Dual-/multi-organelle-targeted AIE probes associated with oxidative stress for biomedical applications
Yuanyuan
You
*a,
Songling
Lin
a,
Chengwei
Tang
b,
Yuchao
Li
b,
Dingyuan
Yan
c,
Dong
Wang
 c and
Xiaohui
Chen
c and
Xiaohui
Chen
 *b
*b
aSchool of Pharmacy, Guangdong Medical University, Dongguan, 523808, China. E-mail: youyy@gdmu.edu.cn
bInstitute of Laboratory Medicine, School of Medical Technology, Guangdong Medical University, Dongguan, 523808, China. E-mail: xhchen@gdmu.edu.cn
cCenter for AIE Research, College of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, China
First published on 2nd August 2024
Abstract
In situ monitoring of biological processes between different organelles upon oxidative stress is one of the most important research hotspots. Fluorescence imaging is especially suitable for biomedical applications due to its distinct advantages of high spatiotemporal resolution, high sensitivity, non-invasiveness, and in situ monitoring capabilities. However, most fluorescent probes can only achieve light-up imaging of single organelles, thus the combined use of two or more probes is usually required for monitoring biological processes between organelles, which can suffer from tedious staining and washing procedures, increased cytotoxicity and poor photostability. Exogenetic oxidants can affect broad-spectrum subcellular organelles, which are not conducive to in situ monitoring of biological processes between specific organelles. To tackle these challenges, a series of dual-/multi-organelle-targeted aggregation-induced emission (AIE) probes associated with oxidative stress have been designed and developed in the past few years. Herein, the recent progress of these AIE probes is summarized in biomedical applications, such as apoptosis monitoring, interplay between organelles, microenvironmental changes of organelles, organelle morphology tracking, precise cancer therapy, and so forth. Moreover, the further outlook for dual-/multi-organelle-targeted AIE probes is discussed, aiming to promote innovative research in biomedical applications.
1. Introduction
Organelles, including mitochondria, lysosomes, lipid droplets (LDs), endoplasmic reticulum (ER), and nucleus, are functionally substructural units within the cytoplasm, which play an extremely important role in the physiological activities of cells.1,2 For example, mitochondria provide energy for cell activities by synthesizing ATP and are the power factories of cells.3,4 Lysosomes containing a variety of hydrolases are responsible for decomposing various metabolites and functionally damaged organelles, which are known as the digestion machines within the cells.5,6 LDs are important storage sites for intracellular neutral lipids, which are closely related to membrane synthesis and protein degradation.7,8 ER can provide reaction space for various enzyme-catalyzed reactions and possess protein handling, modification and folding capacity.9–11 Moreover, the organelles under exogenous stimulation (such as oxidative stress induced by oxidants) can exhibit morphological changes, interact with other organelles, and display abnormal physiological functions leading to cell apoptosis.12–15 Therefore, specific imaging of organelles can in situ real-time monitor their morphology, distribution and movement changes, which is of great research significance for studying physiological processes of life activities.Fluorescence imaging allows us to observe some processes that were previously invisible.16–18 Moreover, benefiting from high spatiotemporal resolution, high sensitivity, non-invasiveness, and in situ and real-time monitoring, fluorescence imaging is especially suitable for biomedical applications.19–21 Conventional fluorescent probes with hydrophobic characteristics exist in the form of aggregates in the biological system.22 However, they suffer from aggregation-caused quenching (ACQ) and reduced photodynamic activity in the aggregated state, which are not conducive to biological imaging and phototherapy.23,24 Fortunately, Tang et al.25 first proposed the concept of aggregation-induced emission (AIE) in 2001, injecting new vitality into biomedicine. AIE probes exhibit non-emissive or weakly emissive in dilute solutions but exhibit bright fluorescence in the aggregated state, which can be attributed to twisted conformations, rotor structures and restriction of intramolecular motion or vibration.26,27 Moreover, AIE probes also possess unique and excellent photophysical properties, such as large Stokes shift, high signal-to-noise ratio, poor photobleaching, easy modification, and good photodynamic activity in the aggregated state, which are particularly suitable for biomedical applications.28–30
Recently, a series of mono-organelle-targeted AIE probes have been developed, such as mitochondria-targeted cationic AIE probes containing triphenylphosphine and pyridinium salts,31,32 lysosomes-targeted AIE probes containing morpholine groups,33,34 ER-targeted AIE probes containing glibenclamide and sulfanilamide groups,35,36 Golgi apparatus-targeted AIE probes containing cyano and pyridinium salts,37,38 nucleus-targeted AIE probes,39,40 lipid droplet-targeted AIE probes,41,42 and so forth. Aiming to simultaneously monitor biological processes of dual-/multi-organelles, commercially available ACQ probes for targeting special organelles are also needed to be used.43 However, the combined use of mono-organelle-targeted AIE probes and ACQ probes suffers from some drawbacks, such as tedious staining and washing procedures, increased cytotoxicity, and lack of long-term tracking of biological processes due to poor photostability.44,45 Additionally, the investigation of physiological changes of organelles under oxidative stress usually requires exogenous oxidants, which will affect broad-spectrum subcellular organelles and thus seriously restrict the biomedical applications.13,46,47 Intriguingly, organelle-targeted AIE photosensitizers in the aggregated state can efficiently generate reactive oxygen species (ROS) to in situ induce oxidative stress against specific organelles, while other organelles or biomacromolecules are not affected, which is particularly suitable for studying the activity of life under oxidative stress.48,49 Therefore, dual-/multi-organelle-targeted AIE photosensitizers have broad application prospects in monitoring biological processes and cancer therapy.
We summarize the design and synthesis of dual-/multi-organelle-targeted AIE probes with in situ oxidative stress induction ability, and their research progress in monitoring apoptosis, interplay between organelles, microenvironments of organelles, organelle morphology tracking, precise cancer therapy, and so forth. Moreover, the challenges and prospects of novel dual-/multi-organelle-targeted AIE probes in biomedicine applications are discussed.
2. The design strategies of dual-/multi-organelle-targeted AIE probes
Due to the excellent photophysical properties and easy modification of the AIE molecular skeleton, a series of dual-/multi-organelle-targeted AIE probes were synthesized (Fig. 1).50,51 The current design strategies mainly include (1) double ligand modification. Two specific targeting ligands are modified on the AIE molecular skeleton to achieve dual-organelle-targeted imaging. For example, Chen et al.52 developed a dual organelle-targeted AIE probe containing phosphonate and pyridinium, which could simultaneously target the plasma membrane and mitochondria. (2) Self-assembled nanoparticles. Tang et al.53 reported dual-organelle-targeted nanoparticles (AIE-Mito-TPP/AlPcSNa4 NPs) through the self-assembly of the positively charged AIE-Mito-TPP and the negatively charged AlPcSNa4via electrostatic, hydrophobic, and π–π interactions. (3) Lipophilic regulation. The organic dyes whose log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (n-octanol/water partition coefficient) value is larger than 5 are suitable for lipid staining, and LDs are derived from the ER containing neutral lipids.54,55 Tang et al.56 designed and synthesized a photoactivatable AIE probe TPA-DHPy with a LogP of 6.22, which was converted into TPA-Py through the photooxidative dehydrogenation reaction under irradiation, showing light-up imaging of LDs and ER. (4) Enzymatic activation. The polarity of the AIE probe can significantly change under enzymatic catalysis, and the probe can be transferred from a specific organelle to other organelles. Dong et al.57 designed and synthesized the esterase-responsive AIE probe Mito-TTPE showing positive charge and red fluorescence emission, which could light up mitochondria at the initial stage. Subsequently, Mito-TTPE was able to transform into the corresponding pyridine counterpart LD-TTP under mitochondrial esterase activation, which could exhibit light-up imaging of LDs. (5) pH sensitivity. For example, Zhao et al.58 developed a pH-sensitive ratiometric AIE probe LD-L for dual-color imaging of LDs and lysosomes. (6) Photoactivation. Alkyl-substituted tetrahydropyridine derivatives can be transformed into the corresponding pyridinium salt derivatives through oxidation reactions, which lead to obvious changes in the polarity and fluorescence emission.59,60 Wang et al.61 designed and synthesized a photoactivatable AIE probe THTTVP based on tetrahydropyridine, showing light-up fluorescence imaging of lysosomes, LDs and mitochondria under white light irradiation. (7) Oxidative stress. The mitochondrial membrane potential decreases significantly under oxidative stress, and the cationic AIE probe can dissociate from the mitochondria and target the nucleus. Tang et al.62 synthesized a type of AIE probe containing four pyridinium salts to achieve light-up imaging of mitochondria. ROS generated under light irradiation could effectively induce oxidative stress to disrupt mitochondrial function, reduce mitochondrial membrane potential, and subsequently light up the nucleus. (8) All in one. AIE probes with different targeting functions are simultaneously added to cells to achieve multi-organelle-targeted light-up imaging.63 (9) The AIE probe first lights up the lysosomes, and then one part of AIE probe escaped from lysosomes can selectively accumulate in other organelles, achieving dual-organelle-targeted imaging.64
P (n-octanol/water partition coefficient) value is larger than 5 are suitable for lipid staining, and LDs are derived from the ER containing neutral lipids.54,55 Tang et al.56 designed and synthesized a photoactivatable AIE probe TPA-DHPy with a LogP of 6.22, which was converted into TPA-Py through the photooxidative dehydrogenation reaction under irradiation, showing light-up imaging of LDs and ER. (4) Enzymatic activation. The polarity of the AIE probe can significantly change under enzymatic catalysis, and the probe can be transferred from a specific organelle to other organelles. Dong et al.57 designed and synthesized the esterase-responsive AIE probe Mito-TTPE showing positive charge and red fluorescence emission, which could light up mitochondria at the initial stage. Subsequently, Mito-TTPE was able to transform into the corresponding pyridine counterpart LD-TTP under mitochondrial esterase activation, which could exhibit light-up imaging of LDs. (5) pH sensitivity. For example, Zhao et al.58 developed a pH-sensitive ratiometric AIE probe LD-L for dual-color imaging of LDs and lysosomes. (6) Photoactivation. Alkyl-substituted tetrahydropyridine derivatives can be transformed into the corresponding pyridinium salt derivatives through oxidation reactions, which lead to obvious changes in the polarity and fluorescence emission.59,60 Wang et al.61 designed and synthesized a photoactivatable AIE probe THTTVP based on tetrahydropyridine, showing light-up fluorescence imaging of lysosomes, LDs and mitochondria under white light irradiation. (7) Oxidative stress. The mitochondrial membrane potential decreases significantly under oxidative stress, and the cationic AIE probe can dissociate from the mitochondria and target the nucleus. Tang et al.62 synthesized a type of AIE probe containing four pyridinium salts to achieve light-up imaging of mitochondria. ROS generated under light irradiation could effectively induce oxidative stress to disrupt mitochondrial function, reduce mitochondrial membrane potential, and subsequently light up the nucleus. (8) All in one. AIE probes with different targeting functions are simultaneously added to cells to achieve multi-organelle-targeted light-up imaging.63 (9) The AIE probe first lights up the lysosomes, and then one part of AIE probe escaped from lysosomes can selectively accumulate in other organelles, achieving dual-organelle-targeted imaging.64
3. Biomedical applications
3.1. Apoptosis monitoring
Apoptosis is one of the major cell death mechanisms.65,66 Benefiting from the high-precision spatiotemporal control of light, photodynamic therapy can effectively induce cell apoptosis to achieve precise cancer therapy.67 However, in situ real-time monitoring of the apoptosis process remains challenging. To address these issues, Tang et al.62 synthesized an optimized agent (TPE-4EP +) through a molecular engineering strategy, which exhibited AIE characteristics and excellent ROS generation efficiency (Fig. 2A–C). Due to the high mitochondrial membrane potential (∼ –220 mV) of cancer cells, the cationic AIE photosensitizer TPE-4EP+ can achieve light-up fluorescence imaging of mitochondria. Intriguingly, a gradually increased fluorescence was observed inside cell nuclei by prolonging the light irradiation time from 0 to 5 min (Fig. 2D), indicating that TPE-4EP+ could be transferred from mitochondria to the nucleus due to the reduced mitochondrial membrane potential under ROS-induced oxidative stress. After HeLa cells were co-stained with FITC-annexin V and PI, only the green fluorescence signal of annexin V could be observed, but the fluorescence signal of PI was not exhibited, indicating early apoptosis. Prolonging the light irradiation time from 5 to 7 min, only bright fluorescence signal of PI could be observed, suggesting that the cells undergo late apoptosis due to cell membrane damage. Therefore, the AIE probe TPE-4EP+ could realize the in situ real-time monitoring of apoptosis, thereby tracking the progress of photodynamic therapy. | ||
| Fig. 2 (A) Chemical structure of TPE-4EP+ and schematic illustration of apoptosis monitoring of TPE-4EP+ under irradiation. (B) The singlet oxygen (1O2) generation ability under irradiation for different times. (C) Plot of PL intensity at 605 nm versus the ctDNA. (D) The confocal laser scanning microscopy (CLSM) images of HeLa cells treated with TPE-4EP +, followed by co-staining with FITC-annexin V and PI.62 Copyright 2019 American Chemical Society. (E) Chemical structure of TTVPHE. (F) The plot of relative maximum emission intensity (I/I0) of TTVPHE with the increasing toluene fraction. (G) The ROS generation ability under irradiation for 0–4 min. (H) The CLSM images, (I) TEM images, and (J) TUNEL assay of QBC939 cells treated with TTVPHE under irradiation for 0–15 min.68 Copyright 2019 Royal Society of Chemistry. | ||
Subsequently, Li et al.68 designed and synthesized AIE photosensitizers for targeting mitochondria and nuclear membranes under oxidative stress, showing efficient ROS generation ability (Fig. 2E–G). Under dark conditions, TTVPHE could light-up rod-shaped mitochondria. After 10 min of white light irradiation, the shape of mitochondria transformed into swelling circles, which was attributed to the destruction of mitochondrial function under oxidative stress (Fig. 2H). Meanwhile, increased red fluorescence was observed on the cell nuclear membrane. Under ROS-induced oxidative stress, the TTVPHE probe could be transferred from mitochondria to the nuclear membrane, which is attributed to the reduced mitochondrial membrane potential and increased permeability of the nuclear membrane. The results of high-resolution TEM and TUNEL assays further verified that ROS generated under white light irradiation could effectively induce apoptosis in cancer cells. Therefore, dual-organelle-targeted AIE photosensitizers with in situ induction of oxidative stress under light irradiation have broad application prospects in tracking the therapeutic effect by monitoring cell apoptosis.
3.2. Monitoring of organelle interplay
The interplay monitoring between organelles under oxidative stress is of great significance for studying various physiological processes.69,70 In order to simultaneously light-up different organelles, the cells are stained with two or more fluorescent probes, which will suffer from complicated staining and increased cytotoxicity. Meanwhile, intracellular oxidative stress induced by external inducers (such as H2O2) can affect a variety of organelles and is not conducive to the interplay monitoring between specific organelles.71 Therefore, the dual-organelle-targeted AIE probes with photodynamic activity are particularly suitable for in situ monitoring of organelle interplay under ROS induced-oxidative stress.The photoactivatable AIE probes based on the photooxidative dehydrogenation mechanism are ideal tools for organelle studies due to their high spatiotemporal controllability and non-toxic byproducts generation under irradiation.72,73 Moreover, the photoactivatable AIE probes based on photooxidative dehydrogenation reactions can achieve transformation of polarity from lipophilicity to hydrophilicity and significant red-shifted emission wavelength, which is particularly suitable for dual-organelle imaging and the in situ monitoring of the organelle interplay. Wang et al.61 developed a photoactivatable AIE probe THTTVP based on tetrahydropyridine, which exhibited strong lipophilicity and green fluorescence emission. Upon photoactivation, THTTVP could be transformed into the corresponding pyridinium salt molecule TTVP through the photooxidative dehydrogenation reaction, showing good hydrophilicity, red fluorescence emission and excellent photodynamic activity (Fig. 3A). It is noteworthy that the little overlap of fluorescence emission spectra between THTTVP and TTVP is especially suitable for the in situ monitoring of organelle interplay under ROS-induced oxidative stress. THTTVP could rapidly accumulate in lysosomes with turn-on green fluorescence (Fig. 3B). Under white light irradiation, the generated TTVP can destroy the integrity of lysosomes through photodynamic therapy, followed by lighting up mitochondria and cytomembrane with red emission, and the THTTVP escaped from lysosomes could selectively target LDs with green emission. Due to significant fluorescence spectral difference between THTTVP and TTVP, the interplay process between LDs and mitochondria under oxidative stress can be in situ real-time monitored. As shown in Fig. 3C, the number of LDs increased significantly and subsequently fused into larger LDs (∼ 1.10 mm), and the mitochondrial swelling could be clearly exhibited under ROS-induced oxidative stress. In addition, the yellow fluorescent spots are attributed to the close spatial contact between LDs with green fluorescence and mitochondria with red fluorescence (Fig. 3C). These results demonstrate that photoactivatable AIE probes can be used to in situ monitor interplay between LDs and mitochondria under oxidative stress.
 | ||
| Fig. 3 (A) Schematic illustration of photoactivable AIE probe THTTVP for the in situ monitoring of interplay between LDs and mitochondria under oxidative stress. (B) CLSM images of HeLa cells treated with THTTVP, followed by staining with LysoTracker. (C) CLSM images of HeLa cells treated with THTTVP under white light irradiation for 0–5 min, followed by incubation under dark conditions.61 Copyright 2022 Royal Society of Chemistry. | ||
3.3. Microenvironment monitoring
LDs are derived from the ER, both of which are two considerably important and closely related components.74 The polarity microenvironments of nascent LDs, mature LDs and ER are obviously different due to their differences in water contents.75 Moreover, LDs and ER are supremely sensitive to ROS-induced oxidative stress, which can significantly alter their polarity microenvironment and effectively destroy the normal function of LDs and ER.76,77 The development of AIE probes that can simultaneously target both LDs and ER are especially suitable to monitor their microenvironment changes under oxidative stress. Tang et al.56 designed and developed a photoactivatable AIE probe based on dihydropyridine, namely TPA-DHPy, which can be converted into the corresponding pyridine counterpart TPA-Py through the photooxidative dehydrogenation reaction (Fig. 4A). Moreover, TPA-Py exhibited significantly polar sensitivity, excellent photodynamic activity and AIE characteristics. TPA-DHPy could quickly enter HeLa cells and gradually light up the cells with the red fluorescence signal by prolonging the irradiation time (Fig. 4B). By co-staining with the commercial LDs-targeted probe BODIPY and the ER-targeted probe ER-Tracker, the high Pearson's correlation coefficients were observed for BODIPy/TPA-DHPy (0.84) and ERTracker/TPA-DHPy (0.81) after photoactivation, respectively, indicating the excellent LDs- and ER-targeted imaging of TPA-DHPy under light irradiation (Fig. 4C). Furthermore, multi-color cellular imaging of LDs and ER could be clearly observed in the lambda mode of CLSM, indicating the obvious polarity differences between LDs and ER (Fig. 4D). By prolonging the irradiation from 20 to 40 scans, the number of yellow- and red-color LDs increased under excessive ROS-induced oxidative stress, suggesting the significant changes in polar microenvironments and the high sensitivity of LDs against oxidative stress due to the excellent ROS generation ability of TPA-Py under white light irradiation and highly unsaturated lipids of LDs. In addition, the swelling of the ER was clearly observed, suggesting the effective destruction of ER function under oxidative stress. Therefore, TPA-Py generated after photoactivation can not only show the polarity differences between LDs and ER but also in situ real-time monitor their changes in the polar microenvironment under oxidative stress.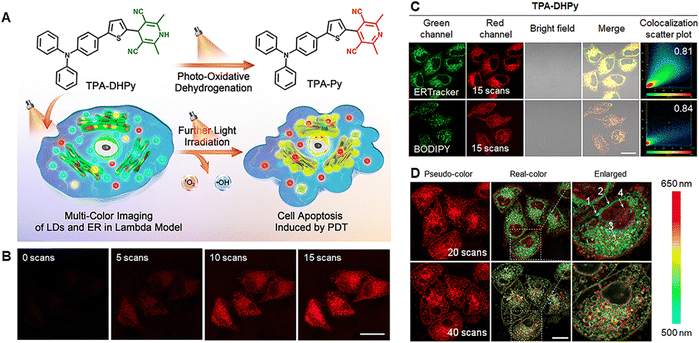 | ||
| Fig. 4 (A) Schematic illustration of photoactivable AIE probe TPA-DHPy for in situ monitoring the microenvironment changes of both LDs and ER under oxidative stress. (B) The CLSM images of HeLa cells treated with TPA-DHPy under irradiation for 0–15 scans. (C) The cellular distribution of TPA-DHPy after irradiation. (D) The CLSM images in the Lambda model of HeLa cells treated with TPA-DHPy under oxidative stress.56 Copyright 2022 Elsevier. | ||
3.4. Organelle morphology tracking
Organelle morphology is susceptible to ROS-induced oxidative stress.56 Tang et al.53 developed mitochondria- and lysosomes-targeted nanoprobe AIE-Mito-TPP/AlPcSNa4 NPs, which could quickly enter into cells and were disassembled in the acidic microenvironment of lysosomes. AIE-Mito-TPP escaped from lysosomes could selectively target mitochondria with light-up green fluorescence, while the hydrophilic photosensitizer AlPcSNa4 still accumulated in lysosomes, which exhibited the in situ monitoring of drug release processes. Moreover, AlPcSNa4 photosensitizers were distributed into the whole cell under irradiation, suggesting that ROS generated by hydrophilic photosensitizer AlPcSNa4 under light irradiation can effectively disrupt the integrity of lysosomes. By prolonging the irradiation time from 0 to 4 min, the red fluorescence signal gradually disappeared, which was not conducive to long-term monitoring of lysosomes. Subsequently, Tang et al.73 further developed a photoactivatable AIE probe TPA-DHPy-Py with high photooxidative dehydrogenation efficiency through molecular engineering strategies, which could be effectively transformed into the corresponding pyridine counterpart TPA-Py-Py via the photochemical reaction under white light irradiation, showing red fluorescence emission and excellent photodynamic activity (Fig. 5A–B). After treatment with TPA-DHPy-Py, MDA-MB-231 cells could quickly light up under light irradiation due to excellent photoactivatable efficiency of TPA-DHPy-Py. By co-staining with commercial BODIPY and ERTracker, the high co-localization coefficients were observed (Fig. 5C), suggesting the dual-organelle-targeted imaging of LDs and ER. The dual-organelle-targeted imaging ability of TPA-DHPy-Py can be attributed to the reasonable n-octanol/water partition coefficient and the derivation of LDs from the ER containing neutral lipids.27,55,78 Moreover, a number of small LDs (∼0.5 μm) could gradually fuse into larger LDs (∼2.0 μm) by prolonging the irradiation time, while ER swelling was also gradually observed (Fig. 5D), which could be attributed to the effective disruption of LDs and ER functions under ROS-induced oxidative stress. Therefore, these results suggest that the fusion of LDs and ER swelling induced by oxidative stress could be real-time monitored. | ||
| Fig. 5 (A) Schematic illustration of the photoactivatable AIE probe for light-up imaging of LDs and ER under light irradiation. (B) The ROS generation ability of 1,4-dihydropyridine derivatives. (C) The cellular distribution of TPA-DHPy-Py after irradiation. (D) The CLSM images of MDA-MB-231 cells treated with TPA-DHPy-Py upon light irradiation for different time intervals.73 Copyright 2022 Wiley-VCH. | ||
3.5. Cancer therapy
Cytoplasmic drug delivery has poor therapeutic effects on cancer due to defects in drug efflux.79,80 Organelle-targeted drug delivery systems can effectively kill cancer cells and improve the therapeutic effect of cancer.81 In particular, ROS-inducted oxidative stress can effectively destroy the normal functions of cellular organelles under light irradiation, thereby killing cancer cells.82 The development of dual-organelle-targeted AIE photosensitizers has emerged. Dong et al.57 designed a cationic AIE theranostic probe Mito-TTP with D-π-A configuration, which exhibited near-infrared fluorescence emission, highly efficient photodynamic activity and typical AIE characteristics (Fig. 6). Mito-TTPE could selectively accumulate in mitochondria with light-up fluorescence, which was attributed to the electrostatic interaction between positively charged Mito-TTPE and the negatively charged mitochondrial membrane. Subsequently, a part of Mito-TTPE could transform into lipophilic LD-TPP via esterase hydrolysis, resulting in light-up imaging of LDs with blue fluorescence. Moreover, Mito-TTPE and LD-TPP could also effectively disrupt the normal functions of mitochondria and LDs under ROS-induce oxidative stress upon light irradiation, respectively. The red fluorescence signal of PI was also observed, which could be attributed to the effective disruption of cell membrane integrity under photodynamic therapy. In addition, the significant fluorescence spectral difference between Mito-TTPE and LD-TPP is particularly suitable for studying the interplay between LDs and mitochondria under oxidative stress. | ||
| Fig. 6 (A) Chemical structure of Mito-TTPE. (B) The CLSM images of HeLa cells treated with Mito-TTPE, followed by co-staining with MitoTracker and Nile Red, respectively. (C) ROS and (D) 1O2 generation abilities of Mito-TTPE and LD-TPP under white light irradiation. (E) The CLSM images of HeLa cells treated with Mito-TTPE under white light irradiation for 0 and 30 min, respectively, followed by co-staining with Calcein-AM and PI. (F) The cell viability of HeLa cells treated with Mito-TTPE under dark conditions and irradiation, respectively.57 Copyright 2022 Royal Society of Chemistry. | ||
In order to achieve in vivo image-guided precise cancer therapy, Meng et al.83 developed deep-red AIE nanocrystal DTPA-BS-F NCs exhibiting a high fluorescence quantum yield (Fig. 7A). DTPA-BS-F NCs could quickly enter into the cancer cells through endocytosis and accumulate into lysosomes and nuclei with turn-on red fluorescence (Fig. 7B), which was attributed to the matched size between DTPA-BS-F NCs and nuclear pore complexes. Moreover, the 1O2 generation efficiency of DTPA-BS-F NCs under light irradiation was also evaluated by using the indicator 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA). As shown in Fig. 7C and D, the AIE photosensitizer (DTPA-BS-F) exhibited poor 1O2 generation ability in the THF solution, while a high singlet oxygen generation efficiency (69%) in the aggregated state was observed, which was especially suitable for effective phototherapy of cancer. Furthermore, the phototherapy efficiency of the DTPA-BS-F NCs was systematically investigated both in vitro and in vivo. DTPA-BS-F could effectively kill cancer cells in vitro and inhibit the growth of tumors in vivo by destroying the normal functions of lysosomes and nuclei.
 | ||
| Fig. 7 (A) Chemical structure of DTPA-BS-F and a schematic illustration of the all-in-one theranostic platform for cancer therapy. (B) The CLSM images of HeLa cells treated with different AIE probes, followed by co-staining with Hoechst 33342 and LysoTracker Green. The ROS generation abilities of different photosensitizers in the THF solution (C) and in the aggregated state (D). (E) The cell viability of HeLa cells treated with different concentrations of DTPA-BS-F under dark conditions and white light irradiation. (F) The tumor volume index of mice during the treatment process.83 Copyright 2022 American Chemical Society. | ||
Immunogenic cell death (ICD) has emerged as a novel strategy for tumor immunotherapy in recent years, which can transform immunosuppressive “cold” tumors into immune-activatable “hot” tumors.84 ER oxidative stress and mitochondrial damage can effectively induce ICD to activate tumor-reactive T cells, exerting the function of an anti-tumor immune response. In 2024, Zhang et al. designed and developed a photoactivatable ICD inducer THTTPy-PTSA based on the photooxidative dehydrogenation reaction mechanism, which exhibited green fluorescence emission and ER-targeting ability (Fig. 8A).85 Under light irradiation, THTTPy-PTSA could be converted into the corresponding pyridinium salt molecule TTPy-PTSA through photooxidative dehydrogenation, showing red fluorescence emission, photodynamic activity and mitochondrial-targeting properties. As shown in Fig. 8B, a high overlap was observed between THTTPy-PTSA and ER tracker red, suggesting the ER-targeted imaging of THTTPy-PTSA. Under light irradiation, THTTPy-PTSA could achieve light-up fluorescence imaging of mitochondria, which could be attributed to the effective transformation from THTTPy-PTSA into positively charged TTPy-PTSA through photooxidative dehydrogenation (Fig. 8C). The photoactivatable THTTPy-PTSA was capable of effectively inducing ER stress and mitochondrial damage, significantly increasing the release of tumor-associated antigens and damage-associated molecular patterns (DAMPs). Moreover, the in vivo anti-tumor activity of THTTPy-PTSA was verified in animal vaccine models (Fig. 8D and E), providing a new strategy for tumor immunotherapy.
 | ||
| Fig. 8 (A) Chemical structures of THTTPy-PTSA before and after photoactivation. The CLSM images of 4T1 cells co-stained with (B) THTTPy-PTSA + ER tracker red and (C) THTTPy-PTSA after photoactivation + Mito tracker green. (D) Tumor volume of mice in different treatment groups. (E) Photographs of the tumors.85 Copyright 2024 Wiley-VCH. (F) Chemical structures of TPAQ, TPATQ and TPITQ. (G) PL spectra of DPITQ vs. the solvent composition changes of the MeOH/water mixture. (H) The CLSM images of 4T1 cells treated with DPITQ for 10–180 min. (I) In vivo NIR-II fluorescence images of the 4T1-bearing nude mice and ex vivo NIR-II fluorescence images of tumors and different organs. (J) Tumor volume of mice in different treatment groups.86 Copyright 2024 Wiley-VCH. | ||
Second near-infrared (NIR-II, 1000–1700 nm) AIE probes with high tissue penetration depth have been widely used for organelle-targeted cancer therapy. Tang et al. synthesized a plasma membrane- and mitochondria-targeted NIR-II AIE probe using a molecular engineering strategy, which exhibited typical AIE properties and phototherapeutic activity (Fig. 8F and G).86 At the in vitro cellular level, DPITQ could specifically target the plasma membrane and mitochondria of cancer cells due to its positive charge and appropriate hydrophobicity (Fig. 8H). Under 635 nm laser irradiation, DPITQ could effectively destroy the normal functions of the plasma membrane and mitochondria, leading to simultaneous pyroptosis and apoptosis under hypoxic conditions, thereby significantly killing cancer cells in vitro. Moreover, with 4T1-bearing nude mice as a in vivo model, DPITQ NPs could effectively locate the tumor through NIR-II fluorescence imaging, followed by precise phototherapy to eliminate the tumor cells (Fig. 8I and J). Therefore, dual-organelle-targeted NIR-II AIE phototherapeutic agents are expected to provide a new strategy for imaging-guided PDT and PTT against cancer.
Compared with mono-/dual-organelle-targeted AIE probes, multi-organelle-targeted AIE probes can significantly improve cancer therapy effects. Tang et al.63 synthesized mitochondria-targeted TFPy, cell membrane-targeted TFVP, and lysosome-targeted TPE-TFPy, showing AIE features and excellent photodynamic activity in the aggregated state (Fig. 9A). These AIE probes were quickly taken up by cancer cells and achieved light-up fluorescence imaging of specific organelles. Moreover, after HeLa cells were simultaneously treated with these three AIEgens (TFPy, TFVP, and TPE-TFPy), the cellular mitochondria, lysosomes and cell membranes could be lit up with red fluorescence (Fig. 9B). The photodynamic activities of these AIE probes were measured using the 1O2 indicator ABDA. Compared with the Ce6 photosensitizer, all the three AIE probes exhibited higher 1O2 generation efficiency in the aggregated state (Fig. 9C), which is attributed to the ACQ effect of Ce6 quenching photosensitization in aggregation.87 It has been confirmed through in vitro cell and in vivo animal experiments that the “three-in-one” group could effectively kill cancer cells in vitro and inhibit the growth of tumors in vivo under light irradiation, showing 1 + 1 + 1 > 3 synergistic photodynamic therapy effect (Fig. 9D–F). Meanwhile, the three AIE probes also showed good biocompatibility.
 | ||
| Fig. 9 (A) Schematic illustration of multi-organelle-targeted “three-in-one” probes for synergistic photodynamic therapy. (B) CLSM images of HeLa cells treated with TFPy + MitoTracker, TPE-TFPy + LysoTracker, TFVP + CellMask and three AIEgens + Hoechst 33258, respectively. (C) 1O2 generation ability of different photosensitizers. Cell viabilities of HeLa cells treated with different concentrations of AIEgens under (D) dark conditions and (E) white light irradiation. (F) Relative tumor volume of mice in different treatment groups.63 Copyright 2020 Wiley-VCH. (G) Schematic illustration of sequential destruction of multi-organelles under oxidative stress. (H) CLSM images of A375 cells treated with THTTPy, followed by staining with commercial organelle-targeted probes under dark conditions and irradiation. (I) Cell viability of A375 cells treated with different concentrations of THTTPy under dark conditions and light irradiation. (J) Tumor volume of mice in different treatment groups.88 Copyright 2023 American Chemical Society. | ||
The sequential destruction of multi-organelles under oxidative stress is also used for precise cancer therapy using a photoactivatable strategy. Wang et al.88 designed and developed photoactivatable AIE probe THTTPy based on tetrahydropyridine, which could be rapidly converted into the corresponding pyridinium salt counterpart TTPy through the photooxidative dehydrogenation reaction, showing red fluorescence emission and excellent photodynamic activity in the aggregated state (Fig. 9G). THTTPy can be effectively taken up by cancer cells and accumulated into cellular lysosomes and LDs in the initial stage, showing light-up green fluorescence emission (Fig. 9H). Subsequently, a portion of THTTPy after photoactivation could be transformed into TTPy, which could disrupt the integrity of lysosomal membranes under ROS-induced oxidative stress upon light irradiation, followed by accumulation into mitochondria with turn-on red fluorescence. Moreover, the normal functions of mitochondria and LDs were effectively destroyed under ROS-induced oxidative stress by further prolonging the light irradiation, suggesting that THTTPy can achieve sequential destruction of multiple organelles. Furthermore, the effective killing of cancer cells in vitro and significant inhibition in tumor growth was also achieved (Fig. 9I–J). Therefore, multi-organelle-targeted AIE probes associated with oxidative stress are highly promising in precise cancer therapy.
4. Conclusions and perspectives
This review highlights the recent advancements for biomedical applications of dual-/multi-organelle-targeted AIE probes showing effective oxidative stress under light irradiation. Various design strategies have been used for developing these AIE probes, including double ligand modification, self-assembly of two mono-organelle-targeted probes, lipophilic regulation, photo/pH/enzyme activation, oxidative stress, and so on. Benefiting from unique photophysical properties and enhanced photodynamic activity in the aggregated state, these dual-/multi-organelle-targeted AIE probes are widely applied in the imaging-guided in situ monitoring of biological processes under oxidative stress and in precise cancer therapy. Overall, significant development of dual-/multi-organelle-targeted AIE probes associated with oxidative stress has been exhibited in biomedical applications.Although dual-/multi-organelle-targeted AIE probes have made great progress for biomedical applications, they still suffer from some challenges, such as limited interplay monitoring due to similar emission spectra in different organelles, lack of single molecule AIE probes targeting multi-organelles simultaneously, short absorption and emission of photoactivatable AIE probes, unclear organelle-targeted mechanism, limited tissue penetration depth, and so forth. Therefore, future efforts should focus on the development of AIE probes that can in situ monitor biological processes among multiple organelles under oxidative stress. Moreover, benefiting from high tissue penetration depth, low tissue absorption and high signal-to-noise ratio, the organelle-targeted second near-infrared (NIR-II, 1000–1700 nm) AIE probes have broad application prospects for image-guided precise cancer therapy under NIR-II light irradiation.89 However, the development of CLSM with NIR-II excited lasers and NIR-II fluorescence imaging ability is not yet perfect, which limits the in situ monitoring of organelle-targeted NIR-II AIE probes in vitro. We believe that greater application prospects of dual-/multi-organelle-targeted AIE probes inducing in situ oxidative stress under irradiation will be exhibited in the biomedical fields through rational molecular design and extensive research efforts.
Author contributions
Conceptualization, Y. Y.; writing – original draft preparation, Y. Y.; writing – review and editing, Y. Y., S. L., C. T., Y. L., D. Y., D. W. and X. C.; supervision, Y. Y. and X. C.; project administration, Y. Y., X. C. and D. W.; funding acquisition, D. W., X. C. and Y. Y. All authors have read and agreed to the published version of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.Acknowledgements
This work was funded by the National Natural Science Foundation of China (22205080), the Guangdong Basic and Applied Basic Research Foundation (2023A1515110122, 2024A1515010677, and 2024A1515012842), and the Doctoral Initial Funding of Guangdong Medical University (4SG24259G, 4SG24210G). The authors also acknowledge the AIE Research Center of Shenzhen University.References
- K. F. Ferri and G. Kroemer, Nat. Cell Biol., 2001, 3, E255–E263 CrossRef CAS PubMed.
- Z. Fang and H. Chen, Adv. Drug Delivery Rev., 2023, 115020 CrossRef CAS PubMed.
- G. Hamilton, Nature, 2015, 525, 444–446 CrossRef CAS PubMed.
- F. J. Bock and S. W. Tait, Nat. Rev. Mol. Cell Biol., 2020, 21, 85–100 CrossRef CAS PubMed.
- J. P. Luzio, P. R. Pryor and N. A. Bright, Nat. Rev. Mol. Cell Biol., 2007, 8, 622–632 CrossRef CAS PubMed.
- S. R. Bonam, F. Wang and S. Muller, Nat. Rev. Drug Discovery, 2019, 18, 923–948 CrossRef CAS PubMed.
- J. A. Olzmann and P. Carvalho, Nat. Rev. Mol. Cell Biol., 2019, 20, 137–155 CrossRef CAS PubMed.
- A. R. Thiam, R. V. Farese Jr and T. C. Walther, Nat. Rev. Mol. Cell Biol., 2013, 14, 775–786 CrossRef CAS PubMed.
- K. Zhang and R. J. Kaufman, Nature, 2008, 454, 455–462 CrossRef CAS PubMed.
- X. Chen and J. R. Cubillos-Ruiz, Nat. Rev. Cancer, 2021, 21, 71–88 CrossRef CAS PubMed.
- A. P. King and J. J. Wilson, Chem. Soc. Rev., 2020, 49, 8113–8136 RSC.
- Y.-H. Lee, F.-Y. Cheng, H.-W. Chiu, J.-C. Tsai, C.-Y. Fang, C.-W. Chen and Y.-J. Wang, Biomaterials, 2014, 35, 4706–4715 CrossRef CAS PubMed.
- D. I. Danylchuk, P.-H. Jouard and A. S. Klymchenko, J. Am. Chem. Soc., 2021, 143, 912–924 CrossRef CAS PubMed.
- N. Rubio, I. Coupienne, E. Di Valentin, I. Heirman, J. Grooten, J. Piette and P. Agostinis, Autophagy, 2012, 8, 1312–1324 CrossRef CAS PubMed.
- G. Filomeni, D. De Zio and F. Cecconi, Cell Death Differ., 2015, 22, 377–388 CrossRef CAS PubMed.
- K. A. Willets, A. J. Wilson, V. Sundaresan and P. B. Joshi, Chem. Rev., 2017, 117, 7538–7582 CrossRef CAS PubMed.
- M. Schäferling, Angew. Chem., Int. Ed., 2012, 51, 3532–3554 CrossRef PubMed.
- C. Li, G. Chen, Y. Zhang, F. Wu and Q. Wang, J. Am. Chem. Soc., 2020, 142, 14789–14804 CrossRef CAS PubMed.
- R. Yuste, Nat. Methods, 2005, 2, 902–904 CrossRef CAS PubMed.
- B.-C. Chen, W. R. Legant, K. Wang, L. Shao, D. E. Milkie, M. W. Davidson, C. Janetopoulos, X. S. Wu, J. A. Hammer III and Z. Liu, Science, 2014, 346, 1257998 CrossRef PubMed.
- J. Qian and B. Z. Tang, Chem, 2017, 3, 56–91 CAS.
- Z. Wang, F. Zhou, C. Gui, J. Wang, Z. Zhao, A. Qin and B. Z. Tang, J. Mater. Chem. C, 2018, 6, 11261–11265 RSC.
- M. Kang, Z. Zhang, N. Song, M. Li, P. Sun, X. Chen, D. Wang and B. Z. Tang, Aggregate, 2020, 1, 80–106 CrossRef.
- D. Wang and B. Z. Tang, Acc. Chem. Res., 2019, 52, 2559–2570 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- Y. Hong, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- Z. Zhao, H. Zhang, J. W. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9888–9907 CrossRef CAS PubMed.
- F. Würthner, Angew. Chem., Int. Ed., 2020, 59, 14192–14196 CrossRef PubMed.
- R. T. Kwok, C. W. Leung, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228–4238 RSC.
- J. Mei, N. L. Leung, R. T. Kwok, J. W. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- Q. Hu, M. Gao, G. Feng and B. Liu, Angew. Chem., Int. Ed., 2014, 53, 14225–14229 CrossRef CAS PubMed.
- D. Wang, M. M. S. Lee, G. Shan, R. T. K. Kwok, J. W. Y. Lam, H. Su, Y. Cai and B. Z. Tang, Adv. Mater., 2018, 30, 1802105 CrossRef PubMed.
- S. Li, X. Ling, Y. Lin, A. Qin, M. Gao and B. Z. Tang, Chem. Sci., 2018, 9, 5730–5735 RSC.
- C. W. T. Leung, Z. Wang, E. Zhao, Y. Hong, S. Chen, R. T. K. Kwok, A. C. S. Leung, R. Wen, B. Li and J. W. Y. Lam, Adv. Healthcare Mater., 2016, 5, 427–431 CrossRef CAS PubMed.
- Z. Zhu, Q. Wang, H. Liao, M. Liu, Z. Liu, Y. Zhang and W.-H. Zhu, Natl. Sci. Rev., 2021, 8, nwaa198 CrossRef CAS PubMed.
- R. Wang, X. Li and J. Yoon, ACS Appl. Mater. Interfaces, 2021, 13, 19543–19571 CrossRef CAS PubMed.
- M. Liu, Y. Chen, Y. Guo, H. Yuan, T. Cui, S. Yao, S. Jin, H. Fan, C. Wang and R. Xie, Nat. Commun., 2022, 13, 2179 CrossRef CAS PubMed.
- P. Xiao, K. Ma, M. Kang, L. Huang, Q. Wu, N. Song, J. Ge, D. Li, J. Dong and L. Wang, Chem. Sci., 2021, 12, 13949–13957 RSC.
- M. Kang, Z. Zhang, W. Xu, H. Wen, W. Zhu, Q. Wu, H. Wu, J. Gong, Z. Wang and D. Wang, Adv. Sci., 2021, 8, 2100524 CrossRef CAS PubMed.
- K. N. Wang, L. Y. Liu, D. Mao, M. X. Hou, C. P. Tan, Z. W. Mao and B. Liu, Angew. Chem., Int. Ed., 2022, 61, e202114600 CrossRef CAS PubMed.
- M. Gao, H. Su, Y. Lin, X. Ling, S. Li, A. Qin and B. Z. Tang, Chem. Sci., 2017, 8, 1763–1768 RSC.
- M. M. Lee, D. M. Lin, J. H. Chau, E. Y. Yu, D. Ding, R. T. Kwok, D. Wang and B. Z. Tang, ACS Nano, 2023, 17, 11039–11053 CrossRef CAS PubMed.
- B. Chen, H. Yuan, W. Zhang, J. Hu, X. Lou and F. Xia, Biosensors, 2022, 12, 667 CrossRef CAS PubMed.
- S. M. Sayed, H.-R. Jia, Y.-W. Jiang, Y.-X. Zhu, L. Ma, F. Yin, I. Hussain, A. Khan, Q. Ma and F.-G. Wu, J. Mater. Chem. B, 2021, 9, 4303–4308 RSC.
- D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441–2453 CrossRef CAS PubMed.
- D. G. Deavall, E. A. Martin, J. M. Horner and R. Roberts, J. Toxicol., 2012, 2012, 645460 Search PubMed.
- R. Franco, O. Schoneveld, A. G. Georgakilas and M. I. Panayiotidis, Cancer Lett., 2008, 266, 6–11 CrossRef CAS PubMed.
- J. Li, Z. Zhuang, Z. Zhao and B. Z. Tang, View, 2022, 3, 20200121 CrossRef CAS.
- H. Wang, Q. Li, P. Alam, H. Bai, V. Bhalla, M. R. Bryce, M. Cao, C. Chen, S. Chen and X. Chen, ACS Nano, 2023, 17, 14347–14405 CrossRef CAS PubMed.
- F. Hu, S. D. Xu and B. Liu, Adv. Mater., 2018, 30, 1801350 CrossRef PubMed.
- X. L. Cai and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 9868–9886 CrossRef CAS PubMed.
- P.-Y. Ho, T. Y. Chou, C. Kam, W. Huang, Z. He, A. H. Ngan and S. Chen, Sci. Adv., 2023, 9, eabn5390 CrossRef PubMed.
- X. Chen, Y. Li, S. Li, M. Gao, L. Ren and B. Z. Tang, Adv. Funct. Mater., 2018, 28, 1804362 CrossRef.
- R. Zhang, G. Niu, Z. Liu, J. H. Chau, H. Su, M. M. Lee, Y. Gu, R. T. Kwok, J. W. Lam and B. Z. Tang, Biomaterials, 2020, 242, 119924 CrossRef CAS PubMed.
- L. Guo, M. Tian, Z. Zhang, Q. Lu, Z. Liu, G. Niu and X. Yu, J. Am. Chem. Soc., 2021, 143, 3169–3179 CrossRef CAS PubMed.
- X. Chen, Z. Zhang, W. Luo, Z. Zhuang, Z. Zhao, L. Wang, D. Wang and B. Z. Tang, Biomaterials, 2022, 287, 121680 CrossRef CAS PubMed.
- Y. Zhang, S. Wang, N. Zhang, X. Wang, Q. Zan, L. Fan, X. Yu, S. Shuang and C. Dong, Mater. Chem. Front., 2022, 6, 333–340 RSC.
- J. Zhuang, Y. Yu, R. Su, Q. Ma, N. Li and N. Zhao, Dyes Pigm., 2023, 208, 110809 CrossRef.
- J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Chem. Rev., 2012, 112, 2642–2713 CrossRef CAS PubMed.
- Y. Hu, S. Y. Yin, W. Liu, Z. Li, Y. Chen and J. Li, Aggregate, 2023, 4, e256 CrossRef CAS.
- Z. Zhang, W. Pan, Y. Xie, K. Liu, M. Gao and Y. Wang, Mater. Chem. Front., 2022, 6, 3662–3668 RSC.
- T. Zhang, Y. Li, Z. Zheng, R. Ye, Y. Zhang, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 5612–5616 CrossRef CAS PubMed.
- W. Xu, M. M. S. Lee, J.-J. Nie, Z. Zhang, R. T. K. Kwok, J. W. Y. Lam, F.-J. Xu, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9610–9616 CrossRef CAS PubMed.
- J. Zhuang, N. Li, Y. Zhang, B. Li, H. Wen, X. Zhang, T. Zhang, N. Zhao and B. Z. Tang, CCS Chem., 2022, 4, 1028–1043 CrossRef CAS.
- X. Zhu and S. Li, Adv. Sci., 2023, 10, 2300824 CrossRef CAS PubMed.
- M. O. Hengartner, Nature, 2000, 407, 770–776 CrossRef CAS PubMed.
- B. A. Carneiro and W. S. El-Deiry, Nat. Rev. Clin. Oncol., 2020, 17, 395–417 CrossRef PubMed.
- T. Zhou, J. F. Zhu, D. Shang, C. X. Chai, Y. Z. Li, H. Y. Sun, Y. Q. Li, M. Gao and M. Li, Mater. Chem. Front., 2020, 4, 3201–3208 RSC.
- H. Xue, E. Ge, W. Ge, J. Li and M. Tian, Anal. Chem., 2022, 94, 9158–9165 CrossRef CAS PubMed.
- K. Wang, S. Ma, Y. Ma, Y. Zhao, M. Xing, L. Zhou, D. Cao and W. Lin, Anal. Chem., 2020, 92, 6631–6636 CrossRef CAS PubMed.
- Y.-H. Liao, J.-T. Wu, I.-C. Hsieh, H.-H. Lee and P.-H. Huang, iScience, 2023, 26, 106005 CrossRef CAS PubMed.
- Y. You, C. Tang, S. Lin, D. Zeng, Y. Cong, D. Wang and X. Chen, Luminescence, 2024, 39, e4645 CrossRef CAS PubMed.
- X. Chen, N. Niu, D. Li, Z. Zhang, Z. Zhuang, D. Yan, J. Li, Z. Zhao, D. Wang and B. Z. Tang, Adv. Funct. Mater., 2023, 33, 2211571 CrossRef CAS.
- L. Galluzzi, J. M. Bravo-San Pedro and G. Kroemer, Nat. Cell Biol., 2014, 16, 728–736 CrossRef CAS PubMed.
- E. Zhao, C. Kam, M.-Y. Wu, E. Wang, J. Wang, B. Z. Tang and S. Chen, CCS Chem., 2022, 4, 515–525 CrossRef CAS.
- H. Yin, L. Xu and N. A. Porter, Chem. Rev., 2011, 111, 5944–5972 CrossRef CAS PubMed.
- I. Tabas and D. Ron, Nat. Cell Biol., 2011, 13, 184–190 CrossRef CAS PubMed.
- G. Niu, R. Zhang, J. P. Kwong, J. W. Lam, C. Chen, J. Wang, Y. Chen, X. Feng, R. T. Kwok and H. H.-Y. Sung, Chem. Mater., 2018, 30, 4778–4787 CrossRef CAS.
- E. L. Giddings, D. P. Champagne, M.-H. Wu, J. M. Laffin, T. M. Thornton, F. Valenca-Pereira, R. Culp-Hill, K. A. Fortner, N. Romero and J. East, Nat. Commun., 2021, 12, 2804 CrossRef CAS PubMed.
- F. Gao, X. Yang, X. Luo, X. Xue, C. Qian and M. Sun, Adv. Funct. Mater., 2020, 30, 2001546 CrossRef CAS.
- X. Ling, L. Huang, Y. Li, Q. Wan, Z. Wang, A. Qin, M. Gao and B. Z. Tang, Mater. Horiz., 2020, 7, 2696–2701 RSC.
- J. Zhou, F. Qi, Y. Chen, S. Zhang, X. Zheng, W. He and Z. Guo, Biosensors, 2022, 12, 1027 CrossRef CAS PubMed.
- R. Xu, W. Chi, Y. Zhao, Y. Tang, X. Jing, Z. Wang, Y. Zhou, Q. Shen, J. Zhang, Z. Yang, D. Dang and L. Meng, ACS Nano, 2022, 16, 20151–20162 CrossRef CAS PubMed.
- P. Meier, A. J. Legrand, D. Adam and J. Silke, Nat. Rev. Cancer, 2024, 24, 299–315 CrossRef CAS PubMed.
- X. Wang, J. Qian, Z. Yang, Y. Song, W. Pan, Y. Ye, X. Qin, X. Yan, X. Huang, X. Wang, M. Gao and Y. Zhang, Adv. Mater., 2024, 36, 2310964 CrossRef CAS PubMed.
- J. Zhuang, Z. Ma, N. Li, H. Chen, L. Yang, Y. Lu, K. Guo, N. Zhao and B. Z. Tang, Adv. Mater., 2024, 36, 2309488 CrossRef CAS PubMed.
- N. Yu, P. Qiu, Q. Ren, M. Wen, P. Geng, D. K. Macharia, M. Zhu and Z. Chen, ACS Nano, 2021, 15, 19793–19805 CrossRef CAS PubMed.
- W. Pan, H. Shao, L. Ma, X. Tong, Z. Zhang, Q. Li, X. Yang, K. Liu, M. Gao and Y. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 37121–37129 CrossRef CAS PubMed.
- Y. Qin, X. Chen, Y. Gui, H. Wang, B. Z. Tang and D. Wang, J. Am. Chem. Soc., 2022, 144, 12825–12833 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |







