Free-base porphyrins with localized NH protons. Can substituents alone stabilize the elusive cis tautomer?†
Abstract
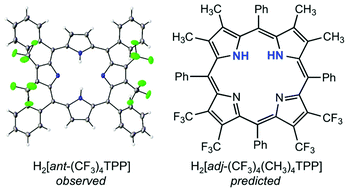
The elusive cis tautomer of free-base porphyrins has recently been isolated and structurally characterized in the form of a supramolecular complex. The question as to whether a suitable set of peripheral substituents might lead to a stable cis tautomer in the absence of supramolecular interactions, however, remains unanswered and is one we have attempted to address here by means of density functional theory calculations. The fact that many antipodally β-tetrasubstituted tetraphenylporphyrin derivatives exhibit localized central protons attached to the β-unsubstituted pyrrole rings led us to surmise that β-tetrasubstitution of adjacent pyrrole rings might lead to a porphyrin cis tautomer, an idea that proved fruitful. Indeed, for the “adjacently” substituted tetraphenylporphyrin derivative H2[adj-(CF3)4(CH3)4TPP], the global energy minimum proved to be a highly saddled cis tautomer, with the trans tautomer about 0.07 eV higher in energy. It is important to underscore, however, that the asymmetric β-substitution pattern is far from the only factor contributing to the stability of the cis tautomer for this porphyrin. A strongly saddled conformation resulting from meso-β steric interactions also helps alleviate the repulsion between the two central NH protons, thereby stabilizing the cis tautomer relative to the trans.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...