A simpler method affords evaluation of π stabilization by phenylalanine of several biochemical carbocations†
Abstract
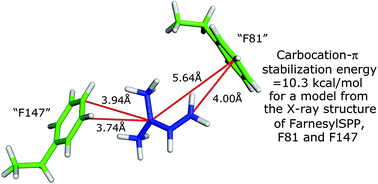
Carbocations are important intermediates in the biosynthesis of terpenes and steroids, and it is challenging to try to understand how these relatively unstable species survive even transiently during biochemical reactions. Carbocation–π interaction with aromatic amino acid residues is an important factor in helping to stabilize these positively charged species. However, the short lifetimes of these active site carbocations makes experimental evaluation of the stabilization afforded by such interaction impossible. Computational studies, however, have provided some insight into this phenomenon. Herein we report a simple, computationally efficient method to estimate such stabilization energies afforded by phenylalanine to biochemical carbocation intermediates. A model is constructed in which the biochemical carbocation is replaced by an appropriate carbocation mimic (t-butyl or dimethylallyl). This substitute carbocation is then aligned with an ethylbenzene serving as a surrogate for each proximate phenylalanine in a geometry that replicates as closely as possible the orientation of that phenylalanine using measurements made on an X-ray structure of an enzyme active site in which a carbocation surrogate is bound. Density functional theory computations on such models were then used to yield estimates of stabilization energies. Application of this method to the tertiary carbocation formed in the reaction catalyzed by geranyl diphosphate C-methyl transferase gave a stabilization energy (−12.3 kcal mol−1) that was essentially identical to that obtained previously by analysis of a much more computationally demanding model of the active site. As a check on the accuracy of the simpler method, it was applied with similar success to the farnesyl cation formed in the reaction catalyzed by aristolochene synthase that is stabilized by cation–π interaction with two phenylalanines. Application of this method is also described to estimate carbocation–π stabilization, by the same two phenylalanines, of the final carbocation intermediate leading to aristolochene through analysis of the X-ray structure of an inhibitor of that carbocation bound in the active site of aristolochene synthase. Finally, the stabilization, by either of two phenylalanines, of six carbocation intermediates in the oxidosqualene cyclase-catalyzed formation of lanosterol is estimated by comparable analysis of an X-ray structure of that reaction product bound in the enzyme active site.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...