA cyclobutanes in cycloaddition chemistry.
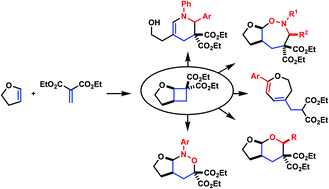
Org. Chem. Front., 2016,3, 1205-1212
https://doi.org/10.1039/C6QO00244G
Chirality transfer in metal-catalysed intermolecular addition reactions involving allenes
This review covers chirality transfer in metal-catalysed intermolecular addition reactions involving allenes, including axial-to-central chirality transfer, asymmetric catalysis and racemization.
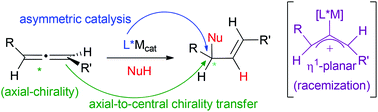
Org. Chem. Front., 2016,3, 1186-1204
https://doi.org/10.1039/C6QO00207B
Synthesis and applications of fluorous phosphines
The synthesis, some of the properties and the applications of fluorous phosphines in biphasic, organometallic and organo-catalysis were reviewed.
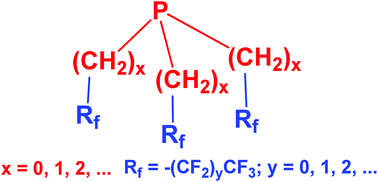
Org. Chem. Front., 2016,3, 1048-1062
https://doi.org/10.1039/C6QO00115G
A highly efficient synthesis of the DEFG-ring system of rubriflordilactone B
A highly efficient synthesis of the DEFG-ring system of rubriflordilactone B via polar-radical-crossover cycloaddition and carbonyl allylation/lactonization is described.
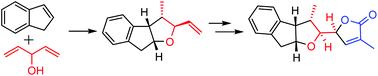
Org. Chem. Front., 2017,4, 47-51
https://doi.org/10.1039/C6QO00241B
Green and practical transition metal-free one-pot conversion of substituted benzoic acids to anilines using tosyl azide
A simple and efficient method for conversion of 2-iodo/2-nitro benzoic acids and dihydropyranone-fused benzoic acids into corresponding anilines, using tosyl azide, under transition metal-free conditions was developed. Steric and electronic effects play crucial role.

Org. Chem. Front., 2016,3, 1680-1685
https://doi.org/10.1039/C6QO00343E
Configuration-guided reactions: the case of highly decorated spiro[cyclopropane-1,2′(3′H)-pyrrolo[1,2-b]isoxazole] derivatives en route to polyhydroxyindolizidines
The formation of structurally differentiated indolizidinones is guided by a diverse behaviour of spirocyclopropaneisoxazolidine precursors under fluorination reactions.
![Graphical abstract: Configuration-guided reactions: the case of highly decorated spiro[cyclopropane-1,2′(3′H)-pyrrolo[1,2-b]isoxazole] derivatives en route to polyhydroxyindolizidines](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00410E&imageInfo.ImageIdentifier.Year=2016)
Org. Chem. Front., 2016,3, 1651-1660
https://doi.org/10.1039/C6QO00410E
Gold-catalyzed cycloisomerization of trifluoromethylated allenols: sustainability and mechanistic studies
Trifluoromethyl-substituted α-allenols are cyclized to the corresponding 2,5-dihydrofurans in the presence of neutral or cationic gold catalysts.

Org. Chem. Front., 2016,3, 1514-1519
https://doi.org/10.1039/C6QO00423G
Manganese-catalysed hydroperoxidation of carbon–carbon double bonds using molecular oxygen present in air and hydroxylamine under ambient conditions
A highly efficient manganese-catalysed hydroperoxidation of carbon–carbon double bonds of enynes as well as styrene derivatives using N-hydroxyphthalimide, N-hydroxybenzotriazole or N-hydroxysuccinimide was developed.
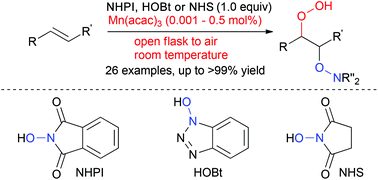
Org. Chem. Front., 2016,3, 1420-1424
https://doi.org/10.1039/C6QO00318D
A new approach toward the synthesis of 2,4-bis(fluoroalkyl)-substituted quinoline derivatives using fluoroalkyl amino reagent chemistry
The present work describes the unprecedented use of Fluoroalkyl Amino Reagents (FARs) to afford 2,4-bis(fluoroalkyl)-substituted quinoline derivatives in two steps under mild reaction conditions, in good yields and with a very good regioselectivity.
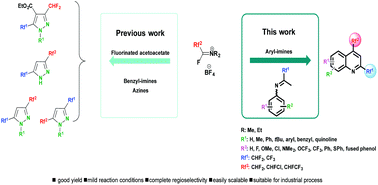
Org. Chem. Front., 2016,3, 1392-1415
https://doi.org/10.1039/C6QO00319B
Iterative catalyst controlled diastereodivergent synthesis of polypropionates
Polypropionates are synthesized using a combination of a copper-catalyzed asymmetric allylic alkylation, ruthenium-catalyzed cross-metathesis and iridium-catalyzed asymmetric allylic etherification.
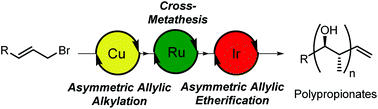
Org. Chem. Front., 2016,3, 1383-1391
https://doi.org/10.1039/C6QO00199H
Synthesis of 2,3-dihydrobenzofurans via the palladium catalyzed carboalkoxylation of 2-allylphenols
A new Pd-catalyzed alkene carboalkoxylation strategy for the preparation of 2,3-dihydrobenzofurans is described.
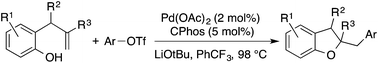
Org. Chem. Front., 2016,3, 1314-1318
https://doi.org/10.1039/C6QO00215C
Towards taxane analogues synthesis by dienyne ring closing metathesis
The synthesis of highly functionalized 16,17,18-trinortaxane analogues based on a dienyne cyclization is described.
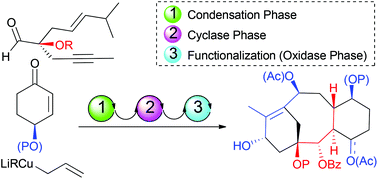
Org. Chem. Front., 2016,3, 1331-1336
https://doi.org/10.1039/C6QO00321D
A tetrasulfate-resorcin[6]arene cavitand as the host for organic ammonium guests
A resorcin[6]arene cavitand bearing sulfate bridges is herein reported. The host is able to interact with ammonium guests through the sulfate bridges.
![Graphical abstract: A tetrasulfate-resorcin[6]arene cavitand as the host for organic ammonium guests](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00336B&imageInfo.ImageIdentifier.Year=2016)
Org. Chem. Front., 2016,3, 1276-1280
https://doi.org/10.1039/C6QO00336B
Homochiral association of binuclear trans-bis(β-iminoaryloxy)palladium(II) complexes doubly linked with m-xylylene spacers: drastic linker-dependence of the association chirality of chiral clothespin-shaped molecules
A significant effect of the linker was observed on association chirality during the interpenetrative dimeric association of clothespin-shaped Pd complexes bearing either rigid or flexible linkers.
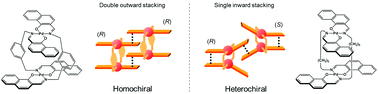
Org. Chem. Front., 2016,3, 1286-1294
https://doi.org/10.1039/C6QO00315J
Fe-Catalyzed reductive NO-bond cleavage – a route to the diastereoselective 1,4-aminohydroxylation of 1,3-dienes
We describe the iron-catalyzed reductive cleavage of N–O-bonds in oxazines using malononitrile as reductant, which – in sequence with a [4 + 2]-cycloaddition – can be considered as an efficient way for the diastereoselective 1,4-aminohydroxylation of 1,3-dienes.
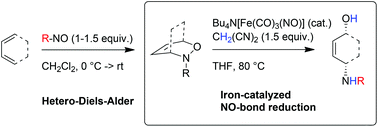
Org. Chem. Front., 2016,3, 1295-1298
https://doi.org/10.1039/C6QO00255B
Versatile and highly efficient oxidative C(sp3)–H bond functionalization of tetrahydroisoquinoline promoted by bifunctional diethyl azodicarboxylate (DEAD): scope and mechanistic insights
The oxidant–base bifunctionality of DEAD realized highly reliable cross dehydrogenative coupling of tetrahydroisoquinoline derivatives via aminal intermediates.
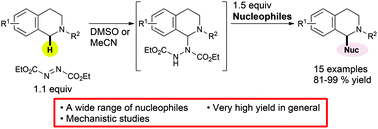
Org. Chem. Front., 2016,3, 1259-1264
https://doi.org/10.1039/C6QO00249H
Switching between intermolecular and intramolecular reactions using flow microreactors: lithiation of 2-bromo-2′-silylbiphenyls
Switching between the intermolecular reaction and the intramolecular reaction was achieved at will using flow microreactors.
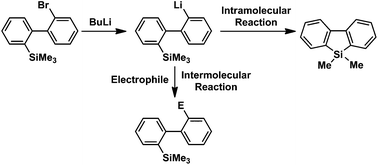
Org. Chem. Front., 2016,3, 1250-1253
https://doi.org/10.1039/C6QO00257A
Considering organic mechanisms and the optimization of current flow in an electrochemical oxidative condensation reaction
Careful consideration of the chemical mechanism for an oxidative condensation reaction led to improved current flow for the electrolysis.
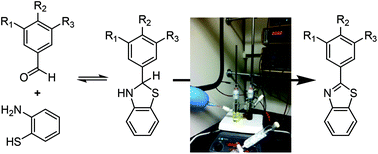
Org. Chem. Front., 2016,3, 1236-1240
https://doi.org/10.1039/C6QO00248J
C–C bond migration in the cycloisomerization of 1,6-enynes
Platinum- and iridium-catalyzed cycloisomerizations of 1,6 enynes that proceed via a C–C bond migration into a carbenoid intermediate are described.
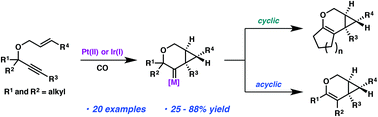
Org. Chem. Front., 2016,3, 1228-1235
https://doi.org/10.1039/C6QO00224B
Catalytic asymmetric direct-type 1,4-addition reactions of simple esters
Catalytic asymmetric 1,4-addition reactions of simple esters have been developed. The desired reactions proceeded smoothly with high enantioselectivities.

Org. Chem. Front., 2016,3, 1241-1245
https://doi.org/10.1039/C6QO00242K
Highly enantioselective Pd-catalyzed indole allylic alkylation using binaphthyl-based phosphoramidite-thioether ligands
A new type of phosphoramidite-thioether ligand has been developed for Pd-catalysed asymmetric C-3 allylic alkylation of indoles with excellent yields and enantioselectivities.

Org. Chem. Front., 2016,3, 1246-1249
https://doi.org/10.1039/C6QO00227G
Rh-catalysed asymmetric conjugate addition of boronic acids to nitroalkenes employing a P-chiral P,π-hybrid ligand
A P-chiral P,π-hybrid ligand was applied to the asymmetric conjugate addition of aryl boronic acids to β-substituted nitroalkenes.
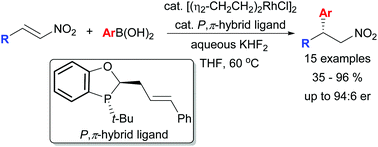
Org. Chem. Front., 2016,3, 1149-1153
https://doi.org/10.1039/C6QO00311G
Silver-mediated oxidative annulation of N-arylthio succinimides with alkynes: direct access to benzo[b]thiophenes
A convenient synthetic route to 2,3-diarylbenzo[b]thiophene derivatives via Ag-catalyzed intermolecular oxidative cyclization between bench-stable N-arylthio succinimides and unactivated internal alkynes is demonstrated herein.
![Graphical abstract: Silver-mediated oxidative annulation of N-arylthio succinimides with alkynes: direct access to benzo[b]thiophenes](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00259E&imageInfo.ImageIdentifier.Year=2016)
Org. Chem. Front., 2016,3, 1126-1130
https://doi.org/10.1039/C6QO00259E
Approach to the synthesis of the C1–C11 and C14–C18 portion of Leucascandrolide A
An iterative asymmetric hydration of dienoates approach to Leucasndrolide A was explored.
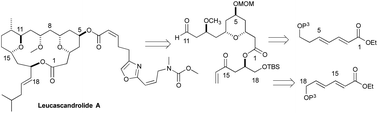
Org. Chem. Front., 2016,3, 1120-1125
https://doi.org/10.1039/C6QO00284F
Synthesis, structure and optical properties of tetraphenylethene derivatives with through-space conjugation between benzene and various planar chromophores
A series of tetraphenylethene derivatives with through-space conjugation and aggregation-enhanced emission properties are synthesized and studied.
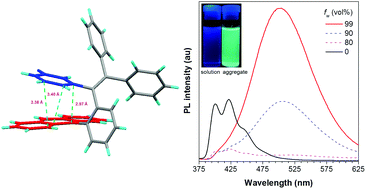
Org. Chem. Front., 2016,3, 1091-1095
https://doi.org/10.1039/C6QO00204H
Diastereo- and enantioselective construction of cyclohexanone-fused spirospyrazolones containing four consecutive stereocenters through asymmetric sequential reactions
The diastereo- and enantioselective synthesis of cyclohexanone-fused spirospyrazolones through an asymmetric Michael/Michael/aldol cascade reaction catalyzed by squaramide and diphenylprolinol silyl ether, followed by a sequential oxidation was developed.

Org. Chem. Front., 2016,3, 1087-1090
https://doi.org/10.1039/C6QO00208K
Concise asymmetric total synthesis of catunaregin
The asymmetric total synthesis of the optically pure catunaregin was accomplished in 7 steps from a known methyl ester using an asymmetric syn-selective aldol reaction and the successive ketalization of a furan diol derivative under acidic conditions.

Org. Chem. Front., 2016,3, 1084-1086
https://doi.org/10.1039/C6QO00213G
Potassium-mediated stereochemical assistance to form one indenonaphthacene isomer from rubrene with complementary diastereoselectivity to the acid based protocol
Complementary diastereoselectivity for C–H and C–C bond formation, in para disposition, leading to one indenonaphthacene derivative: proton versus potassium assistance.
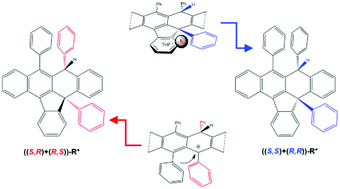
Org. Chem. Front., 2016,3, 1074-1079
https://doi.org/10.1039/C6QO00130K
Synthesis of oxindoles via reductive CO2 fixation
The synthesis of 3-aryl-3-hydroxy-2-oxindoles, which are a structural motif found in various natural products and pharmaceutically active compounds, was conducted via reductive coupling of (2-aminophenyl)(aryl)methanone derivatives and CO2 as a key step. The conditions employing Mg with chlorotrimethylsilane in DMA are the best for the reductive coupling. The reductive coupling and acid-catalyzed lactam formation can be performed in a one-pot reaction to give the oxindoles.

Org. Chem. Front., 2016,3, 929-933
https://doi.org/10.1039/C6QO00107F
Hydrazone–palladium catalyzed annulation of 1-cinnamyloxy-2-ethynylbenzene derivatives
The annulation of 1-cinnamyloxy-2-ethynylbenzene derivatives using a hydrazone–palladium catalyst system proceeded smoothly and gave the corresponding 2-substituted-3-cinnamylbenzofurans in good-to-excellent yields.

Org. Chem. Front., 2016,3, 979-984
https://doi.org/10.1039/C6QO00112B
Reaction of alkenecarboxylic acids with isocyanates via rhodium(III)-catalyzed C–H activation: a versatile route to cyclic imides
A versatile cascade route to cyclic imides via direct functionalization of the β-alkenyl C–H bond of alkenoic acids was developed.

Org. Chem. Front., 2016,3, 971-974
https://doi.org/10.1039/C6QO00119J
Synthesis of unsymmetrical NCN′ and PCN pincer palladacycles and their catalytic evaluation compared with a related SCN pincer palladacycle
A series of unsymmetrical palladacycles have been synthesised and evaluated as catalysts in aldol and vinyl epoxide coupling chemistry.
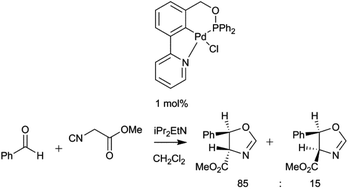
Org. Chem. Front., 2016,3, 957-965
https://doi.org/10.1039/C6QO00198J
Convenient synthesis of pentafluoroethyl thioethers via catalytic Sandmeyer reaction with a stable fluoroalkylthiolation reagent
A Sandmeyer type process is disclosed that allows the straightforward synthesis of pentafluoroethyl thioethers from aromatic amines and tetramethylammonium pentafluoroethylthiolate.

Org. Chem. Front., 2016,3, 949-952
https://doi.org/10.1039/C6QO00194G
Stimuli-responsive supramolecular gel constructed by pillar[5]arene-based pseudo[2]rotaxanes via orthogonal metal–ligand coordination and hydrogen bonding interaction
A supramolecular polyrotaxane constructed by pillar[5]arene-based pseudo[2]rotaxanes via the incorporation of metal–ligand coordination could further self-assemble to form a stimuli-responsive supramolecular gel at high concentration.
![Graphical abstract: Stimuli-responsive supramolecular gel constructed by pillar[5]arene-based pseudo[2]rotaxanes via orthogonal metal–ligand coordination and hydrogen bonding interaction](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00197A&imageInfo.ImageIdentifier.Year=2016)
Org. Chem. Front., 2016,3, 966-970
https://doi.org/10.1039/C6QO00197A
Structure and reactivity of sulfonamide- and acetate-chelated ruthenium alkylidene complexes
The formation of chelated ruthenium alkylidenes with the oxygen of sulfonamides and acetates and their complexation with carbon monoxide have been studied.
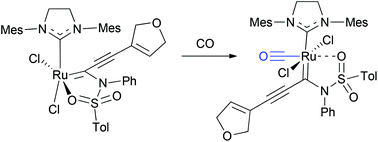
Org. Chem. Front., 2016,3, 939-943
https://doi.org/10.1039/C6QO00148C
Catalytic one-pot synthesis of 4-(hetero)aryl substituted 5-(2-oxoethyl) oxazol-2(3H)-ones by coupling–isomerization–elimination (CIE) sequence
4-(Hetero)aryl substituted 5-(2-oxoethyl) oxazol-2(3H)-ones are readily prepared in a one-pot fashion by a coupling–isomerization–elimination (CIE) sequence from N-Boc protected 1-aryl propargyl carbamates and acid chlorides.
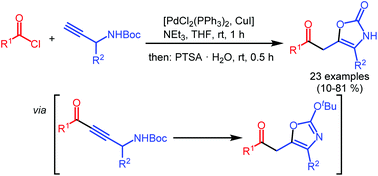
Org. Chem. Front., 2016,3, 887-896
https://doi.org/10.1039/C6QO00138F
Synthesis of 2,5-disubstituted pyrroles via dehydrogenative condensation of secondary alcohols and 1,2-amino alcohols by supported platinum catalysts
2,5-Disubstituted pyrroles are synthesized by acceptorless dehydrogenative reaction of 1,2-aminoalcohols and secondary alcohols by Pt/carbon catalyst.

Org. Chem. Front., 2016,3, 846-851
https://doi.org/10.1039/C6QO00165C
A general photoinduced electron transfer-directed chemoselective perfluoroalkylation of N,N-dialkylhydrazones
The first metal-free, initiator-free, and general photochemical perfluoroalkylation of a variety of N,N-dialkylhydrazones was developed at room temperature in the absence of any external photocatalyst.
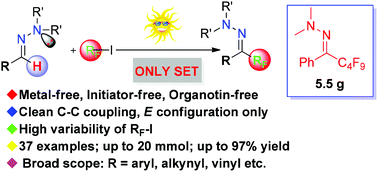
Org. Chem. Front., 2016,3, 841-845
https://doi.org/10.1039/C6QO00158K
Orthogonal cleavage of the 2-naphthylmethyl group in the presence of the p-methoxy phenyl-protected anomeric position and its use in carbohydrate synthesis
Orthogonal removal of naphthylmethyl (NAP) and anomeric O-p-methoxyphenyl (PMP) ethers using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and cerium(IV) ammonium nitrate, respectively, is described.
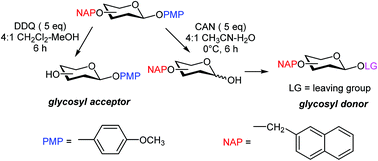
Org. Chem. Front., 2016,3, 753-758
https://doi.org/10.1039/C6QO00144K
Diastereoselective and regiodivergent oxa-[3 + 2] cycloaddition of Achmatowicz products and cyclic 1,3-dicarbonyl compounds
Development of two novel oxa-[3 + 2] cycloaddition reactions of Achmatowicz products with 1,3-dicarbonyl compounds for rapid and highly efficient assembly of polycyclic furopyranones is described. Plausible mechanisms were proposed.
![Graphical abstract: Diastereoselective and regiodivergent oxa-[3 + 2] cycloaddition of Achmatowicz products and cyclic 1,3-dicarbonyl compounds](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00034G&imageInfo.ImageIdentifier.Year=2016)
Org. Chem. Front., 2016,3, 714-719
https://doi.org/10.1039/C6QO00034G
Asymmetric transfer hydrogenation of seven membered tricyclic ketones: N-substituted dibenzo[b,e]azepine-6,11-dione driven by nonclassical CH/O interactions
Enantioselective transfer hydrogenation of dibenzo-fused-azepine-diones: N-substituted dibenzo[b,e]azepin-6-11-dione was achieved by ruthenium catalysis in the presence of formic acid/triethylamine as a mild hydrogen source.
![Graphical abstract: Asymmetric transfer hydrogenation of seven membered tricyclic ketones: N-substituted dibenzo[b,e]azepine-6,11-dione driven by nonclassical CH/O interactions](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00036C&imageInfo.ImageIdentifier.Year=2016)
Org. Chem. Front., 2016,3, 614-619
https://doi.org/10.1039/C6QO00036C
Tandem oxidative radical brominative addition of activated alkynes and spirocyclization: switchable synthesis of 3-bromocoumarins and 3-bromo spiro-[4,5] trienone
A K2S2O8-mediated tandem radical brominative addition of alkynoates, oxidative spiro-cyclization, and 1,2-migration of esters is reported for the synthesis of 3-bromocoumarins with high efficiency.
![Graphical abstract: Tandem oxidative radical brominative addition of activated alkynes and spirocyclization: switchable synthesis of 3-bromocoumarins and 3-bromo spiro-[4,5] trienone](/ta/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6QO00041J&imageInfo.ImageIdentifier.Year=2016)
Org. Chem. Front., 2016,3, 510-515
https://doi.org/10.1039/C6QO00041J
Efficient synthesis of 2-acylquinolines based on an aza-vinylogous Povarov reaction
Povarov-type chemistry gives ready access to 2-acylquinolines.
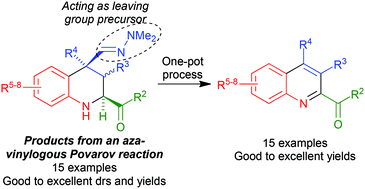
Org. Chem. Front., 2016,3, 412-422
https://doi.org/10.1039/C6QO00037A
Synthesis of fused isoquinolines via gold-catalyzed tandem alkyne amination/intramolecular O–H insertion
A novel gold-catalyzed tandem alkyne amination/intramolecular O–H insertion has been developed. A variety of [1,4]oxazino[3,2-c]isoquinolines are readily accessed under mild reaction conditions by utilizing this strategy, thereby providing an efficient and practical route for the construction of synthetically useful fused isoquinolines.

Org. Chem. Front., 2016,3, 491-495
https://doi.org/10.1039/C6QO00033A
About this collection
We are delighted to present this collection of articles dedicated to Professor Barry Trost (Stanford University) to celebrate his 75th birthday. This themed collection is guest edited by Bernhard Breit from Albert-Ludwigs-Universität Freiburg.