Open Access Article
Open Access ArticleNear-infrared AIEgens with high singlet-oxygen yields for mitochondria-specific imaging and antitumor photodynamic therapy†
Shasha
Zhang
a,
Wenfang
Yang
a,
Xiao
Lu
b,
Xinyi
Zhang
a,
Zhichao
Pan
a,
Da-Hui
Qu
 a,
Dong
Mei
*b,
Ju
Mei
a,
Dong
Mei
*b,
Ju
Mei
 *a and
He
Tian
*a and
He
Tian
 a
a
aKey Laboratory for Advanced Materials, Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, Frontiers Science Center for Materiobiology and Dynamic Chemistry, Institute of Fine Chemicals, School of Chemistry & Molecular Engineering, East China University of Science & Technology, 130 Meilong Road, Shanghai 200237, P. R. China. E-mail: daisymeiju@ecust.edu.cn
bClinical Research Center, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, 56 South Lishi Road, Xicheng District, Beijing 100045, P. R. China. E-mail: meidong@bch.com.cn
First published on 6th June 2023
Abstract
AIE-active photosensitizers (PSs) are promising for antitumor therapy due to their advantages of aggregation-promoted photosensitizing properties and outstanding imaging ability. High singlet-oxygen (1O2) yield, near-infrared (NIR) emission, and organelle specificity are vital parameters to PSs for biomedical applications. Herein, three AIE-active PSs with D–π–A structures are rationally designed to realize efficient 1O2 generation, by reducing the electron–hole distribution overlap, enlarging the difference on the electron-cloud distribution at the HOMO and LUMO, and decreasing the ΔEST. The design principle has been expounded with the aid of time-dependent density functional theory (TD-DFT) calculations and the analysis of electron–hole distributions. The 1O2 quantum yields of AIE-PSs developed here can be up to 6.8 times that of the commercial photosensitizer Rose Bengal under white-light irradiation, thus among the ones with the highest 1O2 quantum yields reported so far. Moreover, the NIR AIE-PSs show mitochondria-targeting capability, low dark cytotoxicity but superb photo-cytotoxicity, and satisfactory biocompatibility. The in vivo experimental results demonstrate good antitumor efficacy for the mouse tumour model. Therefore, the present work will shed light on the development of more high-performance AIE-PSs with high PDT efficiency.
Introduction
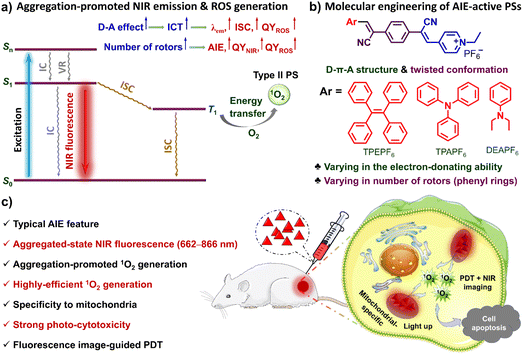
Photodynamic therapy (PDT), due to its site-specificity and non-invasiveness, has become one of the most promising strategies for clinical tumour treatment.1–13 Exploiting photosensitizers (PSs) effectively generating reactive oxygen species (ROS) such as singlet oxygen (1O2) under light irradiation is of paramount importance to the development of PDT.1,3–6,8 Among the PSs developed,1–17 the luminescent ones usable for image-guided PDT are attracting increasing attention.1–5,8,9,11,15–17 For a clinically usable luminescent PS, high ROS generation, long emission wavelength, and organelle specificity are very important factors.2,4 However, most of the existing luminescent PSs still can hardly simultaneously hold these three merits.Small-molecular organic fluorophores with photosensitizing properties stand out among various PSs, due to their satisfactory biocompatibility and biodegradability, easy-to-adjust structures, and optical properties.1–5,8,9,11–13,15–17 However, traditional organic luminescent PSs usually suffer from the aggregation-caused quenching (ACQ) effect in the biological media due to the rigid planar π-conjugated structures and poor water-solubility.13,16,17 The ACQ effect of a luminescent PS usually leads to a reduction in the efficiency of emission and 1O2 generation and subsequently limits its wide application in biomedical fields. In contrast, aggregation-induced emission (AIE)-active luminogens (AIEgens) have shown significant advantages and potential for biomedical applications due to their high brightness, outstanding photostability, and long-term in situ imaging ability.5,8–11,14,15,18–37 What is more attractive is that the strong intra/intermolecular interactions make the AIEgens prone to form tighter aggregates, which could block the nonradiative pathways, increase the quantum yield, stabilize the triplet state, and thus boost the generation of ROS.5,8,37 It is therefore believed that the AIEgen-based PSs are ideal candidates for image-guided PDT.5,9,11,23,25,27,29,37
NIR fluorescence imaging has shown great potential in the diagnosis of diseases such as cancer, due to its negligible bio-substrate autofluorescence interference and deep-tissue penetrating ability.9,11,24,33,36 As such, developing simple and facilely accessible AIE-active NIR PSs is thus urgently needed to promote clinical applications. Moreover, organelle specificity is also conducive to the performance of luminescent PSs. Mitochondria-specific PSs provide a favourable option for high-efficiency PDT,33,34,36 because mitochondria are the energy factories for cells and key regulators of cell death signals.38 On the other hand, mitochondria are the primary targeted sites of 1O2, which are damaged in the early stages of apoptosis induced by PDT. Despite that AIE-active NIR PSs have been developed for mitochondria-specific image-guided PDT,34,36,39–41 currently there is still a lack of simple and economical ones with high ROS yield and mitochondria-targeting ability.
Herein, three NIR AIE-active PSs (i.e., TPEPF6, TPAPF6 and DEAPF6) with mitochondria specificity and ultra-efficient 1O2 generation have been developed through rational molecular engineering (Scheme 1a and b). Impressively, TPEPF6 and TPAPF6 show the highest 1O2 quantum yields reported so far (Table S1†). The in vitro experiments have shown that the present AIE-PSs can be quickly taken up by cancer cells and display bright NIR fluorescence in cells, exhibiting high photostability, good biocompatibility, and preferable localization in mitochondria. In order to simply evaluate the PDT potential of these small-molecular AIE-PSs, we adopted the intratumoral injection to ensure their accumulation in tumours. The in vivo experiments have demonstrated the potential of these PSs to visualize tumours, efficiently destroy cancer cells and prevent the growth of malignant tumours under white-light irradiation (Scheme 1c). This research work is supposed to not only provide powerful alternatives for NIR fluorescence image-guided PDT but also offer insights into the rational design of efficient NIR-luminescent AIE-PSs.
Results and discussion
Molecular design, synthesis, and characterization
As shown in Scheme 1, in each of our AIE-PSs, twisted conformation, extended π-conjugation, donor–acceptor (D–A) electronic structures, as well as the sufficient separation of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) have been ingeniously integrated. Structurally speaking, the twisted conformation with multiple rotors is believed to be responsible for the AIE attribute. To be more specific, the multiple aromatic rings connected through single bonds can rotate or twist around the single bonds and thus can be viewed as rotors. In the solution or molecularly dispersed state, the multiple aromatic rings can rotate freely and vigorously, which dissipates the excited-state energy, boosts the nonradiative decay channels, and thus leads to inefficient or even no emission. In contrast, in the solid or aggregated state, the intense intermolecular interactions exert physical constraints on the intramolecular motions, which activates the radiative decay pathways and results in efficient luminescence. Moreover, because of the repulsive interaction and the steric hindrance, the aromatic rings are tilted out of the plane, leading to a distorted 3D conformation. Such a conformation could prevent the π–π stacking and further hamper the emission quenching. Consequently, the AIE characteristics can be ensured. The extended π-conjugation together with the D–A structure is supposed to account for the NIR fluorescence and ICT effect. In the meantime, the ICT and the small overlap between the HOMO and LUMO, which for one thing reduce the energy gap between the singlet state and the triplet state (ΔEST) and for another promote the intersystem crossing (ISC), are supposed to contribute to the efficient PS effect.5,8,37,42–46 What's more, it has been discovered that the separation of electron–hole transition orbitals is associated with the ICT effect, can give rise to a small ΔEST and accelerate the ISC process, thus conducive to the generation of ROS.5,8,37,47–50Accordingly, molecules holding a D–π–A structure with multiple rotors are facilely constructed. Electron-donating tetraphenylethylene (TPE), triphenylamine (TPA), and diethylaminobenzene (DEA) are respectively conjugated to the same electron-accepting moiety, i.e. (E)-4-(2-cyanovinyl)-1-ethylpyridin-1-ium groups via two simple Knoevenagel condensation reactions by using the intermediate of 2,2′-(1,4-phenylene)diacetonitrile. It thus gives rise to the uncharged molecular skeleton supposed to have an AIE feature and long-wavelength emission. Further salification of the pyridinyl groups successfully yields our targeted molecules TPEPF6, TPAPF6, and DEAPF6 (Scheme 1). The positively charged pyridinium group not only can serve as an electron-accepting unit but also might afford a good mitochondria-targeting function.33,34,40,41 The simple three-step synthetic routes of the designed TPEPF6, TPAPF6, and DEAPF6 are shown in Scheme S1.† The structures of all the intermediates and targeted products were confirmed by 1H NMR, 13C NMR, and HRMS, with the detailed characterization data shown in Fig. S1–S25.†
Theoretical calculations
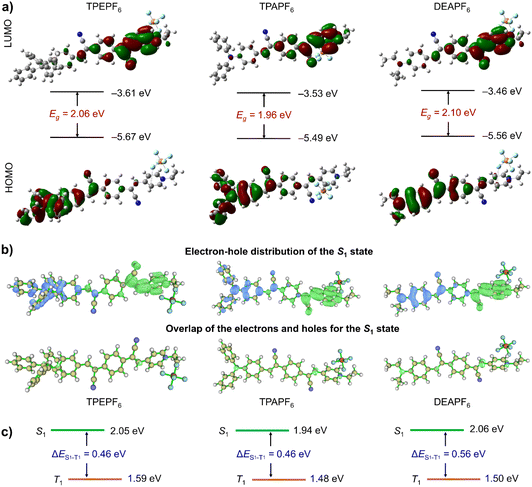
To grasp an understanding on the relationship between the structure and the properties of these three compounds prior to the experiments, the TD-DFT method in the Gaussian 09 software package was first applied to perform the structure optimization and theoretical calculations.51 As revealed by the optimized molecular geometries shown in Fig. 1a, all these compounds adopt a non-planar conformation. It would effectively inhibit the intermolecular π–π stacking and the subsequent fluorescence quenching and hence would allow these compounds to fluoresce intensively in the aggregated state. The energy gaps (Egs) of TPEPF6, TPAPF6, and DEAPF6 were calculated to be 2.06, 1.96, and 2.10 eV, respectively. Moreover, each compound exhibits distinctly different electron distributions between the HOMO and LUMO (Fig. 1a), suggestive of a remarkable ICT effect. The electron clouds on all the LUMOs are mainly distributed over the (Z)-4-(2-cyano-2-phenylvinyl)pyridin-1-ium moiety. Compared with TPEPF6 and TPAPF6, the electron cloud of the HOMO in DEAPF6 is more widely distributed on the benzene in the middle of the 2,2'-(1,4-phenylene)diacrylonitrile group. It means that the charge separation is more thorough in TPEPF6 and TPAPF6 as compared to that in DEAPF6. It implies that these compounds might all possess efficient PS properties, and the PS performances of TPEPF6 and TPAPF6 would probably be better than that of DEAPF6. Taking the efficient ICT effect and small Eg together, NIR fluorescence can be anticipated as well.To gain a deeper insight into their ICT effects, the electron–hole distributions were further analysed using the Multiwfn program.47,48,52 The results are well consistent with those obtained via Gaussian calculations. More specifically, the calculated electron–hole distributions of these molecules in the S1 state show that the electrons are mainly distributed on the (Z)-4-(2-cyano-2-phenylvinyl)pyridin-1-ium group (A), while the holes are primarily distributed on the acrylonitrile-decorated TPE/TPA/DEA group (D). It means that electrons are transferred from the D group to the A group in the S1 state (the top panel of Fig. 1b). Clearly, it is also illustrated that there is a small overlap between the electron and hole distributions in all these three compounds (the bottom panel of Fig. 1b). The overlap between the electron and hole distribution of DEAPF6 is slightly larger than that of TPEPF6 or TPAPF6 according to the Sr and Sm values shown in Table S2.† Combining multiple parameters of electron–hole distribution (Table S2†), all the S1 states feature ICT characteristics, which is beneficial to the reduction of ΔEST.48 The ΔES1−T1 is calculated to be 0.46, 0.46, and 0.56 for TPEPF6, TPAPF6, and DEAPF6, respectively (Fig. 1c and S26†). As shown in Table S3,† all these compounds have small ΔESTs. Moreover, the results of cyclic voltammetry tests (Fig. S27† and Table 1) are in good accordance with those obtained by Gaussian calculations, all suggesting that TPEPF6, TPAPF6, and DEAPF6 might be good photosensitizers. Encouraged by the theoretical results, a series of experiments were implemented to assess the photophysical and photosensitizing properties, the mitochondria-targeting ability, and the in vitro and in vivo PDT effect.
| Comp. | Soln./nm | Aggr./nm | Solid/nm | HOMOe/eV | LUMOf/eV | E g /eV | HOMOh/eV | LUMOh/eV | E g /eV | S1h/eV | T1h/eV | ΔESTh/ev | E SOG /nmol | Relative 1O2 yieldj | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λ abs | λ em | λ em | λ em | ||||||||||||
| a Absorption maximum in DMSO. b Emission maximum in DMSO. c Emission maximum in the DMSO/toluene mixture (1/9, v/v). d Emission maximum in the solid state. e Obtained from the onset of the oxidation voltages using the equation: EHOMO = −e(Eonset ox + 4.4 V). f Obtained from the onset of the reduction voltages using the equation: ELUMO = −e(Eonset re + 4.4 V). g Obtained from the equation: Eg = ELUMO − EHOMO. h Values obtained from DFT calculation using the Gaussian 09 package. i 1O2 generation, which was calculated by monitoring the amount of ABDA consumed per minute in the presence of 10 nmol of AIE-PS under white-light irradiation (25 mW cm−2). [ABDA] = 5 × [AIE-PS]. j The relative 1O2 generation efficiencies were measured under the same conditions as Rose Bengal (RB = 0.75 in water) which was employed as the standard. | |||||||||||||||
| TPEPF6 | 419 | 553 | 662 | 694 | –5.77 | –3.99 | 1.78 | –5.67 | –3.61 | 2.06 | 2.05 | 1.59 | 0.46 | 37.7 | 5.11 |
| TPAPF6 (crystal) | 476 | 583 | 783 | 833 | –5.48 | –3.97 | 1.51 | –5.49 (–5.48) | –3.53 (–3.86) | 1.96 (1.62) | 1.94 (1.65) | 1.48 (1.34) | 0.46 (0.31) | 40.2 | 3.17 |
| DEAPF6 | 495 | 640 | 768 | 866 | –5.59 | –3.40 | 2.19 | –5.56 | –3.46 | 2.10 | 2.06 | 1.50 | 0.56 | 19.8 | 0.88 |
Photophysical properties
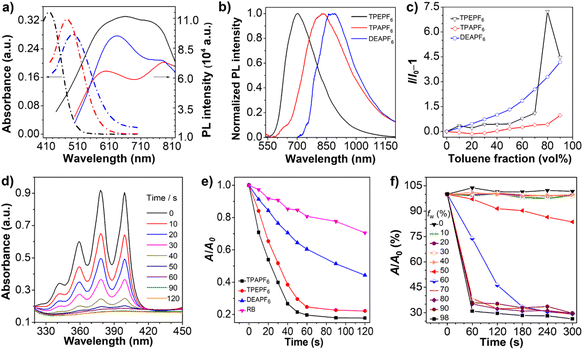
The photophysical properties of TPEPF6, TPAPF6, and DEPPF6 are summarized in Table 1. Their absorption maximum lies at 419, 476, and 495 nm (Fig. 2a), respectively, and the corresponding molar absorption coefficients are determined to be 3.44 × 104, 3.24 × 104, and 2.81 × 104 L mol–1 cm–1. Clearly, the locations of the absorption maxima are compatible with the visible-light excitation source applied in PDT. The emission maximum of TPEPF6, TPAPF6, and DEPPF6 in DMSO is located at 553, 583, and 640 nm, respectively (Fig. 2b). As the solvent polarity increases, their fluorescence intensities gradually decrease with the emission maxima blue- or red-shifted to different degrees (Fig. S28–S30†), reflecting the ICT effect.53,54 Noteworthily, the solvatochromic effect of TPEPF6 and TPAPF6 is much more significant than that of DEAPF6, which agrees well with the theoretical calculation results.Their AIE properties were fully demonstrated with the results depicted in Fig. 2c and S31–S42.† They merely showed weak photoluminescence in DMSO (good solvent) in the range of 500–700 nm. As the fraction of toluene (poor solvent) increased, the emission intensities of all these three fluorogens were boosted significantly, attributed to the aggregation-activated restriction of the intramolecular motions (RIM). The emission peaks of TPEPF6, TPAPF6, and DEAPF6 in the DMSO/toluene (1/9, v/v) mixtures are located at 662, 783, and 768 nm, respectively, suggestive of their NIR-fluorescence properties. Their Stokes shifts are over 200 nm and larger than those of the traditional ACQ fluorophores (usually smaller than 50 nm). The large Stokes shift can effectively reduce self-absorption in fluorescence imaging. The transmission electron microscopy (TEM) and dynamic light scattering (DLS) results (Fig. S43–S46†) verified that these NIR-fluorescent compounds all possess AIE properties. Furthermore, as illustrated in Fig. 2b, the emission peaks of TPEPF6, TPAPF6, and DEAPF6 in the solid state are located at 694, 833, and 866 nm, respectively. And the fluorescence spectra of TPAPF6 and DEAPF6 even extend over 1200 nm, indicating their potential in the second near-infrared (NIR-II) fluorescence imaging. In addition, the average zeta potential of TPEPF6, TPAPF6, and DEAPF6 in DMSO/water (v/v, 1/99) was measured to be 14.9, 27.9, and 38.1 mV, respectively (Fig. S47†). It thus can be speculated that they might be able to target the mitochondria of living cells.
As shown in Fig. S48,† only small changes were observed in the UV-vis spectra of these three AIEgens (10 μM) under white-light irradiation. The absorbance values remain above 70% even after 15 minutes of continuous white-light irradiation (25 mW cm−2), indicative of their satisfactory photostability.
The crystal data and acquisition parameters of TPAPF6 are summarized in Table S4.† As shown in Fig. S49,† TPAPF6 takes on a highly twisted 3D conformation. Abundant intra- and intermolecular short contacts such as C–H⋯π, C–H⋯F, C–H⋯N interactions, etc. significantly stiffen the molecular conformation and restrict the intramolecular motions. Moreover, the distances between the central benzene plane and the pyridinium ring plane of two adjacent molecules are all longer than 3.65 Å, which can prevent the intermolecular π–π stacking, endowing TPAPF6 with outstanding AIE properties.
It is believed that the AIE effect and D–π–A structure tend to promote the generation of ROS.55,56 As depicted in Fig. 2d, e, and S50,† under white-light irradiation, the absorbance of the ROS indicator, i.e., 9,10-anthracenediyl-bis(methylene)dimalonic acid (ABDA), at 378 nm in the presence of any of these AIEgens significantly decreased with the increasing irradiation time. The decomposition efficiencies of ABDA coexisting with these AIEgens were significantly higher than that with the commercial PS, i.e. Rose Bengal (RB). As calculated, 10.0 nmol of TPEPF6 can degrade 37.7 nmol of ABDA per minute, and the same amount of TPAPF6 and DEAPF6 can degrade 40.2 nmol and 19.8 nmol of ABDA per minute, respectively. The decomposition rate constant of ABDA (κABDA) was determined from the curve of ln(A0/A) versus irradiation time (Fig. S51†). The larger the slope, the stronger the ability to generate 1O2. The relative slopes of RB, TPEPF6, TPAPF6, and DEAPF6 are 1.000, 6.597, 8.380, and 2.428, respectively. It suggests that the 1O2 yields of these three AIEgens are all higher than that of RB under parallel conditions. In addition, using RB as a reference (the 1O2 quantum yield is 0.75 in water), the 1O2 quantum yields of TPEPF6, TPAPF6, and DEAPF6 were estimated to be 5.11, 3.17, and 0.88, respectively (Fig. S51†).
The photosensitizing properties evaluation results show good consistency with the results acquired via Gaussian and Multiwfn programs. Obviously, compared with DEAPF6, TPEPF6 and TPAPF6 show higher 1O2 quantum yields, which may be related to their different ICT effects and ISC processes.57 Among all these three AIEgens, the more the rotors, the stronger the 1O2-generating ability is. It might be because the D–π–A-structured AIEgen with more rotors could take on a more twisted conformation which facilitates the electron–hole separation and the differentiation of the electron-cloud distributions at the HOMO and LUMO, thus promoting the ISC. In this manner, the AIE feature not only improves the fluorescence brightness, but also contributes to the generation of ROS in the aggregated state.
Thus, the ability of TPAPF6 to produce 1O2 was studied in mixtures of DMSO/H2O with different water fractions. The results showed that its ability to produce 1O2 is significantly enhanced when the water content exceeds 60% (Fig. 2f and S52†). It is probably because the restriction of intramolecular motions in the aggregated state helps to promote the ISC process, and stabilizes the 1O2. Moreover, the increased amount of oxygen brought by the addition of water might also contribute to the enhanced generation of 1O2.
Mitochondria-specificity and cytotoxicity
Investigation of the intracellular 1O2 generation ability of these three AIE-PSs in 4T1 and SK-OV-3 cells was performed using a SOSG assay.58–60 As shown in Fig. S53–S58,† strong green fluorescence emerged with the prolongation of the light-irradiation time, indicating their efficient 1O2 production in cells. In addition, the bright-field images showed that the cells cultured by TPEPF6 and TPAPF6 almost all became round, with the cytoplasm becoming leaked and the cytoskeleton destroyed. The cells began to fall off from the culture plates after 15 minutes of white-light irradiation. The morphology of the cells treated with DEAPF6 also changed significantly after 30 min of light irradiation as the cells turned rounded and shrunken. All the above results together exhibited that these AIE-PSs can produce 1O2 at high yields under light irradiation, which can effectively kill cells.The mitochondria-targeting abilities of these AIE-PSs were assessed as well. The cells treated with our AIE-PSs were clearly visualized with bright red fluorescence, suggesting they enjoy outstanding cell membrane permeability and cell-imaging capabilities. For both 4T1 and SK-OV-3 cells, the fluorescence from AIE-PSs merges well with the fluorescence signal from MitoTracker® Deep Red FM (Fig. S59†). Their Pearson's correlation coefficients are 0.79, 0.84, and 0.75 in 4T1 cells, and 0.79, 0.81, and 0.85 in SK-OV-3 cells, respectively. It showed that these AIE-PSs all hold the ability to target and image the mitochondria of live cells, which may play an important role in tumour treatment.
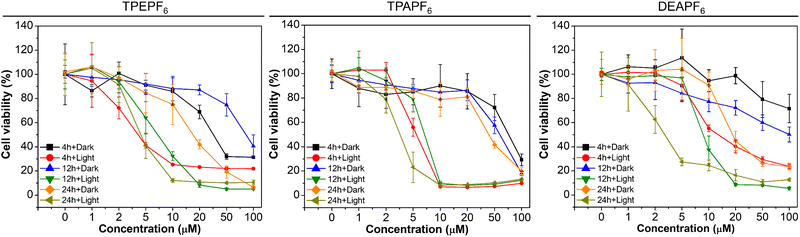
Motivated by the excellent 1O2 generation efficiency, we further investigated the biocompatibility and photodynamic killing activity of these AIE-PSs through a standard Cell-Counting-Kit-8 (CCK-8) assay. As depicted in Fig. 3, these AIE-PSs show low dark cytotoxicity to both cancer cells and normal cells at a concentration of 10 μM, with all the cell viabilities remaining above 80%. Take TPAPF6 for example. Cell viability does not change significantly in the dark even when the concentration of TPAPF6 is increased to 20 μM, suggestive of good biocompatibility. In sharp contrast, when the cells were exposed to white light for 30 min, the cell survival rates decrease sharply with the increasing concentrations of AIE-PSs. For example, TPEPF6, TPAPF6, or DEAPF6 at a concentration of 10 μM could cause severe viability loss after white-light irradiation, with the cell survival rate of 4T1 cells decreasing to only 12%, 8%, and 24%, respectively. The corresponding half inhibitory concentration (IC50) of TPAPF6 to 4T1 cells was as low as 3.98 μM (Table S5†). The IC50 of TPEPF6 to A549 and the one of DEAPF6 to 4T1 was 3.12 μM and 3.79 μM, respectively. All the results suggested that these AIE-PSs hold significant photo-cytotoxicity. Similar cytotoxicity has also been found in all examined cells with different AIE-PSs (Fig. 3, S60, and Table S5†). Pitifully, these AIE-PSs show no obvious difference in the phototoxicity to cancer cells and normal cells. As such, intratumoral injection is utilized for the in vivo experiments.
Tumour visualization and the antitumor effect
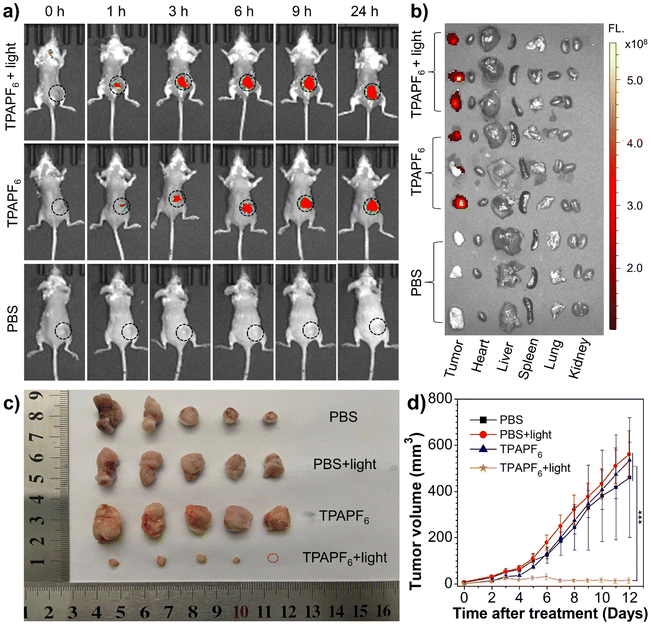
From the viewpoint of excitation/emission wavelength, singlet-oxygen yield, and mitochondria-targeting ability, TPAPF6 holds the best overall performance among all these three AIE-PSs. In other words, although the singlet-oxygen generating ability of TPEPF6 is higher than that of TPAPF6, the absorption and emission maxima of TPAPF6 lie at much longer wavelengths as compared to those of TPEPF6. Moreover, the mitochondria-targeting capability of TPAPF6 is also superior to that of TPEPF6. In view of this, TPAPF6 was selected as a model for the following tumour visualization and antitumor experiments. In vivo treatment experiments were carried out based on the 4T1 breast tumour model. As depicted in Fig. 4a, within 1 h after intratumoral injection of TPAPF6, the red fluorescence signal collected with λem = 700 nm at the tumour sites clearly demonstrated the accumulation of TPAPF6. Notably, fluorescence emitted from the tumour site was still intense at 24 h post-injection. It is suggestive of the high retention of the TPAPF6 nanoaggregates in cells. Similar results were obtained with the fluorescence signals recorded at λem = 760 nm (Fig. S61†), further demonstrating the tumour visualizing ability of TPAPF6 with NIR fluorescence. The mice were sacrificed and the fluorescence signals of major organs and tumours were captured to further study the biodistribution of TPAPF6in vivo (Fig. 4b). Among all the evaluated tissues including tumour, heart, liver, spleen, lung, and kidney, no fluorescence signal was observed except from the tumour.4T1 tumour-bearing mice were applied to evaluate the in vivo therapeutic effect of TPAPF6. As illustrated in Fig. S62, 4c and d, the growth of the tumours on the mice injected with PBS and subjected to light irradiation (i.e., PBS + light group) cannot be hindered. Similar results were also shown by the group injected with TPAPF6 but exempt from light irradiation (TPAPF6 group) and the PBS control group. In sharp contrast, the TPAPF6-treated group showed significantly inhibited tumour growth under white-light irradiation. To further verify the antitumor effect of TPAPF6, 5 mice in each group were sacrificed after 12 days treatment. Then, the tumours were collected, and their volumes and weights were measured (Table S6†). The results shown in Fig. 4c, d, and S63† further proved that TPAPF6 has remarkable antitumor ability via PDT.
Moreover, the body weights of mice in each group were measured to assess the in vivo biocompatibility. It was found that the body weights of mice among different groups changed reasonably within the normal range during the PDT process, reflecting minimal systemic effects (Fig. S64†). The main organs of mice were collected on day 12 after treatment and stained with H&E dyes. Compared with the PBS group, H&E staining images of major organs in each treated group showed no obvious inflammatory lesions or impairment, and no tissue necrosis was found in any of the histological specimens (Fig. S65a†). Besides that, the blood of live mice was collected on day 11 post-treatment and the blood biochemical indexes were analysed. As shown in Fig. S65b and c,† the expression levels of aspartate aminotransferase (AST) and blood urea nitrogen (BUN) showed no distinct discrepancy among the four groups, indicating low side effects and satisfactory biocompatibility of TPAPF6. These results provide preliminary evidence that TPAPF6 would not cause acute toxicity during the treatment period, suggestive of its potential for clinical application.
Conclusions
In summary, a series of D–π–A-structured AIE-active PSs, namely TPEPF6, TPAPF6, and DEAPF6, with the electron-donor varying from TPE to TPA and to DEA were facilely synthesized via simple procedures. Near-infrared emission and high 1O2 production are achieved by increasing the separation degree of electron–hole distribution, enlarging the difference in the distribution of electron clouds at the HOMO and LUMO, and simultaneously reducing the ΔEST. Through the investigation of the structure–property relationship, we found that large π-conjugation, strong D–π–A effect as well as sufficient rotors are essential to achieving NIR-emissive and AIE-active photosensitizers with high 1O2 generation ability. Compared with DEAPF6, TPEPF6 and TPAPF6 show higher 1O2 quantum yields. However, the emission wavelengths of TPAPF6 and DEAPF6 are relatively longer than that of TPEPF6, with the solid-state emission spectra extending over 1200 nm. In other words, the emission and photosensitizing properties can be finely tuned by modulating the electron-donating ability and the number of rotors of the electron-donor. Furthermore, their specific targeting capability to mitochondria has also been proven in living cells. These AIE-PSs all show strong ability to kill cells under white-light irradiation even at a low concentration. More importantly, in vivo experiments demonstrate that TPAPF6 can achieve visualization of tumour sites with the NIR fluorescence in a high-contrast fashion, and in the meantime can effectively eliminate tumours in a PDT manner. Therefore, our work not only provides some clues on the molecular engineering of highly efficient singlet-oxygen-generating PSs based on AIEgens, but also contributes a series of high-performance PSs with great potential to be used in clinical photo-theranostics.Data availability
All the data associated with the research in this manuscript are available on reasonable request and can be acquired from the corresponding authors.Author contributions
J. Mei conceived and designed this work. S. Zhang, W. Yang and Z. Pan conducted the experiments. X. Lu performed the in vivo experiments related to tumour visualization and the therapeutic effect. X. Zhang performed the DFT calculation and electron–hole analysis. J. Mei, D. Mei, S. Zhang, H. Tian and D. H. Qu analysed and interpreted the data. J. Mei, S. Zhang, D. Mei and H. Tian wrote and revised the article. All authors participated in drafting the manuscript and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (21788102, 22275055, 21875064, 81903545, and 21790361), Shanghai Science and Technology Commission Basic Project Shanghai Natural Science Foundation (21ZR1417600), Shanghai Municipal Science and Technology Major Project (2018SHZDZX03), Programme of Introducing Talents of Discipline to Universities (B16017), Shanghai Science and Technology Committee (17520750100), Beijing New-Star Plan of Science and Technology (Z201100006820009), and the Fundamental Research Funds for the Central Universities. The authors thank the Research Center of Analysis and Test of East China University of Science and Technology for help with the characterization. All the mice used were handled strictly in accordance with governmental and international guidelines on animal experimentation. Mice were free to obtain sterile food and water. The animal experiments have been approved by the Laboratory Animal Ethics Committee of East China University of Science and Technology (No. ECUST-2021-03001).Notes and references
- T. C. Pham, V.-N. Nguyen, Y. Choi, S. Lee and J. Yoon, Chem. Rev., 2021, 121, 13454–13619 CrossRef CAS PubMed.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- B. M. Luby, C. D. Walsh and G. Zheng, Angew. Chem., Int. Ed., 2019, 58, 2558–2569 CrossRef CAS PubMed.
- J. H. Correia, J. A. Rodrigues, S. Pimenta, T. Dong and Z. Yang, Pharmaceutics, 2021, 13, 1332 CrossRef CAS PubMed.
- F. Hu, S. Xu and B. Liu, Adv. Mater., 2018, 30, 1801350 CrossRef PubMed.
- M. Lan, S. Zhao, W. Liu, C.-S. Lee, W. Zhang and P. Wang, Adv. Healthcare Mater., 2019, 8, 1900132 CrossRef PubMed.
- R. Vankayala and K. C. Hwang, Adv. Mater., 2018, 30, 1706320 CrossRef PubMed.
- H. Yu, B. Chen, H. Huang, Z. He, J. Sun, G. Wang, X. Gu and B. Z. Tang, Biosensors, 2022, 12, 348 CrossRef CAS PubMed.
- W. Wu, D. Mao, F. Hu, S. Xu, C. Chen, C.-J. Zhang, X. Cheng, Y. Yuan, D. Ding, D. Konng and B. Liu, Adv. Mater., 2017, 29, 1700548 CrossRef PubMed.
- M. Wu, X. Liu, H. Chen, Y. Duan, Y. Liu, J. Liu, Y. Pan and B. Liu, Angew. Chem., Int. Ed., 2021, 60, 9093–9098 CrossRef CAS PubMed.
- J. Dai, Y. Li, Z. Long, R. Jiang, Z. Zhuang, Z. Wang, Z. Zhao, X. Lou, F. Xia and B. Z. Tang, ACS Nano, 2020, 14, 854–866 CrossRef CAS PubMed.
- R. Wang, X. Li and J. Yoon, ACS Appl. Mater. Interfaces, 2021, 13, 19543–19571 CrossRef CAS PubMed.
- P.-C. Lo, M. S. Rodriguez-Morgade, R. K. Pandey, D. K. P. Ng, T. Torres and F. Dumoulin, Chem. Soc. Rev., 2020, 49, 1041–1056 RSC.
- L. Zhang, Y. Li, W. Che, D. Zhu, G. Li, Z. Xie, N. Song, S. Liu, B. Z. Tang, X. Liu, Z. Su and M. R. Bryce, Adv. Sci., 2019, 6, 1802050 CrossRef PubMed.
- Y. Jiang, W. Zhu, Z. Xu, Z. Zhang, S. Tang, M. Fan, Z. Li, J. Zhang, C. Yang, W. C. Law, K. T. Yong, D. Wang, G. Xu and B. Z. Tang, Chem. Eng. J., 2022, 448, 137604 CrossRef CAS.
- M. Chiba, Y. Ichikawa, M. Kamiya, T. Komatsu, T. Ueno, K. Hanaoka, T. Nagano, N. Lange and Y. Urano, Angew. Chem., Int. Ed., 2017, 56, 10418–10422 CrossRef CAS PubMed.
- A. Turksoy, D. Yildiz and E. U. Akkaya, Coord. Chem. Rev., 2019, 379, 47–64 CrossRef CAS.
- J. Mei, Y. Hong, J. W. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- J. Mei, N. L. Leung, R. T. Kwok, J. W. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- X. Cai and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 9868–9886 CrossRef CAS PubMed.
- J. Qian and B. Z. Tang, Chem, 2017, 3, 56–91 CAS.
- H. Gao, X. Zhang, C. Chen, K. Li and D. Ding, Adv. Biosyst., 2018, 2, 1800074 CrossRef.
- H. Ma, C. Zhao, H. Meng, R. Li, L. Mao, D. Hu, M. Tian, J. Yuan and Y. Wei, ACS Appl. Mater. Interfaces, 2021, 13, 7987–7996 CrossRef CAS PubMed.
- W. Xu, M. M. S. Lee, J.-J. Nie, Z. Zhang, R. T. K. Kwok, J. W. Y. Lam, F.-J. Xu, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9610–9616 CrossRef CAS PubMed.
- G. Jiang, C. Li, X. Liu, Q. Chen, X. Li, X. Gu, P. Zhang, Q. Lai and J. Wang, Adv. Opt. Mater., 2020, 8, 2001119 CrossRef CAS.
- H. Chen, S. Li, M. Wu, Kenry, Z. Huang, C. S. Lee and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 632–636 CrossRef CAS PubMed.
- F. Hu, D. Mao, Kenry, X. Cai, W. Wu, D. Kong and B. Liu, Angew. Chem., Int. Ed., 2018, 57, 10182–10186 CrossRef CAS PubMed.
- H. Shi, X. Pan, Y. Wang, H. Wang, W. Liu, L. Wang and Z. Chen, ACS Appl. Mater. Interfaces, 2022, 14, 17055–17064 CrossRef CAS PubMed.
- H. Wen, Z. Zhang, M. Kang, H. Li, W. Xu, H. Guo, Y. Li, Y. Tan, Z. Wen, Q. Wu, J. Huang, L. Xi, K. Li, L. Wang, D. Wang and B. Z. Tang, Biomaterials, 2021, 274, 120892 CrossRef CAS PubMed.
- W. Wu, D. Mao, S. Xu, M. Panahandeh-Fard, Y. Duan, F. Hu, D. Kong and B. Liu, Adv. Funct. Mater., 2019, 29, 1901791 CrossRef CAS.
- Y. Ma, Z. Zhuang, L. Xing, J. Li, Z. Yang, S. Ji, R. Hu, Z. Zhao, Y. Huo and B. Z. Tang, Adv. Funct. Mater., 2021, 31, 2106988 CrossRef CAS.
- Z. Liu, H. Zou, Z. Zhao, P. Zhang, G. G. Shan, R. T. K. Kwok, J. W. Y. Lam, L. Zheng and B. Z. Tang, ACS Nano, 2019, 13, 11283–11293 CrossRef CAS PubMed.
- G. Yuan, C. Lv, J. Liang, X. Zhong, Y. Li, J. He, A. Zhao, L. Li, Y. Shao, X. Zhang, S. Wang, Y. Cheng and H. He, Adv. Funct. Mater., 2021, 31, 2104026 CrossRef CAS.
- Y. Wang, S. Xu, L. Shi, C. Teh, G. Qi and B. Liu, Angew. Chem., Int. Ed., 2021, 60, 14945–14953 CrossRef CAS PubMed.
- W. Liu, Z. Li, Y. Qiu, J. Li, J. Yang and J. Li, ACS Appl. Bio Mater., 2021, 4, 5566–5574 CrossRef CAS PubMed.
- L. Zhang, J. L. Wang, X. X. Ba, S. Y. Hua, P. Jiang, F. L. Jiang and Y. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 7945–7954 CrossRef CAS PubMed.
- S. Liu, G. Feng, B. Z. Tang and B. Liu, Chem. Sci., 2021, 12, 6488–6506 RSC.
- S. Samanta, Y. He, A. Sharma, J. Kim, W. H. Pan, Z. G. Yang, J. Li, W. Yan, L. W. Liu, J. L. Qu and J. S. Kim, Chem, 2019, 5, 1697–1726 CAS.
- C. Zhou, C. Peng, C. Shi, M. Jiang, J. H. C. Chau, Z. Liu, H. Bai, R. T. K. Kwok, J. W. Y. Lam, Y. Shi and B. Z. Tang, ACS Nano, 2021, 15, 12129–12139 CrossRef CAS PubMed.
- C. Wang, X. Zhao, H. Jiang, J. Wang, W. Zhong, K. Xue and C. Zhu, Nanoscale, 2021, 13, 1195–1205 RSC.
- W. Zhuang, L. Yang, B. Ma, Q. Kong, G. Li, Y. Wang and B. Z. Tang, ACS Appl. Mater. Interfaces, 2019, 11, 20715–20724 CrossRef CAS PubMed.
- Y. F. Xiao, W. C. Chen, J. X. Chen, G. Lu, S. Tian, X. Cui, Z. Zhang, H. Chen, Y. Wan, S. Li and C. S. Lee, ACS Appl. Mater. Interfaces, 2022, 14, 5112–5121 CrossRef CAS PubMed.
- Z. Liu, Q. Wang, W. Qiu, Y. Lyu, Z. Zhu, X. Zhao and W.-H. Zhu, Chem. Sci., 2022, 13, 3599–3608 RSC.
- D. Wei, Y. Chen, Y. Huang, P. Li, Y. Zhao, X. Zhang, J. Wan, X. Yin, T. Liu, J. Yin, Z. Liu, Q. Zhang, J. Wang and H. Xiao, Nano Today, 2021, 41, 101288 CrossRef CAS.
- Z. Meng, H. Xue, T. Wang, B. Chen, X. Dong, L. Yang, J. Dai, X. Lou and F. Xia, J. Nanobiotechnol., 2022, 20, 344 CrossRef CAS PubMed.
- L. Liu, X. Wang, L. J. Wang, L. Guo, Y. Li, B. Bai, F. Fu, H. Lu and X. Zhao, ACS Appl. Mater. Interfaces, 2021, 13, 19668–19678 CrossRef CAS PubMed.
- L. Lin, Z. Wang, J. Fan and C. Wang, Org. Electron., 2017, 41, 17–25 CrossRef CAS.
- R. L. X. Gao, M. Barbatti, J. Jiang and G. Zhang, J. Phys. Chem. Lett., 2019, 10, 1388–1393 CrossRef PubMed.
- X. Tang, L. S. Cui, H. C. Li, A. J. Gillett, F. Auras, Y. K. Qu, C. Zhong, S. T. E. Jones, Z. Q. Jiang, R. H. Friend and L. S. Liao, Nat. Mater., 2020, 19, 1332–1338 CrossRef CAS PubMed.
- J. Li, T. Nakagawa, J. MacDonald, Q. Zhang, H. Nomura, H. Miyazaki and C. Adachi, Adv. Mater., 2013, 25, 3319 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta Jr., F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- Z. Liu, T. Lu and Q. Chen, Carbon, 2020, 165, 461–467 CrossRef CAS.
- P. Tan, W. Zhuang, S. Li, J. Zhang, H. Xu, L. Yang, Y. Liao, M. Chen and Q. Wei, Chem. Commun., 2021, 57, 1046–1049 RSC.
- G. Lin, P. N. Manghnani, D. Mao, C. Teh, Y. Li, Z. Zhao, B. Liu and B. Z. Tang, Adv. Funct. Mater., 2017, 27, 1701418 CrossRef.
- H. Zhang, W. Jiang, Y. Peng, J. Yang, X. Chu, Z. Long, R. Li, Q. Liang, H. Suo, S. Wang, M. Yang, J. Qi, D. Ding, Y. W. Yang and B. Wang, Biomaterials, 2022, 286, 121577 CrossRef CAS PubMed.
- W. Wu, L. Shi, Y. Duan, S. Xu, X. Gao, X. Zhu and B. Liu, Chem. Mater., 2021, 33, 5974–5980 CrossRef CAS.
- W. Wu, D. Mao, S. Xu, Kenry, F. Hu, X. Li, D. Kong and B. Liu, Chem, 2018, 4, 1937–1951 CAS.
- W. Sun, L. Luo, Y. Feng, Y. Cai, Y. Zhuang, R. J. Xie, X. Chen and H. Chen, Angew. Chem., Int. Ed., 2020, 59, 9914–9921 CrossRef CAS PubMed.
- D. Zhu, J. Zhang, G. Luo, Y. Duo and B. Z. Tang, Adv. Sci., 2021, 8, 2004769 CrossRef CAS PubMed.
- K. Zhang, Z. Yu, X. Meng, W. Zhao, Z. Shi, Z. Yang, H. Dong and X. Zhang, Adv. Sci., 2019, 6, 1900530 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2165738. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc00588g |
| This journal is © The Royal Society of Chemistry 2023 |