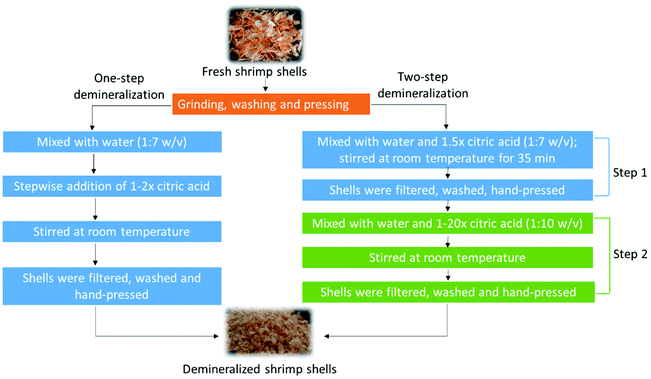
Two-step demineralization of shrimp (Pandalus Borealis) shells using citric acid: an environmentally friendly, safe and cost-effective alternative to the traditional approach
Julia
Pohling
,
Deepika
Dave
 *,
Yi
Liu
*,
Yi
Liu
 ,
Wade
Murphy
and
Sheila
Trenholm
,
Wade
Murphy
and
Sheila
Trenholm
Center for Aquaculture and Seafood Development, Fisheries and Marine Institute of Memorial University of Newfoundland, 155 Ridge Road, St. John's NL, A1C 5R3, Canada. E-mail: Deepika.Dave@mi.mun.ca
First published on 23rd December 2021
Abstract
Removal of minerals from crustacean shells during chitin extraction is traditionally achieved using hydrochloric acid. However, the environmental, health and safety concerns of hydrochloric acid have led to investigation of potential alternatives for this application. In most previously reported studies using other acids, the residual ash content in the demineralized shells could not be reduced to below 1%, which is required for high-grade applications of chitin. In the present study, near-complete demineralization of Pandalus Borealis shells was achieved using citric acid through one-step and two-step processes within 2 hours at room temperature. Fresh shrimp shells were pretreated by thorough grinding and washing. In the one-step demineralization, a residual ash content of 0.59% was obtained by treating the shells with twice the stoichiometric amount of citric acid for 2 h. The residual ash content was further reduced to 0.19% with implementation of a two-step process, in which the majority of minerals in the shells were first dissolved within 35 min using 1.5 times the stoichiometric amount of citric acid, followed by the second demineralization using 8 times the stoichiometric amount of citric acid for 60 min. The two-step process consumed a lower amount of citric acid in comparison to the one-step process (approx. 13.8% less 50% citric acid for every 1000 kg of fresh shrimp shells processed). The low residual mineral content achieved was comparable to the conventional process using hydrochloric acid, indicating the potential of using citric acid for demineralization of shrimp shells to produce premium-quality chitin.
Introduction
Northern shrimp (Pandalus borealis) is an important species in the fishery industry in Newfoundland and Labrador. In 2019, the total landing volume of shrimp was 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 874 t (35.3% of the total shellfish landing) with a landing value of $196 million.1 Approximately one quarter of the harvested shrimp is exported as cooked and peeled. During shrimp processing, a large quantity of shells is produced as by-products, which are an abundant source of chitin. Chitin is a natural polysaccharide that largely exists in fungi and exoskeletons of arthropods (crustaceans and insects). Chitin and its deacetylated derivative, chitosan, have been reported with an impressive range of applications in medical, environmental, cosmetic, food and nutrition industries.2–7
874 t (35.3% of the total shellfish landing) with a landing value of $196 million.1 Approximately one quarter of the harvested shrimp is exported as cooked and peeled. During shrimp processing, a large quantity of shells is produced as by-products, which are an abundant source of chitin. Chitin is a natural polysaccharide that largely exists in fungi and exoskeletons of arthropods (crustaceans and insects). Chitin and its deacetylated derivative, chitosan, have been reported with an impressive range of applications in medical, environmental, cosmetic, food and nutrition industries.2–7
Extraction of chitin from shrimp shells involves the removal of minerals, fats, proteins and pigments. Traditionally, shrimp shells are dried to prevent spoilage, and ground to homogenize the raw materials and reduce its volume in preparation for chitin extraction.8 Minerals (mainly calcium carbonate) are removed through reaction with inorganic or organic acids.7–12 Proteins are usually removed from the shells by incubation in a sodium hydroxide solution at elevated temperatures, and the denatured proteins are separated from chitin through centrifugation. Finally, depigmentation is performed for the chitin obtained after demineralization and deproteination, which is commonly achieved using alcohols, organic solvents or peroxides.
The requirement of residual ash content in food-grade chitin is <2.5%.13 For higher-grade applications, such as production of chitosan, the residual ash content should be <1%.14 Hydrochloric acid (HCl) is the most commonly used acid in demineralization of shrimp shells due to its low cost, wide availability and high efficiency. However, there are many environmental, health and safety concerns due to its extreme corrosiveness and respiratory hazards, particularly when used in remote areas with limited emergency response and water treatment capabilities. Furthermore, the use of HCl for demineralization has been reported to result in damaged molecular structure, reduced molecular weight and decreased degree of acetylation that negatively affect the intrinsic properties of the purified chitin.15 A number of studies have been performed to reduce the amount of hydrochloric acid required to achieve <1% residual ash content.16–19 It was found that traditionally used concentrations of HCl (3–7%) are in a large excess and much lower concentrations are sufficient. Depending on the thickness of shells and particle size, as well as whether deproteination is performed prior to demineralization, the concentration of HCl may be further reduced. For example, in our preliminary study (data not shown) the HCl amount was reduced by >80% through pretreatment of shells, resulting in a significant reduction of overall reaction volume in demineralization.
Organic acids, mainly including formic acid, citric acid, lactic acid and acetic acid, have been investigated as alternatives to HCl for demineralization of crustacean shells.8,20,21 Formic acid is corrosive and has severe health concerns. During the reaction of calcium carbonate with formic acid, calcium formate is produced, which is widely used as a feed additive to provide antifungal and antibacterial effects by acidification.22 However, calcium formate is not approved for use in human food in the EU and many other countries.23 This renders formic acid unsuitable for production of food-grade chitin and food-grade protein concentrates. Acetic acid is relatively harmless in dilutions of <10% (e.g. household vinegar). However, it is a category 2 flammable liquid at higher concentrations and requires dangerous goods transportation, temperature and explosion-proof storage and ventilation, as well as appropriate worker safety measures. Concentrated acetic acid is corrosive to metals and tissues and poses a breathing hazard. In addition, its pungent vinegar odor causes aggravation of airway, and is difficult to remove from chitin after demineralization. Lactic acid has been widely applied in industries including food, pharmaceuticals and cosmetics. It can be used as a descaling agent to remove hard water deposits through reaction with the minerals such as calcium carbonate. However, concentrated lactic acid is corrosive to metals and can cause serious eye damage and skin irritation. Similar to acetic acid, lactic acid also has an acrid odor. It is reported as category 1 flammable, category 2 health hazardous and is toxic to human at high concentrations.
In comparison to other acids commonly used in extraction of chitin, citric acid is by far the least toxic. Citric acid is a naturally occurring metabolic intermediate vital to the tricarboxylic acid cycle respiration pathway in all animal and plant cells. It is non-flammable, non-explosive and safe without reactivity hazards. Citric acid has few health hazards and does not have any known sensitizing mutagenic carcinogenic or reproductive effects. Ameh et al. performed demineralization of shrimp powder (<250 μm particle size) using citric acid with concentrations of 0.1, 0.2, 0.3, 0.4, and 0.5 M at a shell![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio of 1
water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and reported 3% of residual ash in the chitin obtained from the use of the stoichiometric amount of citric acid (0.2 M).9 They also reported the reduction of residual ash levels to around 2% after 15 min of reaction using the two highest concentrations (0.4 and 0.5 M). Zhao et al. subjected dried shrimp shells (particle size 0.355 nm) to 10% citric acid for demineralization and reported 1.85% of residual ash in the chitin.12 Guo et al. extracted chitin from minced shrimp heads (Pandalus Borealis) and reported 2.1% of residual ash in the chitin after treatment of shells with 10% citric acid for 2 hours at a solid
13 and reported 3% of residual ash in the chitin obtained from the use of the stoichiometric amount of citric acid (0.2 M).9 They also reported the reduction of residual ash levels to around 2% after 15 min of reaction using the two highest concentrations (0.4 and 0.5 M). Zhao et al. subjected dried shrimp shells (particle size 0.355 nm) to 10% citric acid for demineralization and reported 1.85% of residual ash in the chitin.12 Guo et al. extracted chitin from minced shrimp heads (Pandalus Borealis) and reported 2.1% of residual ash in the chitin after treatment of shells with 10% citric acid for 2 hours at a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.25.10 Baron et al. treated dried and ground shrimp cuticles from Litopenaeus vannamei with formic, acetic, and citric acid.11 The reaction dynamics were investigated at 9, 15, and 360 min of reaction time at reaction temperatures of 20 °C, 30 °C, and 40 °C. At the stoichiometric ratio, the use of citric acid resulted in the lowest residual minerals of 1% in the demineralized shells.
6.25.10 Baron et al. treated dried and ground shrimp cuticles from Litopenaeus vannamei with formic, acetic, and citric acid.11 The reaction dynamics were investigated at 9, 15, and 360 min of reaction time at reaction temperatures of 20 °C, 30 °C, and 40 °C. At the stoichiometric ratio, the use of citric acid resulted in the lowest residual minerals of 1% in the demineralized shells.
So far, demineralization of shrimp shells has been performed using moderate concentrations of citric acid within 10% (v/v).9–12,20,24 However, to our best knowledge, there have been rare studies reported about demineralization of shells to <1% of residual ash. To our best knowledge, demineralization was performed in a one-step process in all previously reported research on extraction of chitin from shrimp shells, and the acid was in large excess in most studies. In the present study, for the first time, a two-step demineralization process using citric acid was developed to maximize the removal of minerals and minimize the amount of acid required (Fig. 1). Different from the traditional approach of drying and grinding the shells prior to chitin extraction, fresh shrimp shells were used in this study as they were received. The shells were intensively pretreated to remove most loose proteins and reduce the particle size before demineralization. The aim of this study is to achieve near-complete demineralization under non-hazardous and environmentally benign conditions through combination of a thorough pretreatment with a two-step demineralization process. This process was compared to demineralization using HCl with the stoichiometric amount to evaluate the potential of citric acid as an alternative to HCl.
Materials and methods
Chemicals and materials
Fresh shrimp shells were collected from the local shrimp processing plant in Newfoundland. The shells were chilled on ice and transported to the bioprocessing pilot plant, Fisheries and Marine Institute of Memorial University of Newfoundland, and immediately processed once received.Food-grade citric acid and hydrochloric acid (20° Be, industrial grade) were purchased from Eastchem Inc., St John's, Canada. The chemicals used for proximate analysis were purchased from VWR Canada and all in analytical grade.
Proximate analysis
The proximate composition of pretreated shrimp shells was analyzed following the standard method of the Association of Official Analytical Chemists International (AOAC).25 Ash and moisture levels were determined according to standard dry ashing and moisture procedures (AOAC 938.08 and 930.15). The content of lipids was determined using the Soxhlet method (AOAC 948.15). The nitrogen content (NTotal and NChitin) was determined using the Kjeldahl method (AOAC 954.01). NTotal was measured using the pretreated shells. NChitin was measured using the pretreated shells after chemical deproteination (incubation of the pretreated shells in 3.5% NaOH solution at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 solid/solution ratio and 60 °C for 3 hours). For the calculation it was assumed that the chemical deproteination efficiency was 100%, and the obtained chitin was 100% acetylated. The nitrogen content derived from proteins in the pretreated shells (NProtein) was calculated as NTotal–NChitin. The protein content was calculated as NProtein × 6.25.
10 solid/solution ratio and 60 °C for 3 hours). For the calculation it was assumed that the chemical deproteination efficiency was 100%, and the obtained chitin was 100% acetylated. The nitrogen content derived from proteins in the pretreated shells (NProtein) was calculated as NTotal–NChitin. The protein content was calculated as NProtein × 6.25.
Pretreatment of shrimp shells
The shrimp shells were pretreated on pilot-scale to remove loose proteins and other impurities and achieve a homogeneous particle size. An amount of 253.65 kg of shrimp shells was processed via thorough grinding, washing and pressing, resulting in 82.3 kg of pretreated shells with a homogeneous particle size of 1.4 mm. The grinding led to significant reduction of the shell volume which needs to be handled in further downstream processing and the resulting reduction of tank size and chemical use. The weight of shells was reduced by 67.6% (wet basis) after pretreatment. A large amount of loose proteins and moisture were removed from the shells by grinding, washing, and pressing. In addition, some minerals and chitin were also lost into the wastewater. The solid in the wastewater was recovered and freeze-dried, followed by the composition analysis. The solid contained moisture, ash, lipids, proteins, salt, chitin and astaxanthin (the detailed analysis results will be discussed in our future publication). The main difference between a conventional process without shell pretreatment and the process in the present study is that the native shrimp proteins and pigments can be recovered after our pretreatment, which are denatured and lost in the traditional approach.The pretreated shells were stored at −28 °C in 1.5 kg sample size until further use. The same batch of pretreated shells was used for all demineralization experiments.
Determination of the citric acid volume
The amount of citric acid used in demineralization of shrimp shells was calculated using the stoichiometric reaction equation (eqn (1)), based on the assumption that all mineral deposits in the shrimp shells are calcium carbonate. According to the moisture and ash content (Table 1), 200 g of pretreated shells contained 30.94 g of calcium carbonate. The demineralization reaction is an acid/base reaction between calcium carbonate and citric acid, producing calcium citrate, carbon dioxide (CO2) and water (H2O). The overall equation is:| 3 CaCO3 (s) + 2 H3C6H5O7 (aq) ⇔ Ca3(C6H5O7)2 (aq) + 3 H2O (l) + 3 CO2 (g) | (1) |
| Proximate composition (%) | Pretreated shellsa |
|---|---|
| a The moisture content of the pretreated shells (wet weight basis) was 64.53 ± 0.35%. b Undetectable by Soxhlet analysis. c Estimated as (100% – ash – proteins – lipids). The lipid content was assumed as 0–1%. | |
| Ash | 43.61 ± 0.10 |
| NTotal | 4.85 ± 0.06 |
| NChitin | 2.81 ± 0.03 |
| NProtein | 2.04 |
| Proteins | 12.75 |
| Lipids | <1%b |
| Chitin | 42.6–43.6c |
By stoichiometric calculation, the theoretical amount required to demineralize 200 g of pretreated shells is 39.578 g pure citric acid or 79.16 ml of a 50% citric acid solution. In the present study, this amount of citric acid was labelled as “1×”. When using an excess of citric acid, the amount was labelled based on the stoichiometric amount. For example, a 1.5× demineralization would be 200 g shells treated with 118.74 ml of 50% citric acid.
Demineralization of shrimp shells using citric acid
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 in a 2.5 L beaker. The solution was stirred for a few minutes until a homogeneous mixture was obtained. The predetermined volume of 50% citric acid was added stepwise to avoid excessive foaming. The mixture was stirred at room temperature (21 °C) for the predetermined reaction time using a EUROSTAR 60 overhead stirrer (IKA Works Inc, Wilmington, NC, USA) with R 1382 Propeller stirrer, 3-bladed (IKA Works Inc, Wilmington, NC, USA). The pH of the mixture was monitored using a HQ40D portable multi-meter (HACH, London, ON, Canada). In-process samples were taken at various time throughout the demineralization process and strained through a 0.5 mm sieve, thoroughly washed and repeatedly hand-pressed for 5 min under running water. The collected samples were dried at 105 °C for 24 h, and analyzed for residual ash content (dry weight basis).
7 in a 2.5 L beaker. The solution was stirred for a few minutes until a homogeneous mixture was obtained. The predetermined volume of 50% citric acid was added stepwise to avoid excessive foaming. The mixture was stirred at room temperature (21 °C) for the predetermined reaction time using a EUROSTAR 60 overhead stirrer (IKA Works Inc, Wilmington, NC, USA) with R 1382 Propeller stirrer, 3-bladed (IKA Works Inc, Wilmington, NC, USA). The pH of the mixture was monitored using a HQ40D portable multi-meter (HACH, London, ON, Canada). In-process samples were taken at various time throughout the demineralization process and strained through a 0.5 mm sieve, thoroughly washed and repeatedly hand-pressed for 5 min under running water. The collected samples were dried at 105 °C for 24 h, and analyzed for residual ash content (dry weight basis).
The control experiment using HCl was performed by mixing the pretreated shells with the stoichiometric amount of HCl (20° Be) and following the same steps as described above.
In step 2, the remaining mineral deposits in the demineralized shells after 1st step were further removed using 50% citric acid with a concentration ranging from 1× to 20×. A portion of 150 g of shells was mixed with distilled water and the predetermined volume of 50% citric acid to a total volume of 1.5 L. The mixture was stirred constantly at room temperature. The samples were collected at 30 min and 60 min, strained through a 0.5 mm sieve, thoroughly washed and repeatedly hand-pressed for 5 min under running water. The collected samples were dried at 105 °C for 24 h, and analyzed for residual ash content (dry weight basis).
Analysis of degree of acetylation of chitin
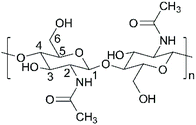
The degree of acetylation (DA) of chitin in the samples obtained after two-step demineralization and enzymatic deproteination was determined using solid-state 1H and 13C Nuclear Magnetic Resonance (NMR) spectroscopy at Centre for Chemical Analysis, Research and Training, Memorial University of Newfoundland. The NMR spectra were obtained at 298 K on a Bruker Avance II 600 spectrometer equipped with a SB Bruker 3.2 mm MAS triple-tuned probe operating at 600.33 MHz for 1H and 150.97 MHz for 13C spectroscopy. The chemical shifts were referenced to tetramethylsilane (TMS) using adamantane as an intermediate standard for 13C spectra. The 13C{1H} cross-polarization (CPMAS) spectra were collected with a Hartmann-Hahn match at 62.5 kHz and 100 kHz 1H decoupling, with a contact time of 2 ms, a recycle delay of 3 s and 1 k scans. The spectra were deconvoluted and integrated using MestReNova.The DA of chitin was calculated using the relative intensities of the resonances of the methyl carbon (ICH3) and other carbons (IC1, IC2, IC3, IC4, IC5 and IC6) in chitin (Fig. 2) following the method developed by Ottøy et al.:26
| DA (%) = ICH3/((IC1 + IC2 + IC3 + IC4 + IC5 + IC6)/6) | (2) |
Statistical analysis
Proximate analysis and one-step demineralization experiments were performed in triplicates. Two-step demineralization experiments were carried out in quadruplicates. The data were analyzed with analysis of variance (ANOVA) at 95% confidence level using Minitab 17.3.1.Results and discussion
Proximate composition
The proximate composition of the pretreated shrimp shells is shown in Table 1. Shahidi and Synowiecki reported that the protein content of dried Pandalus borealis processing materials was 41.90%.27 Kim et al. claimed 12.19% lipids and 44.50% proteins in freeze-dried Pandalus borealis by-products.28 Dave et al. reported that the cooked shells of Pandalus borealis contained 8.12% lipids and 50.65% proteins (dry weight basis).29 The amount of lipids and proteins of the pretreated shells in the present study (<1% and 12.75%, respectively) was significantly lower in comparison to the previously reported research, due tothe removal of loose proteins and lipids by thorough grinding and washing during the pretreatment. The estimated chitin content in the present study is 42.6–43.6%. In the study by Shahidi and Synowiecki, the chitin content in dried Pandalus borealis processing materials was 17.01%.27 Rødde et al. reported 17–20% of chitin in dried Pandalus borealis shells, which contained 33–40% of proteins and 32–38% of ash.30 The level of chitin estimated in the present study is higher compared to the previously reported results, mainly resulting from the decrease of protein content in the pretreated shells.One-step demineralization
One-step demineralization of shrimp shell was performed at different citric acid concentrations ranging from 1× to 2× and compared to a standard demineralization process using 1× HCl. The reaction dynamics and residual ash content after treatment were significantly affected by the citric acid concentration. Table 2 shows the residual ash values in the demineralized shells by using different acid concentrations after 2 h of reaction.| Amount of citric acid (multiples of the stoichiometric amount) | Residual ash (%) |
|---|---|
| 1× | 9.48 ± 0.07 |
| 1.25× | 6.15 ± 0.82 |
| 1.5× | 1.18 ± 0.42 |
| 2× | 0.59 ± 0.05 |
| 1× HCl (control) | 0.29 ± 0.05 |
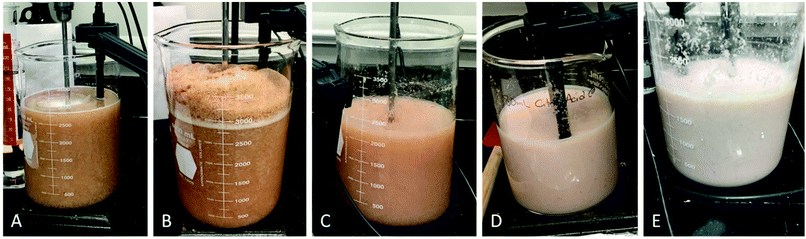
During the demineralization reaction, formation of CO2 resulted in foaming, which needed to be controlled by stepwise addition of the acid as well as adjustment of stirring speed and agitation pattern to break down the foam. Foam formation was started once the pH was reduced to below 5 and lasted for approximately 30 minutes. Afterwards, the foam subsided, and minor bubbling was observed in the first hour. In this laboratory-scale study, the entire amount of citric acid was added within 2–5 minutes, and foaming could be easily controlled by increasing stirring speed and manually disrupting the foam using a spatula. However, when the reaction was performed on pilot scale in a 600 L tank, the surface/volume ratio of the tank was much smaller (7853 cm2/600 L = 13.08) compared to the beaker used on lab scale (176 cm2/2.5 L = 70.4), and the space on the top of the tank was very limited to allow for foam accumulation. Therefore, the demineralization had to be carried out more slowly by stirring and adding the acid at lower speeds to avoid much foaming. In an industrial tank, a foam disruptor would therefore be an important installation to accelerate acid addition and shorten processing time. Fig. 3 illustrates the dynamics of the demineralization process. Before acid addition, the solution of pretreated shells exhibited a light brown color at 200 rpm (Fig. 3A). During the most intense foam formation in the first 10 minutes of the reaction, the foam layer was about 1/3 of the height of the solution (Fig. 3B). The generation of calcium citrate during the reaction leads to processing concerns as it will change shell quality and flow characteristics if it is present in its precipitated form. Due to the large amount of calcium citrate generated (approximately 39–51 g) in the present study and its relatively low solubility in water (0.95 g L−1 at 25 °C), calcium citrate precipitated as the acid was used up during the demineralization process. Precipitated calcium citrate thickened the reaction mixture and thereby interfered draining of the shells. Furthermore, calcium citrate stuck to the shells and could not be washed off, even with thorough agitation and pressing. This residual contamination was presumably reflected in elevated ash values found in samples. Therefore, the high residual mineral content was also prompted from calcium citrate contamination in addition to residual calcium carbonate present in the product. The precipitation was decreased with the use of increased volume of citric acid. Fig. 3C is an example of minor precipitation of calcium citrate in the reaction using 2× citric acid. A color change was observed; however, thickness of the mixture was not affected, and the precipitate could be removed. Fig. 3D illustrates medium precipitation observed using 1.5× citric acid. The color change was more pronounced, and the shells retained a white hue after washing. Fig. 3E shows the thickened mixture obtained using 1× citric acid, which was difficult to drain. The white residues remained on the shells even after thorough washing and pressing, and could only be washed off with an acidic solution.
In the control experiment, demineralization of shrimp shells was performed with 1× HCl for 2 h. The treatment resulted in consistent and near-complete demineralization (0.29% of residual ash). Calcium chloride was formed during the reaction, which is highly soluble in water and therefore did not precipitate. In comparison to citric acid treatment, foam formation occurred more quickly in the early phase of the control experiment, and the speed of HCl addition was adjusted accordingly to avoid overflowing of the reaction vessel. The appearance of the reaction mixture was similar to Fig. 3A. However, the stirring pattern was changed as the demineralized shells were lighter and spongy, thereby requiring an increase in stirring speed towards the end of the reaction.
The reaction processes using 1.25× and 1.5× citric acid were further studied to investigate the demineralization dynamics in relation to precipitation of calcium citrate and pH change (Table 3 and Fig. 4). During the demineralization process, samples were extracted at 15, 30, 45, 60, 90, 120, and 180 min. At each sampling point, the pH was recorded and the ash content of the sample was determined. In the demineralization using 1.25× citric acid, the residual ash content was reduced to 5.3% after 180 min. A color change from brown to light pink/dilute milk was observed within the first 30 min, and thickening of the mixture was observed from 60 to 90 min. In the demineralization using 1.5× citric acid, the lowest residual ash level of 1.18% was obtained at 120 min. However, the residual ash content was increased toward the end of the reaction, possibly due to the accumulation of calcium citrate precipitate on the shells. A color change from brown to light pink/dilute milk was observed between 90 and 120 min, and thickening of the mixture was observed after 120 min. As indicated in Fig. 4B, the majority of the minerals in shrimp shells was removed in the first 30 minutes, with only a small decrease observed between 30 and 180 min.
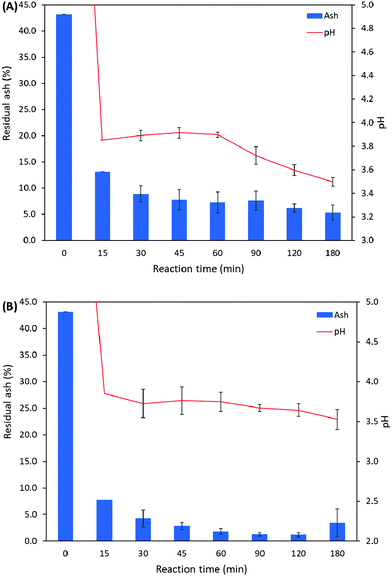 | ||
| Fig. 4 Residual ash content in demineralized shrimp shells using (A) 1.25× and (B) 1.5× citric acid. | ||
| t (min) | Amount of citric acid (multiples of the stoichiometric amount) | |||
|---|---|---|---|---|
| 1.25× | 1.5× | |||
| pH | Residual ash (%) | pH | Residual ash (%) | |
| 0 | 9.40 ± 0.05 | 43.61 | 9.33 ± 0.12 | 43.61 |
| 15 | 3.85 | 13.12 ± 0.14 | 3.59 | 7.78 ± 0.07 |
| 30 | 3.89 ± 0.05 | 8.87 ± 1.58 | 3.73 ± 0.18 | 4.27 ± 1.60 |
| 45 | 3.91 ± 0.05 | 7.75 ± 1.92 | 3.76 ± 0.17 | 2.82 ± 0.69 |
| 60 | 3.90 ± 0.02 | 7.24 ± 2.01 | 3.75 ± 0.12 | 1.83 ± 0.57 |
| 90 | 3.72 ± 0.08 | 7.62 ± 1.79 | 3.67 ± 0.04 | 1.24 ± 0.33 |
| 120 | 3.60 ± 0.05 | 6.15 ± 0.82 | 3.64 ± 0.08 | 1.18 ± 0.41 |
| 180 | 3.50 ± 0.04 | 5.30 ± 1.44 | 3.53 ± 0.13 | 3.45 ± 2.62 |
During the demineralization process, the initial pH was drastically decreased upon acid addition. Afterwards, there was a slight increase of pH due to the consumption of citric acid. Citric acid is a polyprotic acid, and its first proton is released much faster than the second and third ones (eqn (3)–(5), Ka1 = 7.4 × 10−4, Ka2 = 1.7 × 10−5, Ka3 = 5.4 × 10−7). Therefore, calcium carbonate was mainly reacted with the first proton of citric acid in the early stage of the reaction. Afterwards, the slow and constant release of protons from the dihydrogen citrate (C6H7O7−) and monohydrogen citrate (C6H6O72−) dominated the whole process, resulting in the steady decrease of pH of the mixture (Fig. 4).
| C6H8O7 (aq) + H2O (l) ⇔ C6H7O7− (aq) + H3O+ (aq) | (3) |
| C6H7O7− (aq) + H2O (l) ⇔ C6H6O72− (aq) + H3O+ (aq) | (4) |
| C6H6O72− (aq) + H2O (l) ⇔ C6H5O73− (aq) + H3O+ (aq) | (5) |
Alkhaldi et al. reported that the reaction between calcium carbonate and citric acid is a complicated process composed of multiple steps (eqn (6)–(8)).31 When a large excess of citric acid is added (e.g. 2× citric acid), the dissociation of citric acid to dihydrogen citrate (C6H7O7−) is significantly promoted due to the high concentration of citric acid and the consumption of protons by calcium carbonate. Consequently, a large quantity of C6H7O7− is generated, which abundantly reacts with calcium ions (Ca2+). Therefore, most Ca2+ exist in the reaction mixture in the form of Ca(C6H7O7)+ with little formation of calcium citrate (Ca3(C6H7O7)2).
| CaCO3 (s) + 2H3O+ (aq) ⇔ Ca2+ (aq) + 3H2O (l) + CO2 (g) | (6) |
| Ca2+ (aq) + C6H7O7− (aq) ⇔ Ca(C6H7O7)+ (aq) | (7) |
| Ca2+ (aq) + 2Ca(C6H7O7)+ (aq) ⇔ Ca3(C6H5O7)2 (aq) + 4H+ | (8) |
As indicated in the present study, during the demineralization using 1× citric acid, calcium citrate precipitated quickly within the first 10 min. The shell solution became milky, very thick and difficult to stir. Draining of the shells was hampered by the precipitate that stuck to the shells and clogged pores of the strainer sieve. The calcium citrate precipitate could not be removed by rinsing the shells with water, and resulted in an elevated residual ash level of 9.48% (Table 2). Therefore, the concentration of 1× citric acid was unsuitable for demineralization. The use of 1.25× citric acid was also not suitable for effective demineralization due to fast precipitation and high residual ash content (5.30%) in the demineralized shells. Although the use of 1.5× citric acid resulted in a lower residual ash content (1.18%) after 120 min of demineralization, the onset and severity of precipitation during the reaction was observed inconsistent among the replicates. In order to obtain consistent results, the reaction would have to be stopped prior to approximately 60 min. By that time, the average residual ash content was 1.83%, which was above the acceptable specification of 1% for high-grade applications of chitin. Among the four concentrations of citric acid investigated, only 2× citric acid resulted in residual ash content of below 1% (0.59%, Table 2), which fulfilled the required specification for high-grade applications of chitin and therefore suitable for the one-step demineralization process. However, the use of such an excess of acid reduces the economic viability of the process. With the aim to reduce the amount of citric acid used and improve the demineralization efficiency, a two-step demineralization process was designed and investigated.
Two-step demineralization
In the present study, the two-step demineralization was performed to minimize both consumption of acid and contamination of the demineralized shells with calcium citrate precipitate. As indicated by the results from one-step demineralization, the use of 1.5× citric acid reduced the mineral content in the shells by 90% (4.27 ± 1.60% residual ash) within 30 minutes, while the onset of precipitate formation was observed at around 60 min. Therefore, the reaction conditions of this process was used as the first step in the two-step demineralization process to remove most of the minerals in the shells. After step 1 of demineralization, the average residual ash content in the demineralized shells (74.26% of moisture) was 2.80 ± 0.47%, corresponding to a reduction of mineral content by 93.5% (original ash content of 43.61%). The shells were further treated with citric acid with a concentration ranging from 1× to 20× (Table 4 and Fig. 5). The lowest residual ash content was achieved using 14× citric acid for 30 min (0.32%) and 8× citric acid for 60 min (0.19%) of demineralization. However, it should be noted that the residual ash content from 30 min of demineralization using 8× citric acid (0.34%) was similar to the lowest value from using 14× citric acid (0.32%), and the difference might be due to data deviation or variation of ash content in the shrimp shell samples. The demineralized shells were significantly softer in comparison to the shells obtained from one-step demineralization, possibly due to the lower content of residual ash or calcium citrate. The specific reason will be further investigated in future studies, and this softer property of the shells from two-step demineralization will be studied about its influence on downstream processing steps (e.g. enzymatic deproteination or deacetylation) towards production of high-purity chitin and chitosan.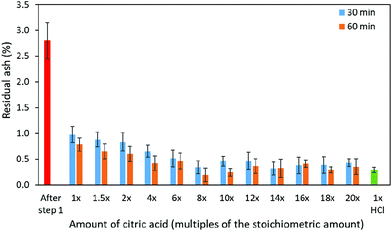 | ||
| Fig. 5 Residual ash content of demineralized shrimp shells after step 2 of two-step demineralization. | ||
| Residual ash (%) | |
|---|---|
| Pretreated shells | 43.61 ± 0.10 |
| After step 1 | 2.80 ± 0.47 |
| Amount of citric acid (multiples of the stoichiometric amount) | 30 min | 60 min |
|---|---|---|
| 1× | 0.98 ± 0.16 | 0.78 ± 0.13 |
| 1.5× | 0.88 ± 0.14 | 0.65 ± 0.15 |
| 2× | 0.84 ± 0.18 | 0.60 ± 0.15 |
| 4× | 0.65 ± 0.12 | 0.42 ± 0.14 |
| 6× | 0.51 ± 0.16 | 0.47 ± 0.15 |
| 8× | 0.34 ± 0.12 | 0.19 ± 0.13 |
| 10× | 0.46 ± 0.09 | 0.24 ± 0.07 |
| 12× | 0.47 ± 0.17 | 0.36 ± 0.14 |
| 14× | 0.32 ± 0.12 | 0.35 ± 0.13 |
| 16× | 0.38 ± 0.16 | 0.37 ± 0.06 |
| 18× | 0.37 ± 0.18 | 0.31 ± 0.08 |
| 20× | 0.43 ± 0.07 | 0.35 ± 0.15 |
A one-way ANOVA was performed to analyze the influence of citric acid concentration on step 2 of demineralization. As shown in the interval plot (Fig. 6A), when the reaction was performed for 30 min, the intervals of the first three groups (1×, 1.5× and 2× citric acid) do not overlap with the intervals of the groups of 8× and higher citric acid. This indicates that the average residual ash levels obtained using citric acid with low concentrations (1×, 1.5× and 2×) was statistically different (p < 0.05) from the values obtained using the high concentrations (8× and higher). Therefore, although the residual ash content was effectively decreased from 2.8% to just below 1% in step 2 by using citric acid with a low concentration within 2×, a significant further reduction in mineral content can be achieved by increasing the acid concentration to 8× or higher. When the reaction time was 60 min, this difference in residual ash content between using the low and high concentrations of citric acid was visible as a trend (Fig. 5), but not statistically significant (p > 0.05, Fig. 6B). To investigate the effect of reaction time, a 2-sample t-test was performed for the two groups of 30 and 60 min reactions for each citric acid concentration. No statistically significant difference was observed between 30 and 60 min incubation time for all citric acid concentrations.
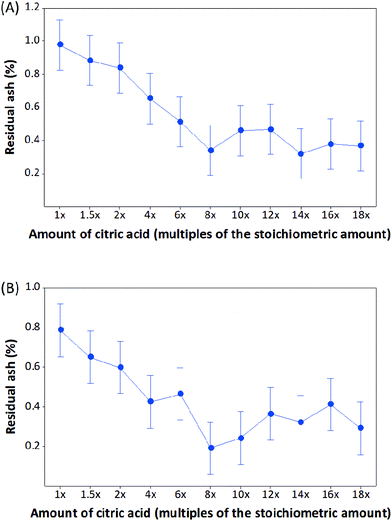 | ||
| Fig. 6 Interval plot of residual ash content of demineralized shrimp shells after (A) 30 min and (B) 60 min of step 2 of two-step demineralization. | ||
As indicated in Table 4, with the increase of citric acid concentration to 10× and higher, the residual ash content showed some fluctuations and was higher after some treatments in comparison to the lowest value obtained from the use of 8× citric acid. This was possibly due to the variation of ash content in the shrimp shell samples. Since the residual ash content was decreased to the lowest level when using 8× citric acid, it was assumed that the majority of the minerals in the samples has been reacted and the further increase of citric acid concentration didn't result in consumption of more minerals. In order to objectively determine the overall achievable lowest residual ash content using the two-step citric acid process, the average value from reactions using 8× and higher concentrations of citric acid was calculated, which was 0.39 ± 0.13 and 0.31 ± 0.13% for reactions after 30 and 60 min, respectively. Although the difference of 0.8% residual ash between 30 and 60 min was slight, it can be important in the production of high-quality chitin and chitosan using gentle processes. Moreover, further downstream processing, such as enzymatic deproteination, will be facilitated by the low content of residual ash in the demineralized shells, since mineral deposits may physically hinder the accessibility of the proteins in the shell to the enzyme. Therefore, 60 min was selected to use in step 2 of demineralization.
Based on the results from the present study, the following 2-step process was determined as the best alternative to the traditional HCl demineralization of shrimp shells: in step 1, the pretreated shrimp shells are mixed with 1.5× citric acid and stirred for 35 min of incubation, followed by a brief wash; in step 2, 8× citric acid was added to the collected shells and the mixture was incubated for 60 min, followed by thorough washing and pressing. This process will result in soft demineralized shells with a residual ash content of approximately 0.31% (reduction by 99.3%), which is comparable to the result from one-step demineralization using 1× HCl (0.29%, Table 2).
The degree of acetylation (DA) of chitin in the demineralized shrimp shells was determined using solid-state 1H and 13C NMR spectroscopy (Table 5). However, the proteins in the demineralized shrimp shells interfered with the analysis and resulted in a DA value significantly above 100%. Therefore, the samples were deproteinated to remove the proteins. Since alkaline deproteination will promote deacetylation of chitin, an enzymatic deproteination process was adopted (the detailed method will be discussed in our future publication). As indicated in Table 5, the chitin in the samples after demineralization and deproteination had a DA of 104.67%, indicating that no significant deacetylation of chitin occurred during the whole process including pretreatment of shrimp shells, demineralization and deproteination. The DA was slightly higher than 100% possibly due to the small amount of protein residue in the samples.
| Sample treatment | After demineralization | After demineralization and deproteination |
|---|---|---|
| DA (%) | 117.00 ± 9.09 | 104.67 ± 1.88 |
Techno-economic considerations of demineralization processes
Although there is no significant difference in residual ash content in demineralized shells obtained from two-step process using citric acid and one-step process using HCl, the use of citric acid would be far superior compared to HCl in terms of production cost and requirements for environmental, health and safety (EHS) provisions. The cost drivers for chitin production mainly include costs for chemicals and other reagents. Quotes for citric acid and HCl in intermediate quantities (citric acid: 70 × 25 kg bags daily; HCl: 12 × 240 kg drums daily) was obtained from a local chemical supplier, and the cost for different demineralization processes was estimated (Table 6). It should be noted that as prices for chemicals can be significantly decreased with the increase of purchased amount, this cost estimation is likely overpriced and thereby may not represent the actual production cost. However, for comparison purposes, it can be observed that for every 1000 kg of shrimp shells, the amount of citric acid used is reduced by 35.49 L using the two-step process compared to the one-step process. Considering the large processing volume of shrimp shells on industrial scale, this decrease in citric acid consumption is significant.| Amount | Traditional demineralization using HCla | Optimized demineralization using HClb | One-step demineralization using citric acid | Two-step demineralization using citric acid |
|---|---|---|---|---|
a The traditional process in which the shells are demineralized without pretreatment and using 3.5% HCl (v/v) (the typical range: 3–7%) at a shell/solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (w/v) (the typical range: 1 8 (w/v) (the typical range: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15–1 15–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20).8,34
b Based on our preliminary study in which the stoichiometric amount of HCl was used at a shell/solution ratio of 1 20).8,34
b Based on our preliminary study in which the stoichiometric amount of HCl was used at a shell/solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (w/v).
c Estimated based on 40% reduction of weight as observed after step 1.
d Calculated based on a shell/solution ratio of 1 7 (w/v).
c Estimated based on 40% reduction of weight as observed after step 1.
d Calculated based on a shell/solution ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v).
e Estimated based on residual ash content of 2.8% after step 1. 10 (w/v).
e Estimated based on residual ash content of 2.8% after step 1.
|
||||
| Weight of shrimp shells (kg) | 1000 | 1000 | 1000 | 1000 |
| Weight of pretreated shrimp shells (kg) | — | 324.46 | 324.46 | 324.46 |
| Reaction volume (step 1) (L) | 8000 | 2271 | 2271 | 2271 |
| Volume of acid (step 1) (L) | 747 (20°Be HCl) | 115 (20°Be HCl) | 256.82 (50% citric acid) | 192.61 (50% citric acid) |
| Weight of shells after step 1 (kg)c | — | — | — | 194.68 |
| Reaction volume (step 2) (L) | — | — | — | 1948d |
| Volume of acid (step 2) (L) | — | — | — | 28.72e (50% citric acid) |
| Total amount of acid required | 747 L (20°Be HCl) | 115 L (20°Be HCl) | 256.82 L (50% citric acid) or 128.41 kg (citric acid) | 221.33 L (50% citric acid) or 110.67 kg (citric acid) |
| Cost of acid ($ kg−1) | 1.29 | 1.29 | 3.20 | 3.20 |
| Total cost of acid ($) | 964.00 | 148.00 | 410.91 | 354.14 |
| Residual ash (%) | <1 | 0.29 ± 0.05 | 0.59 ± 0.05 | 0.31 ± 0.13 |
Although the cost from using citric acid is significantly higher than using HCl, there are multiple cost drivers that only apply to HCl and result in extra expense. HCl is extremely corrosive and has sufficient volatility to constitute an inhalation danger due to its vapor pressure of >10 mmHg (1.33 kPa) at 25 °C.32 It is considered as an environmental hazard and is corrosive to aquatic life due to a pH < 2. According to Transport Canada, the implementation of an Environmental review and Emergency Response Assistance Plan is required for HCl of above the threshold quantity (3 tons). Specialized storage, ventilation and spill prevention system are required to use HCl. Since diluted HCl is very expensive when purchased in large amounts, HCl concentrate is usually purchased for large-scale production, which then requires specialized solution preparation system. As a reducing agent, HCl causes corrosion even in high-grade stainless steel and acid fumes. Even used after dilution, HCl can condense on cold metal surfaces and gradually causes corrosion of plant systems, pumps, fittings, monitoring systems and electrical controls. Therefore, the significantly shorter life span of all equipment in the vicinity of the demineralization system must be considered. In addition, worker safety and training must be ensured, and local storage and handling infrastructure must be developed. In remote areas such as Newfoundland, it can be very costly to implement appropriate systems.33 The management of all the above mentioned characteristics are significant cost drivers, especially when considering production facilities in strictly regulated countries.
In contrast, citric acid is a safe and environmental friendly option to implement for the demineralization process. As a common ingredient in many food products, it underlies very few handling requirements. The only safety concern is its mild corrosiveness, and requirement of dust control when citric acid powder is used. It is not considered as an environmental hazard and does not require specialized shipping, handling or storage. The shipping cost is even less when citric acid is purchased as a powder because the product is light. There is no requirement for Environmental review or Emergency Response Assistance Plan. Citric acid is not corrosive to most common food-grade stainless steel equipment, so the life span of the production system can be relatively long. Therefore, citric acid is a promising sustainable and economical alternative to HCl for large-scale demineralization of shrimp shells.
Conclusions
Although many studies have been reported about demineralization of shrimp shells using citric acid, the present study is the first one that used shells of Pandalus Borealis as the raw materials. Pandalus Borealis is one of the largest fishery species in the world, and plays an important role in Newfoundland fishery. Therefore, valorization of its shell waste is of great significance for the development of the blue economy. The shrimp shells were pretreated by thorough grinding and washing, during which the majority of loose proteins were removed and the particle size was decreased. A demineralization level of 98.6% was achieved through a one-step process using 2× citric acid for 2 h of reaction. In order to further decrease the mineral content and reduce the consumption of citric acid, a two-step process was performed. The shells were treated with 1.5× citric acid for 35 min first, and then 8× citric acid for 60 min, resulting in a reduction of minerals by 99.6%. Although the low residual ash content in the treated shells from both processes fulfilled specifications for high-grade applications of chitin (residual ash content <1%), the two-step process resulted in a lower residual ash content without longer reaction time required compared to the one-step process, and the demineralized shells obtained were noticeably softer. Even though the general specification for ash content in chitin and chitosan is <1% in the field of biomedical production, it is always desirable to achieve higher purity of the product. In addition, the amount of citric acid consumed can be reduced by approximately 35.49 L of 50% citric acid for every 1000 kg of fresh shrimp shells using the two-step process compared to the one-step process. Therefore, the two-step process is of more interest, and will be further investigated towards its influence on production of high-purity chitin and chitosan.The low levels of residual mineral content obtained from both processes were comparable to the conventional demineralization using HCl. The economic viability of the demineralization processes using citric acid and HCl was compared. Since citric acid is more environmentally friendly and has fewer health and safety concerns, the overall cost of production in a long term might be less by using citric acid. Therefore, the two-step demineralization process using citric acid is a promising alternative to the traditional HCl process for demineralization of shrimp shells during production of premium-quality chitin.
Besides shrimp shells, crab shells are also frequently used for extraction of chitin. The developed process possibly can be implemented to obtain the crab chitin. However, based on the authors’ experience, a large quantity of shrimps is sold peeled, so shrimp shells can be easily collected from farms or processing plants. In comparison, most crabs are sold with the shells, which makes the collection of shells difficult. As shrimp shells are much softer and thinner compared to crab shells, the initial grinding in the pretreatment step of the present process will be much easier for shrimp shells. In addition, the thinner shrimp shells result in larger surface area when reacting with the acid, thus leading to faster and more complete demineralization.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors would like to thank Dr Céline Schneider (Research Laboratory Associate, Centre for Chemical Analysis, Research and Training, Memorial University of Newfoundland) for the NMR analysis. This work was supported by Weston Loblaws Seeding Innovation Fund (SFI18-0217 – Memorial University).References
- Fisheries and Land Resources, Seafood industry year in review 2019, https://www.gov.nl.ca/ffa/files/2019-SIYIR-WEB.pdf, (accessed 22 January 2021).
- M. Hayes, Marine Bioactive Compounds: Sources, Characterization and Applications, Springer, New York, 2011 Search PubMed.
- I. Hamed, F. Özogul and J. M. Regenstein, Trends Food Sci. Technol., 2016, 48, 40–50 CrossRef CAS.
- F. Khoushab and M. Yamabhai, Mar. Drugs, 2010, 8, 1988–2012 CrossRef CAS PubMed.
- B. Bellich, I. D'Agostino, S. Semeraro, A. Gamini and A. Cesàro, Mar. Drugs, 2016, 14, 99 CrossRef PubMed.
- F. Shahidi, J. K. V. Arachchi and Y.-J. Jeon, Trends Food Sci. Technol., 1999, 10, 37–51 CrossRef CAS.
- E. Khor and A. C. A. Wan, Chitin: Fulfilling a Biomaterials Promise, Elsevier Ltd., Oxford, 2nd edn, 2014 Search PubMed.
- I. Younes and M. Rinaudo, Mar. Drugs, 2015, 13, 1133–1174 CrossRef CAS PubMed.
- A. O. Ameh, M. T. Isa, D. Abutu and A. Danlami, Leonardo Electron. J. Pract. Technol., 2014, 99–108 Search PubMed.
- N. Guo, J. Sun, Z. Zhang and X. Mao, J. Ocean Univ. China, 2019, 18, 719–726 CrossRef CAS.
- R. Baron, M. Socol, A. Arhaliass, S. Bruzac, K. Le Roux, J. Rodriguez del Pino, J. P. Bergé and R. Kaas, Process Biochem., 2015, 50, 2215–2223 CrossRef CAS.
- D. Zhao, W.-C. Huang, N. Guo, S. Zhang, C. Xue and X. Mao, Polymers, 2019, 11, 409 CrossRef PubMed.
- A. M. Mizani and B. M. Aminlari, in Proceedings of European Congress of Chemical Engineering, Copenhagen, 2007, pp. 1–8 Search PubMed.
- M. S. Hossain and A. Iqbal, J. Bangladesh Agric. Univ., 2014, 12, 153–160 CrossRef.
- A. Percot, C. Viton and A. Domard, Biomacromolecules, 2003, 4, 12–18 CrossRef CAS PubMed.
- A. Tolaimate, J. Desbrieres, M. Rhazi and A. Alagui, Polymer, 2003, 44, 7939–7952 CrossRef CAS.
- I. Younes, S. Hajji, M. Rinaudo, M. Chaabouni, K. Jellouli and M. Nasri, Int. J. Biol. Macromol., 2016, 84, 246–253 CrossRef CAS PubMed.
- H. A. S. Al Hoqan, N. AL-Shaqsi, M. A. Hossain and M. A. Al Sibani, Carbohydr. Res., 2020, 492, 108001 CrossRef PubMed.
- T. S. Trung, L. H. Tram, N. Van Tan, N. Van Hoa, N. C. Minh, P. T. Loc and W. F. Stevens, Carbohydr. Res., 2020, 489, 107913 CrossRef PubMed.
- T. Setoguchi, T. Kato, K. Yamamoto and J. Kadokawa, Int. J. Biol. Macromol., 2012, 50, 861–864 CrossRef CAS PubMed.
- N. S. Mahmoud, A. E. Ghaly and F. Arab, Am. J. Biochem. Biotechnol., 2007, 3, 1–9 CrossRef CAS.
- Global Calcium Formate Market 2019 by Manufacturers, Regions, Type and Application, Forecast to 2024, Absolute Reports, 2019, https://www.absolutereports.com/global-calcium-formate-market-13893578 Search PubMed.
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), EFSA J., 2014, 12, 3898 Search PubMed.
- L. D. Tolesa, B. S. Gupta and M.-J. Lee, Int. J. Biol. Macromol., 2019, 130, 818–826 CrossRef CAS PubMed.
- Association of Official Analytical Chemists, Official Methods of Analysis of AOAC International, Association of Official Analytical Chemists, Gaithersburg, MD, USA, 17th edn, 2000 Search PubMed.
- M. H. Ottøy, K. M. Vårum and O. Smidsrød, Carbohydr. Polym., 1996, 29, 17–24 CrossRef.
- F. Shahidi and J. Synowiecki, J. Agric. Food Chem., 1991, 39, 1527–1532 CrossRef CAS.
- S. Kim, N. Y. Yoon, K. Shim and C. Lim, Fish. Aquat. Sci., 2016, 19, 29 CrossRef.
- D. Dave, Y. Liu, J. Pohling, S. Trenholm and W. Murphy, Bioresour. Technol. Rep., 2020, 11, 100535 CrossRef.
- R. H. Rødde, A. Einbu and K. M. Vårum, Carbohydr. Polym., 2008, 71, 388–393 CrossRef.
- M. H. Alkhaldi, H. A. Nasr-El-Din and H. Sarma, SPE J., 2010, 15, 704–713 CrossRef CAS.
- OxyChem, Hydrochloric Acid Handbook, https://www.oxy.com/ourbusinesses/chemicals/products/documents/hydrochloricacid/hydrochloric_acid_handbook.pdf, (accessed 12 July 2021).
- Environment and Climate Change Canada, Technical Guidelines for the Environmental Emergency Regulations, 2019, https://publications.gc.ca/collections/collection_2020/eccc/En4-386-2020-eng.pdf, (accessed 13 July 2021).
- L. Pighinelli, J. Broquá, B. Zanin, A. Flach, C. Mallmann, F. Taborda, L. Machado, S. Alves, M. Silva and R. Dias, Am. J. Biomed. Sci. Res., 2019, 3, 307–314 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |