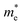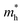Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Crystal structure engineering of metal halide perovskites for photocatalytic organic synthesis†
Chunhua
Wang
a,
Yang
Ding
b,
Biao
Liu
 c,
Bo
Weng
c,
Bo
Weng
 *d,
Johan
Hofkens
*d,
Johan
Hofkens
 *ae and
Maarten B. J.
Roeffaers
*ae and
Maarten B. J.
Roeffaers
 *d
*d
aDepartment of Chemistry, KU Leuven, Celestijnenlaan 200F, Leuven 3001, Belgium. E-mail: johan.hofkens@kuleuven.be
bLaboratory of Inorganic Materials Chemistry (CMI), University of Namur, 61 rue de Bruxelles, B-5000, Namur, Belgium
cHunan Key Laboratory for Super-microstructure and Ultrafast Process, School of Physics and Electronics, Central South University, Changsha 410083, P. R. China
dcMACS, Department of Microbial and Molecular Systems, KU Leuven, Celestijnenlaan 200F, Leuven 3001, Belgium. E-mail: maarten.roeffaers@kuleuven.be; bo.weng@kuleuven.be
eMax Planck Institute for Polymer Research, Ackermannweg 10, Mainz 55128, Germany
First published on 15th February 2023
Abstract
Engineering crystal structure of Cs3BiBr6 and Cs3Bi2Br9 is theoretically and experimentally demonstrated to modulate their photocatalytic performance. This work offers insights into the structure-photoactivity relationships of metal halide perovskites (MHPs) and provides a guideline for exploiting MHPs toward efficient photocatalytic organic synthesis.
Semiconductor-based photocatalysis has attracted growing attention in solar-to-chemical energy conversion.1 The basic principle of photocatalysis starts at the electronic band structure of the semiconductors, which dominates the optoelectronic properties and drives the charge carrier that underpin their photocatalytic properties.2 Consequently, the semiconductor crystal structure, which determines the electronic band structures, as well as the photogenerated charge carrier dynamics, is a key factor affecting their photocatalytic performance.3 For example, anatase TiO2 has often been reported to exhibit better photoactivity than the rutile crystal phase, originating from the difference in photogenerated charge generation and transfer.4,5 Similarly, tetragonal BiVO4 was reported to be less active as a photocatalyst than monoclinic scheelite BiVO4.6 In short, the photocatalytic performance of semiconductors can be regulated via crystal structure engineering.
Inspired by the great advances in solar cells,7,8 metal halide perovskites (MHPs) have recently emerged as promising photocatalysts due to their excellent optoelectronic properties.9–11 Among them, lead-free Bi-based compounds such as Cs3Bi2Br9 show excellent stability.12 However, most MHP materials generally exhibit modest photocatalytic performance due to ineffective charge separation and sluggish surface reaction. To improve photoactivity, cocatalyst loading9,13 and heterojunction construction10,11 are effective. On the other hand, the crystalline structure of MHPs has been shown to play a key role in modulating photoactivity.13 For instance, the incorporation of heteroatoms Ag13 and Sb14 into Cs3Bi2Br9 leads to form Cs2AgBiBr6 and Cs3SbBiBr9, which could improve the photoactivity as compared with Cs3Bi2Br9. However, Cs2AgBiBr6 and Cs3SbBiBr9 are intrinsic different from Cs3Bi2Br9, making it difficult to directly uncover the relationship between crystal structures and photocatalytic performance of MHPs. Therefore, it is anticipated to explore the exact effect of crystal structure on photoactivity based on the MHPs with same elements while different crystalline structures.
Herein, the effect of crystal structure in Cs3BiBr6 and Cs3Bi2Br9 on the photocatalytic performance is investigated. Density functional theory (DFT) calculations reveal that the photogenerated charge carriers in Cs3Bi2Br9 have a reduced effective mass, lower exciton binding energy, and longer carrier lifetime than that in Cs3BiBr6. Photoelectrochemical characterizations further confirm that Cs3Bi2Br9 exhibits improved charge transfer efficiency as compared with Cs3BiBr6. When applied to photocatalytic benzyl alcohol (BA) oxidation, Cs3Bi2Br9 displays enhanced photocatalytic performance compared to Cs3BiBr6. Further decoration of these MHPs with Pd nanocubes as cocatalysts, the BA conversion over 1 wt% Pd/Cs3Bi2Br9 (82.3%) is over twice than that of 1 wt% Pd/Cs3BiBr6 (39.5%). This work offers mechanistic insights into the structure-photoactivity of MHPs and provides a guideline for rational design of efficient MHP photocatalysts.
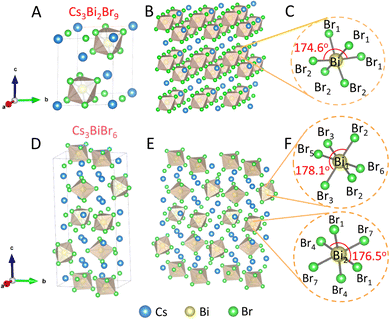
DFT calculations were first performed to understand the crystal structures of Cs3Bi2Br9 and Cs3BiBr6. For Cs3Bi2Br9, within the unit cell (Fig. 1A, Table S1, ESI†), eight Cs+ ions occupied the corners and two other Cs+ ions located on the body diagonal of the unit cell,13 and Bi atoms occupy partial Br6 holes in an ordered arrangement (Fig. 1B), where three short and three long Bi–Br bonds with shorter linkages are shared between neighboring octahedra in each layer (Fig. 1C, Table S2, ESI†).13 The local coordination environment of the [BiBr6]3− octahedra is irregular and leads to a trigonal distortion, forming a two-dimensional perovskite structure. Cs3BiBr6, by contrast, contains two Bi atoms, three Cs atoms, and seven Br atoms in an asymmetric unit (Fig. 1D). These two Bi atoms are independent (Fig. 1F), where each Bi atom is coordinated by six Br atoms and the bond lengths of Bi–Br are different (Table S2, ESI†), and the bond angle between Br–Bi–Br in Cs3BiBr6 is distorted.15 The [BiBr6]3− octahedra exist in isolation and no link between these octahedra, forming a zero-dimensional perovskite structure (Fig. 1E).15,16 Then, the band structures and density of states (DOS) of both materials were studied. From the energy band structures (Fig. S1, ESI†), the conduction band minimum (CBM) and valence band maximum (VBM) of Cs3Bi2Br9 are more dispersive than those of Cs3BiBr6, hinting that the Cs3Bi2Br9 has lower effective masses of electrons and holes (vide infra).13 For Cs3Bi2Br9, the VBM primarily consists of Br 4p states and Bi 6p orbitals, mixed with a few Bi 6s states (Fig. S1B, ESI†), and the CBM is dominated by Bi 6p and Br 4p orbital interactions.17 For Cs3BiBr6, the VBM is mainly composed of Br 4p orbitals, and the CBM consists of an admixture of Bi 6p and Br 4p orbitals (Fig. S1D, ESI†).
The photoelectric properties of Cs3Bi2Br9 and Cs3BiBr6 can be assessed by physical factors such as exciton binding energy, effective mass, charge mobility, and charge carrier relaxation time.13 These parameters were calculated and listed in Table 1. The exciton binding energy (Eb) of Cs3Bi2Br9 is calculated to be 0.278 eV, much smaller than that of Cs3BiBr6 (0.505 eV), which is beneficial for the separation of photogenerated excitons and extraction of charge carriers.13 Besides, Cs3Bi2Br9 shows a significant drop in effective mass of charge carriers as compared to Cs3BiBr6; calculated electron effective mass 0.57 me (Cs3Bi2Br9) vs. 1.42 me (Cs3BiBr6) and the effective hole mass reduces from 6.32 mh to 5.46 mh. Generally, the smaller the effective mass of charge carriers, the faster the transfer rate of charges.10 Thus, the lower effective mass in Cs3Bi2Br9 links to higher mobility which is beneficial for charge carriers migration.13 This is confirmed by the calculated carrier mobility, where the value of Cs3Bi2Br9 is higher than that of Cs3BiBr6. Also, their different deformation potential constants (Fig. S2, ESI†) agree with the results of effective mass and carrier mobility. Furthermore, Cs3Bi2Br9 possesses a longer carrier relaxation time than Cs3BiBr6, which responds to the prolonged carrier lifetime in Cs3Bi2Br9.
Next, Cs3Bi2Br9 and Cs3BiBr6 were synthesized (see ESI†) for further analysis.18 XRD results in Fig. 2A show that both Cs3Bi2Br9 and Cs3BiBr6 exhibit strong diffraction peaks, without shift compared to theoretical data. Cs3Bi2Br9 has a trigonal crystalline structure,19 while Cs3BiBr6 crystallizes in an orthorhombic phase.15 Raman spectroscopy (Fig. 2B) reveals the modes at 167 and 192 cm−1 in Cs3Bi2Br9 belonging to the stretching vibrations of Bi–Br bonds in the [BiBr6]3− octahedra.20 For Cs3BiBr6, although it also contains [BiBr6]3− octahedra, the complex groups are isolated and do not share corners, yielding peaks at different frequencies, i.e. 160 and 135 cm−1. By contrast, Cs3BiBr6 showed a red-shift (33 cm−1) for the terminal Bi–Br stretching modes of the [BiBr6]3− octahedra compared to Cs3Bi2Br9, which suggests weaker Bi–Br bonds in Cs3BiBr6,16 in line with the slightly longer Bi–Br bond lengths (Table S2, ESI†).
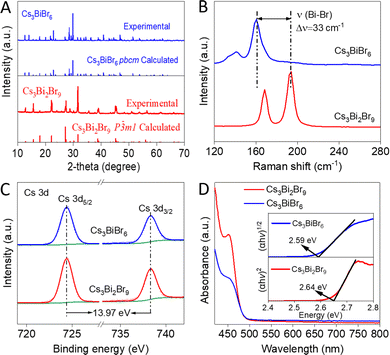 | ||
| Fig. 2 (A) XRD patterns, (B) Raman spectra, (C) high-resolution XPS spectra of Cs 3d core levels, and (D) DRS spectra of Cs3Bi2Br9 and Cs3BiBr6 materials. The inset in Fig. 2D shows the bandgaps of both materials. | ||
XPS was performed to investigate the chemical structure and valence state of both materials. The signals of Cs 3d (Fig. 2C), Bi 4f and Br 3d (Fig. S3A and B, ESI†) are the same, confirming their expected valence states (+1, +3, and −1, respectively) to be consistent with the formula.15,16 The stoichiometry in Cs3Bi2Br9 was calculated to be Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi
Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br = 3
Br = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.21
2.21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.20, and for Cs3BiBr6 the ratio is Cs
9.20, and for Cs3BiBr6 the ratio is Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi
Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br = 3
Br = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.22
1.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.37 (Table S3, ESI†), close to their theoretical values. SEM images (Fig. S4, ESI†) show that both materials have a similar particle size of about 500 nm. EDX spectroscopy further confirms the presence of Cs, Bi and Br elements (Fig. S4E and F, ESI†). Nitrogen physisorption data (Fig. S5, ESI†) shows that the surface area of both materials is similar (Table S4, ESI†).
6.37 (Table S3, ESI†), close to their theoretical values. SEM images (Fig. S4, ESI†) show that both materials have a similar particle size of about 500 nm. EDX spectroscopy further confirms the presence of Cs, Bi and Br elements (Fig. S4E and F, ESI†). Nitrogen physisorption data (Fig. S5, ESI†) shows that the surface area of both materials is similar (Table S4, ESI†).
UV-Vis DRS was employed to determine their absorption properties (Fig. 2D). Note that although Cs3Bi2Br9 and Cs3BiBr6 have different elemental compositions and structures, they have in common [BiBr6]3− octahedra, and their optical properties are largely determined by the Bi3+ cation levels, perturbed slightly by the environment surrounding [BiBr6]3-octahedra.20,21 The absorption onset of Cs3Bi2Br9 is measured at 470 nm (2.64 eV),18 and for Cs3BiBr6 it is 479 nm (2.59 eV),15 which is consistent with their colors (Fig. S6, ESI†). The extracted bandgap of Cs3Bi2Br9 agrees with DFT calculation as well as reported results,18 while Cs3BiBr6 shows a difference (0.6 eV) with the calculated one which could be attributed to the DFT limitations.15 Mott–Schottky analyses (Fig. S7, ESI†), combined with UV-Vis data, show that CB and VB edge potentials of Cs3Bi2Br9 and Cs3BiBr6 are −0.57 and 2.07 eV, and −0.69 and 1.9 eV (vs. NHE), respectively. Steady-state PL spectrum (Fig. S8, ESI†) shows that Cs3Bi2Br9 has an emission centered at around 469 nm, which almost coincides with its bandgap (2.64 eV), corresponding to near band edge emission.13 The emission peak of Cs3BiBr6 shows a slight blue shift, which could be attributed to its indirect bandgap property.22
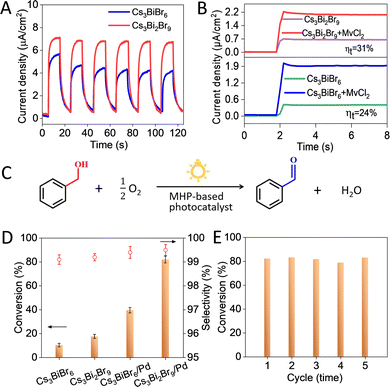
Photoelectrochemical measurements were further performed to reveal the picture of charge separation and transfer.23Fig. 3A shows the transient photocurrent responses of both materials, where a higher photocurrent is observed for Cs3Bi2Br9 than that of Cs3BiBr6, suggesting the efficient charge separation in Cs3Bi2Br9.24 To better understand the difference, EIS experiments were performed.23 Clearly, Cs3Bi2Br9 presents a smaller semicircle in the Nyquist plots (Fig. S9, ESI†), confirming the efficient transfer of charge carriers in Cs3Bi2Br9.24–26 This is further corroborated by the surface charge transfer efficiency (ηt) measurements25,27 (Fig. 3B), the ηt of Cs3Bi2Br9 and Cs3Bi2Br9 is calculated to be 24% and 31%, respectively. To conclude, the above observations disclose that Cs3Bi2Br9 would empower faster charge carriers injection into the redox couple during the photocatalytic reaction.10,24
The selective photocatalytic oxidation of benzyl alcohol (BA) was used as model reaction to compare the performance of Cs3Bi2Br9 and Cs3BiBr6 (Fig. 3C, see Scheme S1, ESI†); the band edge energies of both materials are suitable for the involved redox potentials (+ 0.68 eV).28 Cs3Bi2Br9 shows a higher BA conversion (17.5%) than Cs3BiBr6 (10.2%) after solar light irradiation of 6 h (Fig. 3D), with an excellent selectivity (over 99%) towards benzaldehyde (BAD). To further enhance photoactivity, Pd nanocubes were deposited on MHPs surfaces. The 1 wt% Pd/Cs3Bi2Br9 composite converts 82.3% BA, over 2-fold enhancement than 1 wt% Pd/Cs3BiBr6 (39.5%). This is attributed to the fact that Pd serves as an electron reservoir to promote the transfer of photogenerated charge carriers, thus improving the conversion of BA.27 Besides, a comparison of Cs3Bi2Br9/Pd with reported photocatalysts for selective BA oxidation shows the excellent performance of this material (Table S5, ESI†). Moreover, recycling tests show that no activity loss for 1 wt% Pd/Cs3Bi2Br9 is observed after 5 successive cycles (Fig. 3E). Additionally, the catalyst is stable after exposing in air for 15 days (Fig. S10, ESI†), and no crystal structure changes are observed in XRD patterns (Fig. S11, ESI†).
To elucidate the reation mechanism over Pd/Cs3Bi2Br9 sample, control experiments using different radical scavengers were performed (Fig. S12, ESI†). When hole scavenger ammonium oxalate was added, the conversion rate shows a sharp drop, indicating the key role of photogenerated holes in driving this reaction.10 Contrarily, the performance remains with electron (potassium persulfate) or ˙O2− (1,4-benzoquinone) scavengers.10,29 This follows that O2 was trapped by electrons to produce ˙O2− radicals, while the generated ˙O2− is not directly involved in the BA oxidation process.23 Moreover, when the O2 is replaced by argon (Ar), an obvious reduction in the activity was observed, showing that O2 reacted with electrons which can effectively promote the separation of charge carriers so that this reaction can proceed smoothly. In addition, the presence of hydroxyl radicals (˙OH−) scavenger, t-butanol, has a negligible effect on the performance since no ˙OH− existed in this reaction system.23 Based on these experiments, we propose a plausible mechanism (Fig. S13, Scheme S1, ESI†). Under light illumination, Cs3Bi2Br9 absorbs solar light to produce electron-hole pairs. The photogenerated electrons migrate to the surface of Pd and reduce O2 to ˙O2−, and the generated holes activate BA and generate the BAD product.23
In summary, the effect of the crystal structure of Cs3BiBr6 and Cs3Bi2Br9 on optoelectronic properties and ultimately photocatalytic performance, was investigated theoretically and experimentally. DFT results showed that Cs3Bi2Br9 has a smaller carrier effective mass, lower exciton binding energy, and higher charge mobility than Cs3BiBr6. Furthermore, the more efficiency in charge separation within the Cs3Bi2Br9 than Cs3BiBr6 was experimentally confirmed. As a result, Cs3Bi2Br9 material exhibited a boosted photocatalytic BA oxidation performance, over twice higher than Cs3BiBr6. This work systematically illustrates the crystal structure-photoactivity relationship of MHPs and offers a guideline for exploiting excellent MHP photocatalysts.
This work was financially supported by the Research Foundation - Flanders (FWO grants G098319N, 1280021N), the KU Leuven Research Fund (C14/19/079, iBOF-21-085 PERSIST), KU Leuven Industrial Research Fund (C3/19/046), the Flemish government through long term structural funding Methusalem (CASAS2, Meth/15/04), and MPI financial support to J.H. as an MPI fellow. C.W. acknowledges the financial support from the China Scholarship Council (201806370198). Open Access funding provided by the Max Planck Society.
Conflicts of interest
There are no conflicts to declare.References
- Y. Ding, S. Maitra, S. Halder, C. Wang, R. Zheng, T. Barakat, S. Roy, L.-H. Chen and B.-L. Su, Matter, 2022, 5, 2119–2167 CrossRef CAS.
- Y. He, Y. Liu, C. Li, X.-B. Chen, Z. Shi and S. Feng, ACS Appl. Energy Mater., 2022, 5, 8923–8929 CrossRef CAS.
- Y. Ding, S. Maitra, C. Wang, S. Halder, R. Zheng, T. Barakat, S. Roy, L. H. Chen and B. L. Su, Interdiscip. Mater., 2022, 1, 213–255 CrossRef.
- T. Luttrell, S. Halpegamage, J. Tao, A. Kramer, E. Sutter and M. Batzill, Sci. Rep., 2014, 4, 1–8 CrossRef PubMed.
- J. Zhang, P. Zhou, J. Liu and J. Yu, Phys. Chem. Chem. Phys., 2014, 16, 20382–20386 RSC.
- G. L. Chiarello, A. D. Paola, L. Palmisano and E. Selli, Photochem. Photobiol. Sci., 2011, 10, 355–360 CrossRef CAS PubMed.
- C. Wang, C. Zhang, S. Wang, G. Liu, H. Xia, S. Tong, J. He, D. Niu, C. Zhou, K. Ding, Y. Gao and J. Yang, Sol. RRL, 2018, 2, 1700209 CrossRef.
- M. A. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef CAS.
- M. Roy, S. Ghorui, Bhawna, J. Kangsabanik, R. Yadav, A. Alam and M. Aslam, J. Phys. Chem. C, 2020, 124, 19484–19491 CrossRef CAS.
- C. Wang, H. Huang, B. Weng, D. Verhaeghe, M. Keshavarz, H. Jin, B. Liu, H. Xie, Y. Ding and Y. Gao, Appl. Catal., B, 2022, 301, 120760 CrossRef CAS.
- H. Huang, D. Verhaeghe, B. Weng, B. Ghosh, H. Zhang, J. Hofkens, J. A. Steele and M. B. Roeffaers, Angew. Chem., 2022, e202203261 CAS.
- W. Xiang, S. Liu and W. Tress, Energy Environ. Sci., 2021, 14, 2090–2113 RSC.
- M. Shi, G. Li, W. Tian, S. Jin, X. Tao, Y. Jiang, E. A. Pidko, R. Li and C. Li, Adv. Mater., 2020, 32, e2002137 CrossRef PubMed.
- M. Shi, H. Zhou, W. Tian, B. Yang, S. Yang, K. Han, R. Li and C. Li, Cell Rep. Phys. Sci., 2021, 2, 100656 CrossRef CAS.
- Y. Tang, M. Liang, B. Chang, H. Sun, K. Zheng, T. Pullerits and Q. Chi, J. Mater. Chem. C, 2019, 7, 3369–3374 RSC.
- H. Yang, T. Cai, E. Liu, K. Hills-Kimball, J. Gao and O. Chen, Nano Res., 2020, 13, 282–291 CrossRef CAS.
- T. Geng, S. Wei, W. Zhao, Z. Ma, R. Fu, G. Xiao and B. Zou, Inorg. Chem. Front., 2021, 8, 1410–1415 RSC.
- C. Wang, B. Weng, Y. Liao, B. Liu, M. Keshavarz, Y. Ding, H. Huang, D. Verhaeghe, J. A. Steele and W. Feng, Chem. Commun., 2022, 58, 10691–10694 RSC.
- B. Yang, J. Chen, F. Hong, X. Mao, K. Zheng, S. Yang, Y. Li, T. Pullerits, W. Deng and K. Han, Angew. Chem., Int. Ed., 2017, 56, 12471–12475 CrossRef CAS PubMed.
- M. N. Tran, I. J. Cleveland and E. S. Aydil, J. Mater. Chem. C, 2020, 8, 10456–10463 RSC.
- S. E. Creutz, H. Liu, M. E. Kaiser, X. Li and D. R. Gamelin, Chem. Mater., 2019, 31, 4685–4697 CrossRef CAS.
- J. Eichhorn, S. P. Lechner, C.-M. Jiang, G. F. Heunecke, F. Munnik and I. D. Sharp, J. Mater. Chem. A, 2021, 9, 20653–20663 RSC.
- H. Huang, C. Zhou, X. Jiao, H. Yuan, J. Zhao, C. He, J. Hofkens, M. B. Roeffaers, J. Long and J. A. Steele, ACS Catal., 2019, 10, 1439–1443 CrossRef.
- B. Weng, Q. Quan and Y.-J. Xu, J. Mater. Chem. A, 2016, 4, 18366–18377 RSC.
- Y. Ding, S. Maitra, C. Wang, R. Zheng, M. Zhang, T. Barakat, S. Roy, J. Liu, Y. Li and T. Hasan, J. Energy Chem., 2022, 70, 236–247 CrossRef CAS.
- T. Chen, B. Weng, S. Lu, H. Zhu, Z. Chen, L. Shen, M. B. Roeffaers and M.-Q. Yang, J. Phys. Chem. Lett., 2022, 13, 6559–6565 CrossRef CAS PubMed.
- C. Wang, B. Weng, M. Keshavarz, M.-Q. Yang, H. Huang, Y. Ding, F. Lai, I. Aslam, H. Jin and G. Romolini, ACS Appl. Mater. Interfaces, 2022, 14, 17185–17194 CrossRef CAS PubMed.
- Y. Wu, X. Ye, S. Zhang, S. Meng, X. Fu, X. Wang, X. Zhang and S. Chen, J. Catal., 2018, 359, 151–160 CrossRef CAS.
- H. Huang, H. Yuan, J. Zhao, G. Solís-Fernández, C. Zhou, J. W. Seo, J. Hendrix, E. Debroye, J. A. Steele, J. Hofkens, J. Long and M. B. J. Roeffaers, ACS Energy Lett., 2018, 4, 203–208 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc00468f |
| This journal is © The Royal Society of Chemistry 2023 |