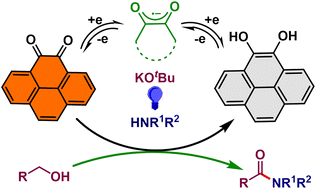
Herein, we describe a cofactor-inspired oxidation methodology where an aromatic dione molecule is used under aerobic conditions. Under photocatalytic conditions, pyrenedione produces amides from alcohols and amines via dehydrogenative intermolecular coupling at room temperature. The synthetic utility of this developed protocol has been examined over a large array of substrates involving a variety of alcohols and amines to synthesize both secondary and tertiary amides in good to excellent yields. This is a significant improvement over multiple transition metal-catalyzed methods following acceptorless dehydrogenation or dehydrogenative coupling methods, performed at very high temperatures. The presented reaction is completely driven by a radical generated by the photoexcitation of pyrenedione and its subsequent reduction by KOtBu. An array of control reactions in tandem with intermediate isolation suggests the operational mechanism and establishes the semiquinonate's preeminent role in steering two consecutive dehydrogenations under very mild reaction conditions.