Deconvolution of fast exchange equilibrium states in NMR spectroscopy using virtual reference standards and probability theory†
Abstract
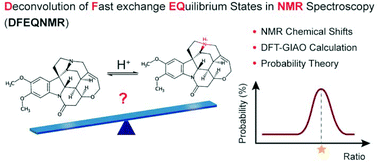
A methodology for deconvolution of fast exchange equilibrium states in NMR spectroscopy (DFEQNMR) was developed based on DFT-GIAO NMR chemical shift prediction and a probability theory algorithm. Proof-of-concept studies were performed to estimate the protonation state of N-containing organic molecules involving fast proton exchange equilibrium and evaluate the solution tautomerism of a purine derivative. DFT–GIAO calculations were optimized to achieve good accuracy in 13C, 1H and 15N chemical shift prediction for protonated species. The probability theory algorithm enabled the determination of solution species ratios and yielded 95% confidence regions by comparing experimental and simulated chemical shift data sets. The calculation showed good accuracy for model partial salts with various functionalities and application in structure elucidation of complex natural product partial salts was also demonstrated. This method showed promising potential in acquisition of important insight into fast exchange equilibrium systems with only one experimental NMR chemical shift data set.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...