Open Access Article
Open Access ArticleInfluences of climate change on long-term time series of persistent organic pollutants (POPs) in Arctic and Antarctic biota†
Katrin
Vorkamp
 *a,
Pernilla
Carlsson
b,
Simonetta
Corsolini
*a,
Pernilla
Carlsson
b,
Simonetta
Corsolini
 c,
Cynthia A.
de Wit
c,
Cynthia A.
de Wit
 d,
Rune
Dietz
e,
Matthew O.
Gribble
d,
Rune
Dietz
e,
Matthew O.
Gribble
 f,
Magali
Houde
g,
Vrinda
Kalia
h,
Robert J.
Letcher
i,
Adam
Morris
j,
Frank F.
Rigét
f,
Magali
Houde
g,
Vrinda
Kalia
h,
Robert J.
Letcher
i,
Adam
Morris
j,
Frank F.
Rigét
 e,
Heli
Routti
e,
Heli
Routti
 k and
Derek C. G.
Muir
k and
Derek C. G.
Muir
 l
l
aAarhus University, Department of Environmental Science, Frederiksborgvej 399, 4000 Roskilde, Denmark. E-mail: kvo@envs.au.dk
bNorwegian Institute for Water Research (NIVA), Fram Centre, Tromsø, Norway
cUniversity of Siena, Department of Physical, Earth and Environmental Sciences, Siena, Italy
dStockholm University, Department of Environmental Science, Stockholm, Sweden
eAarhus University, Department of Ecoscience, Roskilde, Denmark
fUniversity of Alabama at Birmingham, School of Public Health, Birmingham, AL, USA
gEnvironment and Climate Change Canada, Montréal, QC, Canada
hColumbia University, Department of Environmental Health Sciences, New York, NY, USA
iEnvironment and Climate Change Canada, Ottawa, ON, Canada
jNorthern Contaminants Program, Crown-Indigenous Relations and Northern Affairs, Gatineau, QC, Canada
kNorwegian Polar Institute, Fram Centre, Tromsø, Norway
lEnvironment and Climate Change Canada, Burlington, ON, Canada
First published on 21st September 2022
Abstract
Time series of contaminants in the Arctic are an important instrument to detect emerging issues and to monitor the effectiveness of chemicals regulation, based on the assumption of a direct reflection of changes in primary emissions. Climate change has the potential to influence these time trends, through direct physical and chemical processes and/or changes in ecosystems. This study was part of an assessment of the Arctic Monitoring and Assessment Programme (AMAP), analysing potential links between changes in climate-related physical and biological variables and time trends of persistent organic pollutants (POPs) in Arctic biota, with some additional information from the Antarctic. Several correlative relationships were identified between POP temporal trends in freshwater and marine biota and physical climate parameters such as oscillation indices, sea-ice coverage, temperature and precipitation, although the mechanisms behind these observations remain poorly understood. Biological data indicate changes in the diet and trophic level of some species, especially seabirds and polar bears, with consequences for their POP exposure. Studies from the Antarctic highlight increased POP availability after iceberg calving. Including physical and/or biological parameters in the POP time trend analysis has led to small deviations in some declining trends, but did generally not change the overall direction of the trend. In addition, regional and temporary perturbations occurred. Effects on POP time trends appear to have been more pronounced in recent years and to show time lags, suggesting that climate-related effects on the long time series might be gaining importance.
Environmental significancePersistent organic pollutants (POPs) undergo long-range transport and accumulate in Arctic ecosystems. Time series of POPs in Arctic wildlife are an important tool in the management of chemicals, providing data for risk assessments and evaluations of the effectiveness of regulations, as well as information on chemical exposure of consumers of Arctic animals. Climate change can affect these POP time series, via changes in the physical environment and ecosystem structures. This article shows relationships between POP concentrations in polar wildlife and physical and/or biological parameters that need to be understood for a correct interpretation of POP time trends. It is part of the themed issue “Influence of climate change on persistent organic pollutants and chemicals of emerging concern in the Arctic”. |
1. Introduction
Several Arctic countries have established monitoring programmes for persistent organic pollutants (POPs) in the Arctic, in response to increased awareness of the long-range transport of POPs to the Arctic and their accumulation in Arctic food chains. Some of these programmes have now been in operation since the early 1990s or even longer and have generated long-term time series data of POP concentrations for selected Arctic species.1 In Antarctica, some monitoring data are available for POPs, generated by specific countries, but no systematic POP monitoring programmes exist as yet.2 Time series have also been derived from both Arctic and Antarctic sample collections in Environmental Specimen Banks (ESB), such as the Biorepository of the National Institute for Standards and Technology (NIST) of the USA or the ESB at the Swedish Museum of Natural History.3–5The POP time series from the Arctic, derived in these research projects or national monitoring programmes, have been analysed in circumpolar assessments under the auspices of the Arctic Council's Arctic Monitoring and Assessment Programme (AMAP).1,6 The most recent assessment generally found that the temporal trends differed between POPs depending on the date of their regulation: those POPs that were subject to regulation by national initiatives in the 1970s–1980s, such as polychlorinated biphenyls (PCBs) and dichlorodiphenyltrichloroethane (DDT), showed decreasing trends, generally beginning prior to the implementation of the United Nations Stockholm Convention on POPs in 2004.1,6,7 Compounds that came under regulation later, such as polybrominated diphenyl ethers (PBDEs) or perfluorooctane sulfonate (PFOS) regulated in 2009, showed mixed time trends, including shifts from increasing to decreasing concentrations.1,6 A few time series for chemicals under regulation in this time frame, including hexachlorobenzene (HCB) and β-hexachlorocyclohexane (HCH), were still increasing in concentration.1,6
As many Arctic species are part of indigenous peoples' traditional diets, monitoring of contaminant levels in biota also provides information important for food security and contaminant exposure to humans.8,9 In addition, long-term trends of POPs can be used to evaluate the effectiveness of global and regional regulations intended to decrease the concentrations of POPs in the environment. Similarly, Arctic time series can also be used to detect increasing trends of unregulated compounds in the Arctic environment and provide support for new regulatory actions.
The use of POP time trend data in chemicals management is based on the underlying premise that concentrations in biota are mainly determined by the amount of the compound emitted to the environment during production and use and therefore, can be controlled through regulations. Thus, increasing or decreasing concentrations of POPs in fish and wildlife tissues are mainly assumed to reflect changes in their primary emissions. However, contaminant levels in biota also integrate processes in the physical environment and in ecosystems, including changes related to climate change.10 Consequently, time series of POPs in Arctic biota could also be influenced by the direct and indirect environmental changes caused by a warming climate.11 Disturbances in the direct link between primary emissions and concentrations in biota can therefore complicate the interpretation of POP time series for effectiveness evaluations or risk assessments.
A correct description of the concentration developments of POPs in Arctic wildlife requires a better understanding of the effects of climate change on the fate of POPs, including their transport to and distribution in the Arctic as well as the exposure of Arctic animals to POPs. The limited availability of consistent, long-term monitoring data for pollutants has been identified as the largest barrier to studies investigating these influences of climate change on POP temporal trends.12 The long-term monitoring of POPs in Arctic biota, established by several Arctic countries decades ago, enables such investigations. In this article, we have reviewed and assessed studies from the Arctic that have examined associations between time series of POP concentrations and climate parameters, including physical parameters such as temperature and sea-ice extent, as well as ecological/biological parameters such as climate-related changes in species distributions and predator–prey relationships (Table 1). The article also includes some information on climate-related effects of POP time trends in Antarctic species, to complement findings from the Arctic. The objective of this review was thus to elucidate effects of climate change on the long-term time series of POPs in Arctic wildlife. While time trends themselves were assessed elsewhere,1,6 we focus on the state of knowledge of climate change-related impacts on these trends of POPs in Arctic wildlife.
| Freshwater environment | Terrestrial environment | Marine environment | ||
|---|---|---|---|---|
| Physical parameters | (Air) temperature, precipitation amounts, North Atlantic Oscillation (NAO) index, Arctic Oscillation (AO) index, turbidity (proxy for permafrost slumps) | Sea-ice cover (proxy for seal availability as prey) | (Air, land surface and seawater) temperature, precipitation amounts, NAO index, AO index, Pacific Decadal Oscillation (PDO) index, Pacific/North American (PNA) pattern, sea-ice cover (extent and time of break-up and freeze-up), salinity | |
| Biological parameters | Fish weight, chlorophyll a/primary productivity, lipid content, stable isotopes of nitrogen (δ15N) | Stable isotopes of carbon and nitrogen (δ13C; δ15N), food sources | Stable isotopes of carbon and nitrogen (δ13C; δ15N), algae blooms, food sources and composition, age, sex, body condition | |
| Species | Zooplankton, Arctic char (Salvelinus alpinus), burbot (Lota lota) | Arctic fox (Vulpes lagopus) | Emerald rockcod (Trematomus bernachii), Adélie penguins (Pygoscelis adeliae) | Common murre (Uria aalge), thick-billed murre (Uria lomvia), glaucous gull (Larus hyperboreus), northern fulmar (Fulmarus glacialis), beluga (Delphinapterus leucas), ringed seal (Pusa hispida), polar bear (Ursus maritimus) |
| Locations | Norway (Bjørnøya), Canada, Greenland | Norway (Svalbard) | Antarctica | Alaska, Norway (Svalbard, Bjørnøya), Canada, Greenland |
2. Approach
This review was conducted as part of the AMAP assessment “POPs and Chemicals of Emerging Arctic Concern (CEACs): influence of climate change”.13 The process started with a workshop in 2019 which was attended by approximately 40 scientists from Europe, North America and Asia, including non-Arctic countries, scrutinizing available information in this field and developing key science questions.14 This article includes peer-reviewed studies and scientific reports published by January 2022 and aims at a comprehensive review of the topic. However, as its focus is on the effects of climate change on long-term POP trends in Arctic biota, rather than a focus on the POP time series themselves, we have only included those studies that examined associations between POP concentrations with biological and/or physical parameters indicative of climate change. A list of the biological and physical parameters included in these studies is given in Table 1, which also provides a summary of the species studied and the parts of the Arctic where the studies took place. While the focus is on POPs in Arctic biota, representing the majority of the availably information, complementary data from the Antarctic have been included as well. These cover marine fish and birds as no freshwater fish or terrestrial mammals exist in Antarctica. The Arctic locations for the studies are shown in Fig. 1. Table S1 of the ESI† provides a list of all abbreviations used in this article. More details of the studies addressing links between temporal trends of POPs in Arctic and Antarctic biota and climate change is given in Table S2 of the ESI.†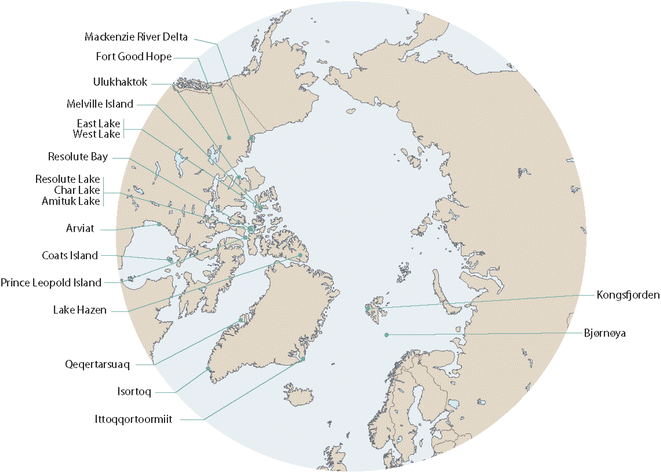 | ||
| Fig. 1 Locations of the studies referenced in this article. The figure was modified from ref. 13, with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
3. Freshwater environment
In remote Arctic lakes, POPs enter the environment primarily through river runoff and atmospheric deposition. Over the last century, POPs have accumulated in lake sediments and catchment areas. Since warming favours POP partitioning from particle and liquid phases to the gas phase, a greater tendency of volatilization from lake water into air has been suggested.11 Changes in air–water exchange will also affect the mass balance between water and sediments.11 In the Canadian Arctic, permafrost thawing has been associated with inputs of POPs to lake systems.153.1 Arctic char
Associations between climate parameters and temporal trends of POPs in Arctic char (Salvelinus alpinus) were investigated in four Canadian High Arctic lakes (Amituk, Hazen, Char, Resolute), an unnamed lake near Isortoq in Greenland and Lake Ellasjøen on Bjørnøya in the Norwegian Arctic (Fig. 1), using multiple regression approaches.13,15–17 The study from Canada investigated the relationship between temporal trends of POPs in fish and biological parameters (fish weight, chlorophyll a as a proxy for primary productivity in the lake) as well as physical parameters (temperature, annual precipitation amounts and interannual atmospheric climate fluctuations such as those represented by the North Atlantic Oscillation (NAO) index).15Temperature was found not to have a significant influence on Arctic char POP concentrations.15 However, air temperatures were used, which fluctuate more than lake water temperatures at the depths where Arctic char are primarily found, thus potentially introducing greater variability into the dataset. Significant negative correlations were detected between primary productivity (expressed as chlorophyll a) and PCB concentrations in the Arctic char of Amituk Lake and Resolute Lake. Relationships between the NAO index of the preceding spring/summer and concentrations of ΣPCBs and ΣDDTs in landlocked Arctic char from Lake Hazen in northern Ellesmere Island are shown in Fig. 2. Concentrations of ΣPCBs, ΣDDTs and ΣHCHs in Arctic char in this lake were positively associated with inter-annual variations of the NAO. Furthermore, concentrations of ΣHCHs were positively correlated with annual precipitation highlighting the importance of wet deposition pathways for HCHs to the Arctic lakes. The inclusion of climate parameters enhanced the explained variability of POP temporal trends in fish by up to 57% in comparison to regression models that did not include the same suite of parameters.15
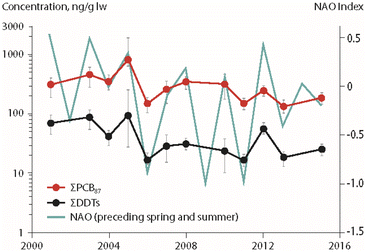 | ||
| Fig. 2 Concentrations of ΣPCBs and ΣDDTs (in ng g−1 lipid weight) in landlocked Arctic char from Lake Hazen (Northern Ellesmere Island, Canada), together with the North Atlantic Oscillation (NAO) index from spring of the year preceding sample collection.15 ΣPCBs represents the sum of 87 congeners. ΣDDTs represents the sum of p,p′- and o,p′-isomers for DDT, DDE and DDD. The figure is based on data from ref. 15. It is reproduced from ref. 13 with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
POP time trends for Arctic char were also analysed for relationships with biological parameters for growth, diet and trophic level (fish length, δ15N, lipid content) and physical climate parameters (air temperature) in two studies from Greenland.16,17 The first study, spanning the period from 1994–2008 and including five years with POP data, showed significant positive correlations between ΣPCBs and ΣDDTs and fish length.16 While air temperatures increased over the study period, lipid contents in fish as well as POP levels (except HCB) decreased, but potential connections between these parameters remained unclear.
An update of this study, covering eight years of POP data (ΣPCBs, PCB-153, ΣDDTs, HCB, α-HCH) collected between 1999 and 2017, included the same biological parameters as well as air temperature of the preceding summer and Arctic Oscillation (AO) index of the preceding winter.17 The study was based on lipid-normalized data. Fish length was again an important parameter for all POPs (except α-HCH), while δ15N values were only correlated to HCB concentrations, in a positive correlation. A positive association was also found between air temperature and concentrations of α-HCH, as shown in Fig. 3, as well as for air temperature and ΣPCBs.
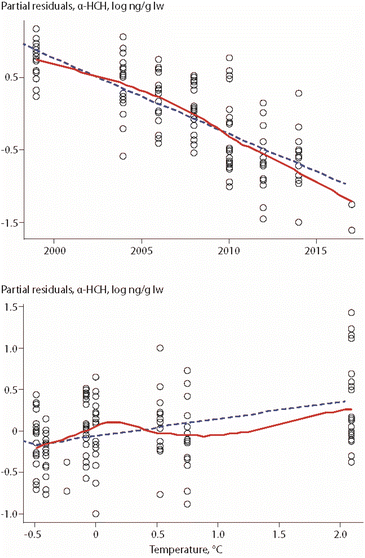 | ||
| Fig. 3 Relationships between α-HCH in Arctic char from Southwest Greenland, year and air temperature of the preceding summer.17 Open circles indicate individual animals, the dashed line shows the least squares regression, and the solid red line shows the smoothed trend. The figure is based on data from ref. 17. It is reproduced from ref. 13 with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
This result is different from findings reported from the Canadian Arctic where no correlations were found between air temperature and POP concentrations.15 Furthermore, while the Canadian study reported positive associations between the NAO index and some POP concentrations in Arctic char, no associations were found between the AO index and contaminants in Arctic char in Greenland.15,17 NAO and AO indices might not be directly comparable; however, these different findings could also indicate local differences between climate-related influences on POP concentrations in the freshwater environment.
Muscle tissue of Arctic char from Lake Ellasjøen (Bjørnøya) was collected from 1998–2017, with data for PCBs and HCB spanning 13 years. The concentrations of ΣPCBs decreased, while HCB concentrations in Arctic char only decreased until 2006 and stabilized thereafter.13 These trends were likely affected by changes in the population of glaucous gulls at Bjørnøya. Gull guano is an important vector of POPs to Lake Ellasjøen.18 Therefore, a decrease in gull numbers, in combination with reduced POP emissions, seems to be the main factor controlling the POP concentrations in Arctic char in Lake Ellasjøen.13 The cause for the decrease of the gull population is subject to ongoing research. The atmospheric temperature at Bjørnøya increased over the sampling period,19 but whether or not this temperature increase influenced POP levels in Arctic char, directly or indirectly via the gull population, is currently unknown.
3.2 Permafrost thawing
The particular role of permafrost thaw on POP trends in freshwater organisms has been studied in two lakes in the Canadian High Arctic, East Lake and West Lake on Melville Island (Fig. 1). Temporal trends of PCBs, organochlorine pesticides and PBDEs were investigated in zooplankton, landlocked Arctic char muscle and char stomach contents.20,21 West Lake is receiving greater inputs of terrestrial carbon from thawing permafrost, leading to higher turbidity. Together with these greater particulate inputs, higher lipid-based concentrations of PCBs, organochlorine pesticides and PBDEs have been observed in char muscle and stomach contents as well as in zooplankton from West Lake, as exemplified for ΣPCBs in Fig. 4.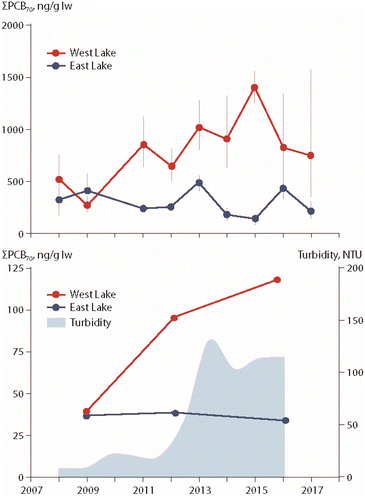 | ||
| Fig. 4 ΣPCB concentrations (in ng g−1 lipid weight) in muscle (upper figure) and pooled stomach contents (lower figure) of landlocked Arctic char from East Lake and West Lake on Melville Island, Canada, together with turbidity of West Lake (lower figure).21 ΣPCBs represents the sum of 70 congeners. The figure is based on data from ref. 21. It is reproduced from ref. 13 with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
The effect of lakeshore permafrost thaw and slumping on POP concentrations in freshwater biota was also studied in lakes of the Mackenzie River Delta Uplands in the Northwest Territories of Canada, although not in relation to a POP time trend.22 Amphipods (Gammarus sp.) in lakes affected by slumps had higher concentrations of POPs than amphipods in lakes without permafrost slumps. This was shown for ΣPCBs, ΣDDTs and ΣHCHs; however, the differences for ΣHCHs were smaller than for the other POPs.
While all studies showed higher POP concentrations in biota from freshwater systems impacted by permafrost thaw and slumping, the conditions differed greatly for the lakes under study. The slump-disturbed lakes of the Mackenzie River Delta Upland were oligotrophic, with reduced levels of dissolved and particulate organic carbon. The opposite was the case for West Lake on Melville Island, which showed greater turbidity and elevated levels of particulate matter than the lake not affected by permafrost thawing. These studies suggest that permafrost thaw can lead to inputs of POPs into remote lake, and accumulation in lake organisms, but pathways may differ.
3.3 Burbot
Potential associations were hypothesized between increases in PCB and DDT liver concentrations in burbot (Lota lota), primary productivity in lake-fed tributaries of the Mackenzie River near Fort Good Hope (Northwest Territories, Canada) and warmer temperatures.23 The study showed two-fold increases in concentrations of total hexachlorinated PCBs and three-fold increases in ΣDDTs in burbot liver over the period 2000–2008. As algal primary productivity also increased over this period, the authors suggested that climate warming could increase primary productivity, leading to higher concentrations of organic matter and, potentially higher availability to the fish.23 This association would be different from the findings reported for Arctic char on negative correlations between primary productions (expressed as chlorophyll a) and PCB concentrations in the char from High Arctic Canadian lakes.15 Subsequent annual sampling at Fort Good Hope showed declining concentrations of ΣPCBs and ΣDDTs in burbot liver from 2008–2012.243.4 Synthesis – freshwater environment
Studies from Canada and Greenland have shown correlations between biological and/or physical climate parameters and POP trends in Arctic char. However, these relationships were not consistent. For example, studies on the oscillation indices included both positive and negative associations with POP levels in freshwater fish: NAO showed a positive association with POP levels in Canadian fish, while AO did not show an association with POP levels in Arctic char from Greenland. It is unclear whether these inconsistencies are related to differences in study design, applicability of the oscillation indices or local differences in underlying processes. In addition, indirect effects may exist on the development of POP levels, for example levels in Arctic char from Bjørnøya seemed to be influenced by the number of gulls in the same location, which may or may not be related to climate change. Permafrost thawing leads to higher inputs of organic matter and POPs into lake systems, as shown in the Canadian Arctic, which is reflected in increasing POP concentrations in lake organisms. The interactions between organic matter and POPs are not well-understood, as also discussed for increasing POP concentrations in burbot, which may be related to increased organic matter in the river. In general, while several observations exist of influences of climate change on POP trends in freshwater biota, underlying mechanisms remain unclear at present.4. Terrestrial environment
4.1 Arctic fox
The only published studies linking long-term POP time trends in terrestrial animals with climate-related changes in the environment have been for Arctic fox (Vulpes lagopus) from Svalbard. Arctic foxes feed on prey from both terrestrial and marine ecosystems.25,26 Temporal trends of lipophilic POPs and perfluoroalkyl substances (PFAS) were studied in young Arctic foxes in relation to their feeding habits and food availability.27,28 The POP study included 141 liver samples collected between 1997 and 2016, while the PFAS study included 113 liver samples collected between 1997 and 2014.27,28 Feeding habits were characterised from stable carbon and nitrogen isotope values in fox muscle tissue. Reindeer and seal availability, i.e. availability of terrestrial and marine food sources, were further approximated from reindeer mortality and sea-ice cover, respectively.Time trends adjusted for diet and food availability (as covariates in the models, besides collection year) were similar to unadjusted time trends for most POPs (Fig. 5). A change was only observed for β-HCH, as no significant trend was found after correction for covariates. In general, POP concentrations were higher in Arctic foxes when a greater percentage of their diet was marine prey. β-HCH concentrations in the liver of Arctic foxes increased with increasing sea-ice cover, which was indicative of a higher availability of seals for Arctic foxes. Likewise, a negative association between β-HCH concentrations in Arctic foxes and reindeer mortality was close to being significant, indicating lower exposure of Arctic foxes to β-HCH when scavenging on reindeer carcasses.27 Similar associations were observed for other, but not for all POPs. The reasons for these differences between compounds remain unclear. A more complex prey composition, also including seabirds and geese, was discussed, besides location of denning habitats, age differences in the foxes and variable snow conditions as factors further influencing POP exposure in the individual foxes.27
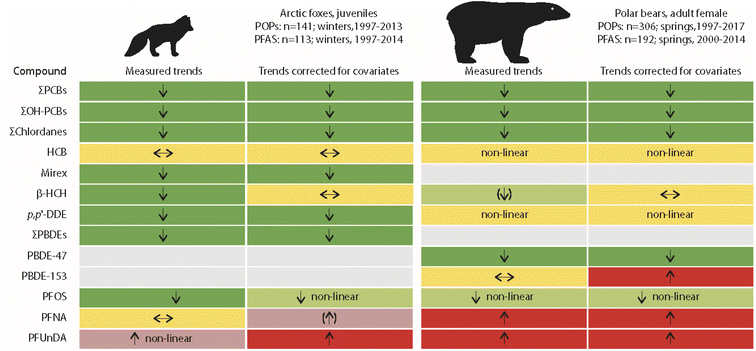 | ||
| Fig. 5 Comparisons of unadjusted and adjusted POP trends in livers of juvenile Arctic foxes and plasma of adult female polar bears from Svalbard.27–29 Arctic foxes: adjusted trends were corrected for climate-related changes in feeding habits and food availability. Polar bears: adjusted trends were corrected for body condition and feeding habits. Green, red and yellow colours indicate decreasing, increasing and no trends, respectively. Arrows in brackets indicate non-significant trends. The figure is based on data from ref. 27–29. It is reproduced from ref. 13 with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
More changes were observed for PFAS in Arctic foxes (Fig. 5). However, the authors concluded that “emission changes dwarfed the influence of feeding habits” on the changes of PFAS concentrations in Arctic foxes.28 PFOS concentrations in Arctic fox liver were positively associated with sea-ice cover, indicative of a higher availability of seals as prey. For the long-chain perfluorocarboxylic acids (PFCAs) (except perfluorotridecanoic acid, PFTrDA), a negative association was found with reindeer mortality. PFOS concentrations, adjusted for variation in feeding habits and food availability, decreased in Arctic foxes from 1997 to 2010 and were stable for the rest of the study period until 2014, whereas the unadjusted concentrations decreased throughout the entire study period (Fig. 5). As PFOS concentrations increased with sea-ice, the adjusted concentrations were higher than the unadjusted concentrations.28 Unadjusted concentrations of PFCAs only increased after 2003, while adjusted concentrations increased over the entire study period. Apparently, this was related to an increase in δ15N after 2003, which will be subject to future research.
4.2 Synthesis – terrestrial environment
The concentrations of several lipophilic POPs and PFAS in Arctic foxes were associated with climate-related variations in food availability and feeding habits. However, these variations only had a minor influence on temporal trends of the contaminants under study.5. Marine environment
5.1 Zooplankton and fish
No systematic Arctic time trends are available for zooplankton that could be analysed for influences from biological and/or physical climate parameters. However, four years of POP data (2007–2009, 2011) were available for zooplankton (Calanus sp.) from Svalbard, i.e. in Kongsfjorden on the west coast of Spitsbergen (Fig. 1). These were studied together with POP data for fish and seabirds with regard to seasonal variations in biomagnification.30–32 Trophic magnification factors (TMFs) were highest in the summer. Furthermore, the species sampled in July included higher numbers of boreal zooplankton and fish species than those sampled in spring and autumn. These seasonal changes, i.e. higher TMFs and more boreal species in the summer samples, could give an indication of future developments in a situation of a warming climate. However, other seasonal changes in the food web (e.g. in lipid dynamics) can also influence biomagnification and consequently, TMFs.33The enantiomeric fractions of chiral pesticides (α-HCH, trans-chlordane, cis-chlordane, oxychlordane) were determined in zooplankton collected from pack-ice north of Svalbard and in three Svalbard fjords that differed in extent and duration of ice coverage and influence of Arctic vs. North Atlantic water masses.34 Enantiomeric fractions in zooplankton varied between fjords and sampling years, due to differences in the timing of seasonal events and environmental conditions at the different locations. For example, the extent of ice cover, timing of the spring algae bloom and annual vertical migration from deep water to surface water were associated with differences in POP concentrations and patterns in zooplankton. The extent of ice cover can reduce air–ocean exchange of POPs, such as α-HCH, while α-HCH and other chiral molecules are degraded in the underlying waters in an enantiomer-selective way, i.e. through biological processes.
Marine fish from the Arctic have not been studied for climate-related changes in long-term POP concentrations. However, data are available for the emerald rockcod (Trematomus bernachii) from Ross Sea in the Antarctic.35–37 Long-term POP data from the early 1980s to 2011 showed a general decrease, but concentration peaks in 2001 and 2005 for PCBs and in 2005 for p,p′-DDE and PBDEs. The concentration peaks were ascribed to the release of POPs trapped in iceberg B15, which calved from the Ross Ice Shelf at the beginning of 2000 and broke apart in 2000, 2002 and 2003.38 In the Arctic, the direct effects of iceberg calving on POP concentrations in the marine environment have not been studied yet.
Some POPs have shown increasing concentrations in Antarctic fish, for example in benthic feeding humped rockcod (Gobionotothen gibberifrons) and two species of icefish (Chaenocephalus aceratus and C. gunnari).39–41 However, it is not known if these increases are related to climate change in any direct or indirect way. Re-mobilization of POPs under warming conditions has been mentioned as a possible explanation, besides secondary sources from environmental POP reservoirs.42,43
5.2 Seabirds
Long-term POP trends in Arctic seabirds exist for a number of species, locations and chemicals.44–46 POP data of seabirds from Alaska, Canada and Bjørnøya (Norway) have been studied in relation to influences from climate change. In addition, POP data in penguin eggs from Antarctica have also been analysed for associations with climate change.Contaminant data for eggs of thick-billed murre (Uria lomvia) and common murre (Uria aalge) collected under the Seabird Tissue Archival and Monitoring Project (STAMP) of Alaska have been studied for relationships between the Pacific Decadal Oscillation (PDO) and POP concentrations.47 Based on eggs collected from coastal areas of the Bering Sea and the Gulf of Alaska between 1999 and 2010, the authors reported highest levels of PCBs and chlorinated pesticides in thick-billed murres when the PDO index was close to zero.47 Lower levels were observed when the PDO index was at an extreme positive or negative value, reflecting warm and cool phases, respectively. These observations corresponded to changes in patterns in δ13C and δ15N values in the thick-billed murre eggs and might thus reflect influences of climate variability on POP levels via shifts in the diet of the birds.
In contrast, in sympatric common murres there were weak or null associations between the PDO index value and POP levels in eggs.47 These different patterns of association observed for the two bird species might reflect foraging differences between the species. Common murres tend to feed in the meso-pelagic zone closer to the colony, while thick-billed murres dive deeper and forage further from shore.48,49
Thick-billed murres breeding in the Canadian Arctic also showed changes in POP exposure as a result of changes in trophic position.50 In eggs collected from Coats Island, a Northern Hudson Bay colony (Fig. 1), between 1993 and 2013, positive relationships were detected between organochlorine chemicals and δ15N. As the birds changed their diet from Arctic cod (Arctogadus glacialis) to capelin (Mallotus villosus), resulting in lower δ15N values, POP concentrations concurrently declined. The diet change was related to changes in ice conditions in Hudson Bay.50 However, an increase in the trophic position of thick-billed murres at the high Arctic colony of Prince Leopold Island (Fig. 1) between 1975 and 2013 was negatively associated with key POPs such as p,p′-DDE and ΣPCBs. This finding was attributed to large reductions in emissions during the 1970s and 1980s, clearly outbalancing a potential increase in POP exposure through a shift in diet.50 After normalization of the POP data to δ15N, general linear models showed significant relationships between POP concentrations in thick-billed murre eggs from Coats Island and some physical climate parameters.13 Models incorporating sampling year with time-lagged AO and NAO indices were consistently among the best ranked models for predicting POP concentrations. Generally, POP concentrations were higher in murre eggs when time-lagged AO and NAO values were used, i.e. 1–3 years previous to sampling years. However, various seasonal relationships with AO and NAO indicated that some POP concentrations decreased in murre eggs when one-year time-lagged summer AO or NAO were greater. Other factors with a positive correlation with POPs in thick-billed murre eggs included greater coverage and earlier freeze-up of sea-ice. Higher air and land surface temperatures were most often related to decreasing concentrations of POPs.
Thick-billed murre eggs from Prince Leopold Island (1975–2014) also revealed correlations between POP concentrations and precipitation amounts, after a time-lag was accounted for.51 Years with increased precipitation were followed by higher concentrations of most compounds (most PCB congeners, p,p′-DDE, dieldrin, chlorobenzenes, octachlorostyrene), but decreased levels of oxychlordane in the murre eggs. Northern fulmars (Fulmarus glacialis) from the same location showed a positive correlation between POP concentrations and NAO index, also including a time-lag. The data suggested that years with NAO+ conditions were followed by higher concentrations of dieldrin, chlorobenzenes, chlordanes and mirex in fulmar eggs.51 However, the majority of variability in the data, in particular for legacy organochlorines, was related to changing emission patterns.
Seabird studies from the Norwegian Arctic focussed on glaucous gull (Larus hyperboreus). Concentrations of POPs (ΣPCBs, HCB, oxychlordane) in gull blood samples from Bjørnøya decreased over the study period (1997–2006), but increased with increasing AO index for the preceding summer and winter.52 In contrast, POP concentrations in gulls decreased with increasing AO index values of the same year. This was consistent with results for thick-billed murres and fulmars from Canada, while thick-billed murres from Alaska showed highest POP concentrations when the PDO index was close to zero.13,47 However, differences between these parameters and studies as well as species-specific differences might limit the comparability of these findings. When AO variation was taken into account in the temporal trend analysis, the POP concentrations in glaucous gulls declined slightly faster than when AO variation was not taken into account. This suggests that climate variability, determined by the AO index in 1997–2006, slowed the decline of POP concentrations in these birds.
The potential effects of climate change on DDT levels have been studied for Adélie penguins (Pygoscelis adeliae) from the Antarctic Peninsula.53 ΣDDT concentrations in Adélie penguin eggs from the Palmer Archipelago did not decrease from the 1970s to the 2000s, which is different from the observations reported for seabirds from the Arctic. The detection of p,p′-DDT in the penguin eggs, despite its worldwide severe restriction, suggests a current source of DDT in the Antarctic marine environment. Although very little recent DDT deposition was reported from the Antarctic, measurable levels were found in Antarctic glacial meltwater.54 The hypothesis of glacial meltwater as a possible secondary source of DDT for the marine environment was supported by measurement-based estimates of 1–4 kg ΣDDT being annually released from Antarctic glacial ablation.53 Since the 1950s, glaciers have retreated by almost 87% from the Antarctic Peninsula.55 Glacial meltwater inputs of DDT have also been linked to increased DDT levels in Antarctic freshwater lakes.56
In summary, the long POP time series available for some Arctic seabirds, as well as research on penguins in the Antarctic, provide useful datasets for studies on effects of climate change. However, as species-specific differences are likely to occur, besides differences in study design, it is difficult to draw any conclusions beyond the specific study, as was also discussed for the freshwater environment. Several studies have shown effects of changes in diet, possibly related to climate change, on POP exposure and consequently, POP levels in the time series. Amongst the physical climate parameters, the large-scale oscillation indices were found to be significantly associated with POP levels, usually with a positive association and a time-lag of at least one year. Other significant physical parameters were sea-ice extent, temperature and precipitation. Glacier melting as a significant secondary source was shown for the Antarctic. In contrast, primary emissions of POPs were still the main driver for the time trends in Arctic seabirds, but rates of concentration changes were found to be affected by climate-related parameters.
5.3 Marine mammals
Studies on the influence of climate change on POP concentrations in marine mammals have included ringed seals (Pusa hispida) from Canada and Greenland, beluga whale (Delphinapterus leucas) from Canada and polar bears (Ursus maritimus) from Canada, Greenland and Svalbard.Ice coverage data were integrated in a long-term study (1993–2008) of POPs in ringed seals from Ulukhaktok in western Canada.57 Higher levels of p,p′-DDE and PCB-153 were reported in seal blubber during years with early ice break-up. The authors suggested that this association could be related to increased foraging during years with long ice-free conditions.57 In another long-term study (1972–2016), several POP concentrations in seals from Resolute Bay, Arviat and the Beaufort Sea (Canada) were positively correlated with sea-ice coverage, suggesting an increased accumulation of POPs in years with greater ice extent.58 The study also showed that the type of ice (new vs. old ice) was differently associated with POP accumulation in seals (Fig. 6). In seals from Resolute Bay and the Beaufort Sea, many POPs were positively associated with multi-year ice coverage, but negatively correlated with new ice coverage.58
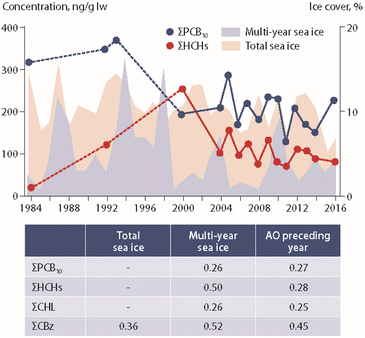 | ||
| Fig. 6 Concentrations of ΣPCBs and ΣHCHs (in ng g−1 lipid weight) in ringed seal blubber from Resolute Bay (Western Lancaster Sound, Canada) in relation to multi-year and total sea-ice coverage.58 Dashed lines indicate higher uncertainty of the curve due to few datapoints. ΣPCB10 shows the sum of ten PCB congeners. The table provides correlation coefficients between POP concentrations and climate parameters. AO: Arctic Oscillation index value. CHL: chlordanes. CBz: chlorobenzenes. The figure is based on data from ref. 58. It is reproduced from ref. 13 with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
A positive association between POP levels and sea-ice extent was also found for ringed seals from Qeqertarsuaq in West Greenland (Fig. 1), spanning a time trend from 1994–2016 with twelve years of data.17 However, these findings contrasted with results from a previous study based on a reduced dataset of nine years with data over the time period 1994–2010.59 The early study showed a negative relationship between the number of days with >50% ice coverage and the concentration of PCB-153 in seals, but not for the other compounds included in the study (PCB-52, p,p′-DDE, HCB, α-HCH, β-HCH).59 In the updated study, sea-ice extent of the preceding year was a major predictive variable for all compounds, except α-HCH, i.e. PCB-52, PCB-153, HCB and ΣDDTs (Fig. 7).17
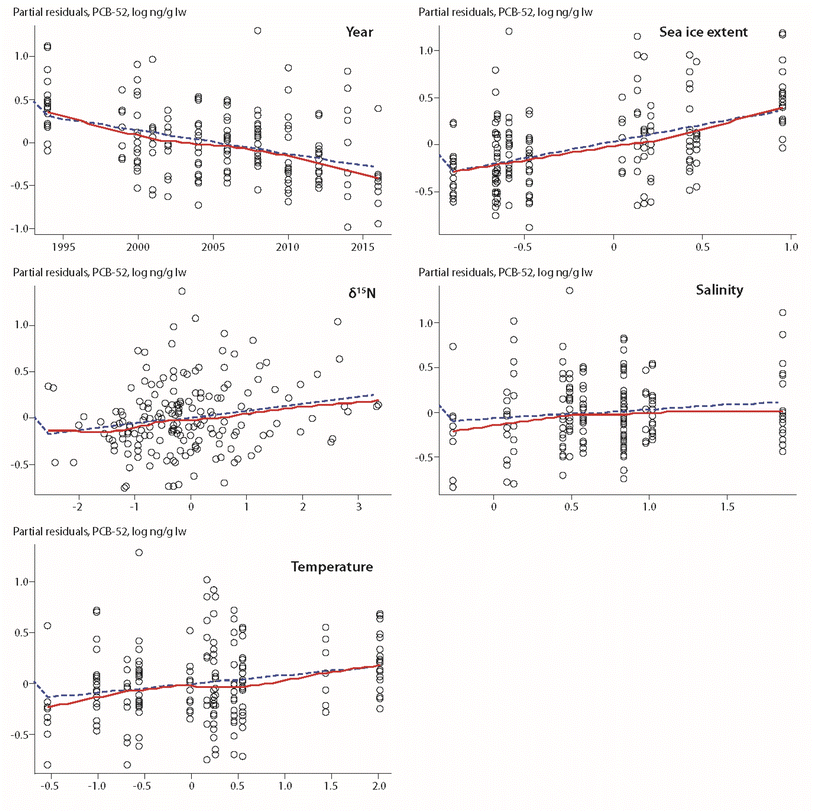 | ||
| Fig. 7 Partial residuals of PCB-52 in ringed seal blubber from West Greenland in relation to biological and physical climate parameters.17 Open circles indicate individual animals, the dashed line shows the least squares regression, the red solid line shows the smoothed trend. The figure is based on data from ref. 17. It is reproduced from ref. 13 with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
For ringed seals from Ittoqqortoormiit in East Greenland, however, sea-ice extent in the preceding year was not an important predictor for any of the compounds.17 This time series covered 14 years with data over the time period 1986–2016. The extent of sea-ice could influence the type and availability of prey, with consequences for POP exposure and lipid stores.17 Less sea-ice increases the air–water exchange of POPs, thus favouring volatilization and reducing the food web availability of volatile compounds such as α-HCH.11,60 At the same time, less sea-ice also decreases the uptake of POPs from ice and by ice-associated organisms. Both processes would lead to a reduction of POP exposure through the marine food web, consistent with positive associations between the extent of sea-ice and POP concentrations in the seals. However, differences in study design, in particular the statistical strength of the time series, might also be of significance for the outcome of the correlation analyses.
Positive associations were also reported between POP levels in ringed seals from Resolute Bay in the Canadian Arctic and the AO index value for the year preceding seal sampling (Fig. 7).58 Relationships were negative when AO information from the year of sampling was used. Correlations were also found between POP concentrations and the Pacific/North American pattern (PNA). In the Hudson Bay Area (Arviat), mainly positive associations were found between levels of POP groups (ΣPCBs, ΣDDTs, ΣHCHs, Σchlordanes) in seal blubber and the AO, NAO and PNA indices for the year of sampling or the preceding year.
POP concentrations in ringed seals from Qeqertarsuaq and Ittoqoortoormiit (Fig. 1) were studied in relation to AO index of the preceding winter.17,59 Both studies from Qeqertarsuaq showed negative associations between the AO index of the preceding winter and the α-HCH concentration in the seals. However, results were different for ringed seals from Ittoqqortoormiit, showing positive associations between the AO index of the preceding winter and concentrations of α-HCH and HCB, which is more in line with the result reported for ringed seals from Canada.58 The reasons for these different results are unknown. An increasing AO index might reflect increased transport of warmer air masses from mid-latitudes to the Arctic, potentially resulting in higher POP transport as well as reductions of sea-ice and enhanced volatilisation of volatile compounds such as α-HCH and HCB from seawater.17 This would likely result in a reduced availability for their uptake into food webs, as discussed for influences from reduced sea-ice extent, but mechanisms remain speculative.
Positive associations were also found between POP concentrations in ringed seals from West Greenland with seawater temperature as well as salinity, both measured in the summer preceding the sampling.17,59 The first study covering the years 1994–2010 showed positive correlations between salinity and the concentration of β-HCH in the ringed seals.59 In the updated study, salinity in the preceding summer was positively correlated with ΣPCBs and ΣDDTs (while β-HCH was not included in this study), as was seawater temperature with ΣPCBs, ΣDDTs, PCB-52 and PCB-153 (Fig. 7). Similarly, seawater temperature was positively correlated with HCB, but not with any other compounds, for ringed seals from Ittoqqortoormiit.17
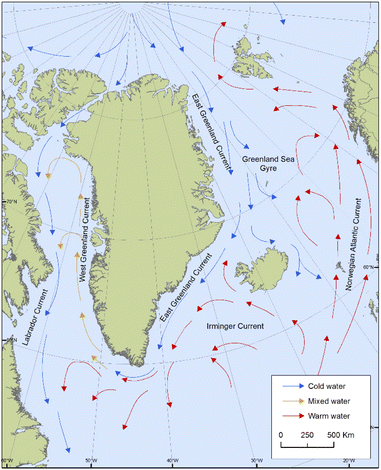
Positive associations shown for POP concentrations in ringed seals with salinity and seawater temperature in West Greenland might be reflective of a greater influx of warmer, higher salinity water, such as that of the Irminger Current, which carries water from the Northeast Atlantic northward (Fig. 8). A relatively larger influx from the Irminger Current compared to water from the Arctic Ocean could have implications for the transport of contaminants to West Greenland. Sea temperature was also an important predictor for HCB in East Greenland seals, which could be related to the air–water exchange processes of relatively volatile compounds like HCB.
Amongst the biological variables, the first study on ringed seals from Qeqertarsuaq showed that trophic position, as indicated by δ15N values, had a strong predictive power for HCB concentrations, indicating a positive association with diet, but the relationship was less strong for the other POPs.59 In the updated study, δ15N values and age were important predictors for PCB-52 (Fig. 6), but not the other contaminants, again showing the importance of the length of the time series for the identified correlations. Positive associations between δ15N values and POP concentrations were found for all compounds studied in the ringed seals from Ittoqqortoormiit.17 Likewise, age was associated positively with all POP concentrations in ringed seals from East Greenland, with the exception of α-HCH, which was negatively correlated with age, and HCB, which did not show a correlation with age. The study also addressed the effect of including biological and climate variables in calculations of annual POP concentration changes.17 This inclusion either reduced or enhanced the annual rate of POP concentration declines by factors ranging from 0.1 to 2.3, i.e. the impact on the overall decrease was relatively small and could change the trends in both directions.
Unlike the more extensively studied ringed seal, beluga was only studied for relationships of POP concentrations with biological parameters.61 PCB concentrations obtained in adult male beluga from the Eastern Beaufort Sea were associated with δ13C values, while dieldrin and mirex were associated with both δ13C and δ15N values. These results could be indicative of a shift in diet, and consequently, contaminant exposure over time, but this has not yet been studied in detail.61 Another study from Canada highlighted that climate-driven processes could influence the exposure of beluga whales to contaminants of emerging concern.62
Climate-related changes in feeding ecology and their implications for POP exposure of polar bears have been discussed for polar bears from the Hudson Bay and East Greenland.63–65 The first study reported for polar bears from Western Hudson Bay (1991–2007) that these bears had consumed less ice-associated bearded seals (Erignathus barbatus) and more open water-associated harbour seals (Phoca vitulina) and harp seals (Pagophilus groenlandicus) in years with shorter periods of ice coverage.63 The prey composition could be derived from stable isotope and fatty acid analysis. This change in diet corresponded with higher concentrations of brominated and chlorinated contaminants in polar bear fat, compared with a theoretical situation of no dietary changes.
Using a similar approach, dietary changes over time were also found for polar bears from East Greenland.64 Over the monitoring period from 1984 to 2011, ringed seals as the main prey species declined from 90% to 34% of the diet, while the consumption of sub-Arctic hooded seals (Cystophora cristata) and, potentially, harp seals increased from 0% to 26% of the bears' diet. An extension of these data to 2016 is shown in Fig. 9.65 The change in prey species was most pronounced for subadult polar bears, which represented the majority of the individuals included in the analysis (Fig. 9). The diet of adult male bears had always consisted of a higher proportion of harp seal, offering a potential explanation for the differences observed between age groups.65
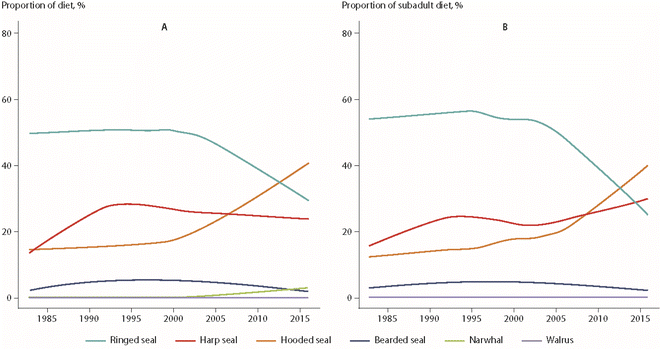 | ||
| Fig. 9 Percentages of prey species of polar bears from East Greenland, based on quantitative fatty acid signature analysis.65 (A) All bears. (B) Juvenile bears. The figure is based on data from ref. 65. It is reproduced from ref. 13 with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
Hooded seal consumption was negatively correlated with NAO, indicating that polar bears consumed more hooded seals in years with lower NAO index values.64 States of a stronger negative NAO index (NAO-) were associated with warmer temperatures and less sea-ice in East Greenland, potentially affecting accessibility of ringed seals on sea-ice. However, no statistical association was found between hooded seal consumption and the area of the East Greenland sea-ice.64 The dietary shift may also reflect better availability of sub-Arctic species moving northward. Their higher contaminant burdens likely influenced POP levels in the polar bears towards higher concentrations (Fig. 10). The parallel time trend for ringed seals, the main polar bear prey species, did not show the same increase in POP concentrations, indicating a different exposure source.65
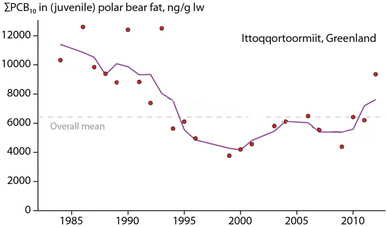 | ||
| Fig. 10 Concentration of ΣPCB (in ng g−1 lipid weight) in fat of juvenile polar bears from East Greenland. The figure is reproduced from ref. 6 with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2016. | ||
Based on the hypothesis that the observed POP time trend perturbations in the polar bears from East Greenland were related to changes in prey composition, POP concentrations were determined for potential polar bear prey species, for samples collected in 2015.65 As shown in Fig. 11, most of these species had higher POP concentrations than ringed seals, which is consistent with the observed changes in diet and POP time trends in the bears. Interestingly, a different pattern was observed for PFAS concentrations, as highest median concentrations of perfluorosulfonic acids and PFCAs were found in ringed seals, followed by harp seals and narwhal (Monodon monoceros).65 Consequently, in years with low percentages of ringed seals as prey species, polar bears had relatively low PFAS levels and higher POP levels. The opposite was the case in years with a high percentage of ringed seals in their prey.
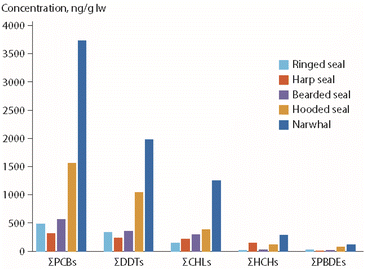 | ||
| Fig. 11 Median POP concentrations (in ng g−1 lipid weight) in blubber of potential prey species as identified for polar bears from East Greenland (Fig. 9).65 CHL: chlordanes. The figure is based on data from ref. 65. It is reproduced from ref. 13 with permission from the Arctic Monitoring and Assessment Programme, copyright 2021. | ||
Two recent studies have investigated temporal trends of POPs (including some PFAS compounds not currently regulated as POPs) in relation to polar bear feeding habits and body condition, for polar bears from Svalbard.28,29 Results for changes in δ13C and δ15N values over time (2000–2017) indicated that the winter diet of the bears shifted towards less marine, less ice-associated and lower trophic level prey items, likely as a consequence of receding sea-ice. Body condition also varied over time (1997–2017), however, including periods of weight loss and weight gain. The temporal development of POPs is summarized in Fig. 5, including effects of adjustments for dietary tracers and body condition. While body condition and diet had a significant influence on POP concentrations in polar bears, the changes introduced by these biological factors were not pronounced enough to significantly alter the time trends for POPs.29,66 Only β-HCH and PBDE-153 showed a change in the overall trend when adjusted for body condition and dietary tracers. For PFAS, the direction of the trends did not change, but the increase was steeper for concentrations of PFCAs adjusted for dietary changes.28 The studies suggest that the long-term trends of these POPs and unregulated PFAS are still predominantly determined by contaminant emissions and that climate-related variations in body condition and feeding habits only have a minor effect.
Focusing on a variety of climate parameters, general linear models indicated subtle adjustments to annual contaminant trends (of PCB-153, PBDE-47, p,p′-DDE, α-HCH and PFOS) in polar bears from Hudson Bay when climate factors were included.13 Many results were similar to those found for thick-billed murres from Hudson Bay, as discussed above.13 Models that incorporated sampling year with time-lagged AO or NAO index values were among the best ranked models for describing contaminant concentrations in polar bears, generally with positive associations between POP concentrations and time-lagged AO or NAO indices. Results for polar bears also varied with sea-ice-related parameters, e.g. later freeze-up dates were correlated with higher concentrations of PBDE-47 and p,p′-DDE. Inverse relationships were found for POP concentrations in the polar bears and time-lagged air and land surface temperatures as well as amount of precipitation in the preceding autumn and winter. Greater snowfall may impede seal predation by the polar bears as has been observed in spring when bear predation on seals was negatively correlated to snow depth.67,68
In summary, the studies on ringed seals and polar bear identify sea-ice as an important parameter in explaining changes in POP concentrations although the results are not consistent and the mechanisms are not understood. For polar bears, significant changes in prey species have been shown, with consequences for POP exposure and noticeable perturbations of the POP time trend. Similarly, the studies from Svalbard have shown changes in feeding habits for the polar bears. As discussed for seabirds, the AO or NAO indices were also correlated with POP concentrations in ringed seals and polar bears, usually with a lag phase. Other relevant parameters include salinity, water and air temperatures and precipitation, but no consistent results emerge as yet. The studies on ringed seals and polar bears from multiple locations would allow a spatial assessment of climate change influences, potentially contributing to a better understanding of the local or regional impacts, as also discussed for Arctic char. However, while methodologies are largely comparable for the established POP time trends, studies of linkages to climate change differ in their approaches and limit comparability at present.
5.4 Synthesis – marine environment
Multiple correlations have been identified between POP concentrations in marine organisms and physical and/or biological parameters. Amongst the physical climate parameters, sea-ice extent (in space and time) and the oscillation indices consistently showed associations with POP concentrations, usually positive ones and, in the case of AO and NAO indices, with a time-lag of a few years. Possible explanations for these observations have been put forward, but a mechanistic understanding is generally lacking. The effects of major iceberg calving events or glacier melts on POP concentrations have been shown for the Antarctic, but not yet for the POP time series from the Arctic. For both seabirds and marine mammals, changes in POP exposure have been related to changes in prey species. Thus, variations in the trophic position as a result of dietary changes can have direct effects on POP levels in time trends, which underlines the importance of characterising trophic position in biota monitoring studies. The current data only show subtle climate-related changes in the long-term POP time series, which can lead to small deviations in declining trends, but which do not change the overall direction of the trend. Thus, reductions in primary emissions seem to remain the main driver of POP temporal trends. However, the data for thick-billed murres and ringed seals suggest that the length of the time series is an important factor in this correlation analysis. As the main change in POP concentrations took place prior to the year 2000, time series including those changes are currently less affected by recently occurring perturbations associated with climate change.6. Conclusions
Datasets from POP monitoring programmes in the Arctic exist that have documented the development of POP concentrations in Arctic wildlife over the last few decades. Some of these datasets have been linked with climate parameters in statistical analyses. So far, this has only been pursued in few studies, leaving a great potential to extend this work and validate the current findings. In addition, if time trends become available for compounds other than well-studied POPs, it will be particularly relevant to assess them for associations with climate-related parameters, as their physical–chemical properties, sources and emissions may deviate from those of POPs. This could provide new insights, especially as current findings might not be valid for compounds with different physical–chemical properties or ongoing primary emissions.A number of studies have identified potential relationships between POP concentrations in Arctic biota and physical climate-related parameters, including climate oscillation indices, sea-ice extent, temperature and precipitation (Fig. 12). However, whether these correlations really represent causal relationships between climate change and POP concentrations in Arctic animals and what processes and mechanisms drive these relationships, is still largely unknown. Further knowledge gaps with regard to the influence of climate change on contaminants in the Arctic were summarized elsewhere.14
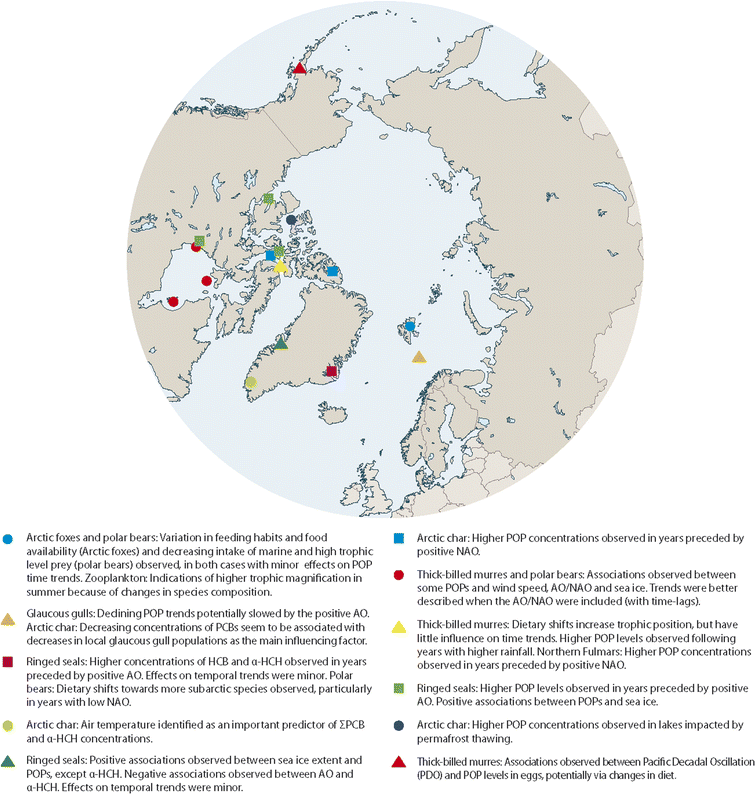 | ||
| Fig. 12 Summary of associations observed for POP concentrations in time series of Arctic biota with physical and/or biological parameters. The figure was modified from ref. 13, with permission from the Arctic Monitoring and Assessment Programme (AMAP), copyright 2021. | ||
In some cases, the strength or direction of the statistical associations varied between locations, species and compounds. Given the relatively low number of studies examining links between climate-related parameters and POP concentrations it is not yet possible to conclude whether these variations indicated locally different phenomena or were mainly caused by differences between the studies, e.g. in the statistical power of a time series or the statistical approach chosen for analysis. However, positive associations between POP concentrations and sea-ice extent as well as oscillation indices, usually with a lag-phase for the independent variables, have been substantiated in a number of studies. Effects of thawing permafrost on POP levels in landlocked lakes of the Canadian Arctic have been shown, presumably via increased inputs of particle-associated POPs. Data from the Antarctic have related peaks in the temporal development of POPs in fish to iceberg calving. These effects have not been studied in other regions yet.
Most studies have shown climate change influences on temporal trends for HCHs and HCB. This could represent a research bias as volatile compounds might be targeted specifically because of an expectation of most pronounced effects in a warming climate. However, the studies generally addressed multiple POPs, including HCHs and HCB. Therefore, the effects found for HCHs and HCB could indicate that processes might be involved where volatility plays a role, for example air–water exchanges as the water becomes warmer and the ice cover is reduced. Again, these mechanisms, linking climate change to alterations in compound availability and uptake in food chains, are far from being understood.
Deviations from generally decreasing trends of POPs were seen in some species in association with ecosystem change and particularly with changes in predator–prey relationships, for example for polar bears increasingly preying on sub-Arctic species with higher POP levels (Fig. 12). Changes in trophic levels were also observed for seabirds. These ecosystems are in turn driven by climate-related changes in the physical environment, such as warming seawater or reduced sea-ice coverage, creating a complexity beyond direct cause–effect relationships. The effects of climate change on accumulation of POPs in Arctic food webs are further explored elsewhere.10
The long-term POP series from the Arctic are important indicators for the effectiveness evaluation of the Stockholm Convention on POPs, based on an assumption of levels in Arctic biota reflecting changes in primary emissions. The results of this assessment suggest that primary emissions are still the main driver of the POP time trends observed in Arctic biota and that their ban leads to decreasing POP concentrations in Arctic biota. However, for some compounds and some species, climate change was identified as an additional factor influencing the rate of concentration declines over time. There are some indications that longer time series are more robust to these climate-related effects which may have been exerting a stronger influence on POP concentrations in biota in more recent years. Studies from Canada indicate a time-lag between changes in climate parameters and POP concentrations, suggesting more pronounced effects in the future. It will be relevant and increasingly important to include climate parameters in monitoring campaigns for POPs, with biological parameters for the characterisations of trophic position, via well-established stable isotope analysis, being particularly pertinent for Arctic wildlife.
7. Recommendations for future research and monitoring
The assessment of associations between physical and/or biological parameters related to climate change and contaminant concentrations in Arctic biota, obtained in long-term national monitoring programmes or from ESB archives, has identified a number of parameters directly or indirectly influencing POP levels in biota. Given the rapid changes in the Arctic, work in this area should be intensified to generate mechanistic understanding and to ensure the validity of the long POP time series in the Arctic. Specifically, the following knowledge gaps have emerged from this assessment of long-term POP time series in relation to climate change, which will benefit from more research and monitoring in the future. A more comprehensive discussion of knowledge gaps, not limited to biota monitoring in the Arctic, is given elsewhere.14• Current monitoring programmes should continue and ensure that auxiliary biological parameters are included, in particular those characterising trophic levels, e.g. δ15N. This will enable a better understanding of effects of dietary changes on POP exposure as well as adjustments of the long-term time series.
• More time series that are available of POPs in Arctic biota should be analysed for associations with climate-related parameters to validate current findings. Extensions to other species and locations will be relevant, to improve our knowledge and achieve larger geographical coverage.
• Studies linking POP trends and climate parameters have been limited to a few compounds so far. The spectrum of chemical and statistical analyses should be expanded to include contaminants with a different use history and other physical–chemical properties than hydrophobic POPs. Extending the list of chemicals could also provide relevant data for identification and risk assessments of CEACs.
• Modelling approaches can support evaluations of climate change on the temporal development of POP concentrations in biota. These can include transport and bioaccumulation models for compounds with different use histories and physical–chemical properties.
• A better use of existing data and research dedicated to mechanistic understanding can improve our understanding of the processes underlying the relationships that have been identified. Oscillation indices and sea-ice coverage seem to be two parameters of recurring significance whose impact on POP trends in biota needs further study. Both parameters showed associations with POP concentrations in biota where these were addressed, with the exception of the study on AO influences on POPs in Arctic char from Greenland.17
• We lack understanding of the geographical extent of certain influences, ranging from specific lakes impacted by permafrost to oscillation indices indicative of large-scale air movements. Coordinated approaches with harmonized research and monitoring strategies could address the significance of local or regional phenomena affecting POP time trends. It will be important to know in the evaluation of time trends what geographical scale they represent.
• Studies in the Arctic and Antarctic could yield complementary information. Both will be needed for a better understanding of the global fate of contaminants. With regard to the topic of this assessment, Antarctic studies may benefit from a more systematic POP monitoring, while Arctic studies should consider the impact of iceberg calving and glacier melting on POP time series.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge Jennifer Balmer for editing the AMAP assessment report and collaboration partners in Arctic communities for providing samples to the national monitoring programmes. All national agencies are acknowledged for their contaminant monitoring programmes. KV received funding from the programme “Miljøstøtte til Arktis” of the Danish Environmental Protection Agency for her contribution to the AMAP assessment report.References
- F. Rigét, A. Bignert, B. Braune, M. Dam, R. Dietz, M. Evans, N. Green, H. Gunnlaugsdóttir, K. S. Hoydal, J. Kucklick, R. Letcher, D. Muir, S. Schuur, C. Sonne, G. Stern, G. Tomy, K. Vorkamp and S. Wilson, Temporal trends of persistent organic pollutants in Arctic marine and freshwater biota, Sci. Total Environ., 2019, 649, 99–110 CrossRef.
- S. Corsolini, Antarctica and NE Greenland: Marine Pollution in a Changing World, in Life Below Water. Encyclopedia of the UN Sustainable Development Goals, ed. W. Leal Filho, A. M. Azul, L. Brandli, A. Lange Salvia and T. Wall, Springer, Cham, 2021, DOI:10.1007/978-3-319-71064-8_150-1.
- P. R. Becker, B. J. Koster, S. A. Wise and R. Zeisler, Biological specimen banking in Arctic research: an Alaska perspective, Sci. Total Environ., 1993, 139–140, 69–95 CrossRef CAS PubMed.
- P. R. Becker and S. A. Wise, The U.S. National Biomonitoring Specimen Bank and the Marine Environmental Specimen Bank, J. Environ. Monit., 2006, 8, 795–799 RSC.
- T. Odsjö, The environmental specimen bank, Swedish Museum of Natural History – a base for contaminant monitoring and environmental research, J. Environ. Monit., 2006, 8, 791–794 RSC.
- AMAP, AMAP Assessment 2015: Temporal Trends in Persistent Organic Pollutants in the Arctic, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2016, vi + 71 pp Search PubMed.
- UNEP, Stockholm Convention on Persistent Organic Pollutants (POPs), Text and Annexes, Revised in 2019, United Nations Environment Programme (UNEP), September 2019 Search PubMed.
- D. Kinloch, H. Kuhnlein and D. C. G. Muir, Inuit foods and diet: a preliminary assessment of benefits and risks, Sci. Total Environ., 1992, 122, 247–278 CrossRef CAS.
- AMAP, Human Health in the Arctic, Summary for Policy Makers, Arctic Monitoring and Assessment Programme (AMAP), Tromsø, Norway, 2021 Search PubMed.
- K. Borgå, M. A. McKinney, H. Routti, K. J. Fernie, J. Giebichenstein, I. Hallanger and D. C. G. Muir, The influence of global climate change on accumulation and toxicity of persistent organic pollutants and chemicals of emerging concern in Arctic food webs, Environ. Sci.: Processes Impacts, 2022 10.1039/d1em00469g.
- J. Ma, H. Hung and R. W. Macdonald, The influence of global climate change on the environmental fate of persistent organic pollutants: a review with emphasis on the northern hemisphere and the Arctic as a receptor, Global and Planetary Change, 2016, 146, 89–108 CrossRef.
- R. W. Macdonald, T. Harner and J. Fyfe, Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data, Sci. Total Environ., 2005, 342, 5–86 CrossRef CAS.
- AMAP, AMAP Assessment 2020: POPs and Chemicals of Emerging Arctic Concern: Influence of Climate Change, Arctic Monitoring and Assessment Programme (AMAP), Tromsø, Norway, 2021, viii + 142 pp Search PubMed.
- C. A. de Wit, K. Vorkamp and D. Muir, Influence of climate change on persistent organic pollutants and chemicals of emerging concern in the Arctic: state of knowledge and recommendations for future research, Environ. Sci.: Processes Impacts, 2022 10.1039/d1em00531f.
- A. Cabrerizo, D. C. Muir, G. Köck, D. Iqaluk and X. Wang, Climatic influence on temporal trends of polychlorinated biphenyls and organochlorine pesticides in landlocked char from lakes in the Canadian High Arctic, Environ. Sci. Technol., 2018, 52, 10380–10390 CrossRef CAS PubMed.
- F. Rigét, K. Vorkamp and D. Muir, Temporal trends of contaminants in Arctic char (Salvelinus alpinus) from a small lake, southwest Greenland during a warming climate, J. Environ. Monit., 2010, 12, 2252–2258 RSC.
- F. Rigét, K. Vorkamp, I. Eulaers and R. Dietz, Influence of climate and biological variables on temporal trends of persistent organic pollutants in Arctic char and ringed seals from Greenland, Environ. Sci.: Processes Impacts, 2020, 22, 993–1005 RSC.
- A. Evenset, J. Carroll, G. N. Christensen, R. Kallenborn, D. Gregor and G. W. Gabrielsen, Seabird guano is an efficient conveyer of persistent organic pollutants (POPs) to Arctic lake ecosystems, Environ. Sci. Technol., 2007, 41, 1173–1179 CrossRef CAS.
- MOSJ, Environmental Monitoring at Svalbard and Jan Mayen, 2021, https://www.mosj.no Search PubMed.
- A. Cabrerizo, D. C. G. Muir, C. Teixeira, S. F. Lamoureux and M. J. Lafrenière, Snow deposition and melting as drivers of polychlorinated biphenyls and organochlorine pesticides in Arctic rivers, lakes, and ocean, Environ. Sci. Technol., 2019, 53, 14377–14386 CrossRef CAS.
- A. Cabrerizo, D. C. Muir, A. O. De Silva, S. Lamoureux and M. Lafrenière, Influence of permafrost disturbances on temporal trends of legacy and emerging persistent organic pollutants in landlocked Arctic char from lakes in the Canadian High Arctic, in Society of Environmental Toxicology and Chemistry (SETAC) Europe 29th Annual Meeting, Helsinki, Finland, 26–30 May 2019 Search PubMed.
- R. D'Onofrio, Effect of permafrost thaw slumps on benthic invertebrates and on concentrations of persistent organic pollutants in lakes of the Mackenzie Delta Uplands, NT, MSc Thesis, Department of Biology, University of Ottawa, Ontario, 2014.
- J. Carrie, F. Wang, H. Sanei, R. W. Macdonald, P. M. Outridge and G. A. Stern, Increasing contaminant burdens in an Arctic fish, burbot (Lota lota), in a warming climate, Environ. Sci. Technol., 2010, 44, 316–322 CrossRef CAS PubMed.
- G. A. Stern, J. Carrie and A. Burt, Temporal trend studies of trace metals and halogenated organic contaminants (HOCs), including new and emerging persistent compounds, in Mackenzie River burbot, Fort Good Hope, NWT, in Synopsis of Research Conducted under the 2013-2014 Northern Contaminants Program, Aboriginal Affairs and Northern Development Canada, Ottawa, Ontario, 2014, pp. 271–280 Search PubMed.
- N. E. Eide, P. M. Eid, P. Prestrud and J. E. Swenson, Dietary responses of arctic foxes Alopex lagopus to changing prey availability across an Arctic landscape, Wildlife Biology, 2005, 11, 109–121 CrossRef.
- D. Ehrich, R. Ims, N. Yoccoz, N. Lecomte, S. Killengreen, E. Fuglei, A. Rodnikova, B. Ebbinge, I. Menyushina, B. Nolet, I. Pokrovsky, I. Popov, N. Schmidt, A. Sokolov, N. Sokolova and V. Sokolov, What can stable isotope analysis of top predator tissues contribute to monitoring of tundra ecosystems?, Ecosystems, 2015, 18, 404–416 CrossRef CAS.
- M. S. Andersen, E. Fuglei, M. König, I. Lipasti, Å. Ø. Pedersen, A. Polder, N. G. Yoccoz and H. Routti, Levels and temporal trends of persistent organic pollutants (POPs) in arctic foxes (Vulpes lagopus) from Svalbard in relation to dietary habits and food availability, Sci. Total Environ., 2015, 511, 112–122 CrossRef CAS PubMed.
- H. Routti, J. Aars, E. Fuglei, L. Hanssen, K. Lone, A. Polder, Å. Ø. Pedersen, S. Tartu, J. M. Welker and N. G. Yoccoz, Emission changes dwarf the influence of feeding habits on temporal trends of per- and polyfluoroalkyl substances in two Arctic top predators, Environ. Sci. Technol., 2017, 51, 11996–12006 CrossRef CAS PubMed.
- A. Lippold, S. Bourgeon, J. Aars, M. Andersen, A. Polder, J. L. Lyche, J. Bytingsvik, B. M. Jenssen, A. E. Derocher, J. M. Welker and H. Routti, Temporal trends of persistent organic pollutants in Barents Sea polar bears (Ursus maritimus) in relation to changes in feeding habits and body condition, Environ. Sci. Technol., 2019, 53, 984–995 CrossRef CAS PubMed.
- I. G. Hallanger, A. Ruus, D. Herzke, N. A. Warner, A. Evenset, E. S. Heimstad, G. W. Gabrielsen and K. Borgå, Influence of season, location, and feeding strategy on bioaccumulation of halogenated organic contaminants in Arctic marine zooplankton, Environ. Toxicol. Chem., 2011, 30, 77–87 CrossRef CAS PubMed.
- I. G. Hallanger, A. Ruus, N. A. Warner, D. Herzke, A. Evenset, M. Schøyen, G. W. Gabrielsen and K. Borgå, Differences between Arctic and Atlantic fjord systems on bioaccumulation of persistent organic pollutants in zooplankton from Svalbard, Sci. Total Environ., 2011, 409, 2783–2795 CrossRef CAS PubMed.
- I. G. Hallanger, N. A. Warner, A. Ruus, A. Evenset, G. Christensen, D. Herzke, G. W. Gabrielsen and K. Borgå, Seasonality in contaminant accumulation in Arctic marine pelagic food webs using trophic magnification factor as a measure of bioaccumulation, Environ. Toxicol. Chem., 2011, 30, 1026–1035 CrossRef CAS PubMed.
- P. Carlsson, J. H. Christensen, K. Borgå, R. Kallenborn, K. Aspmo Pfaffhuber, J. Ø. Odland, L.-O. Reiersen and J. Pawlak, Influence of Climate Change on Transport, Levels, and Effects of Contaminants in Northern Areas, AMAP Technical Report No. 10, Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, 2016 Search PubMed.
- P. Carlsson, N. A. Warner, I. G. Hallanger, D. Herzke and R. Kallenborn, Spatial and temporal distribution of chiral pesticides in Calanus spp. from three Arctic fjords, Environ. Pollut., 2014, 192, 154–161 CrossRef CAS PubMed.
- S. Corsolini, A. Covaci, N. Ademollo, S. Focardi and P. Schepens, Occurrence of organochlorine pesticides (OCPs) and their enantiomeric signatures, and concentrations of polybrominated diphenyl ethers (PBDEs) in the Adélie penguin food web, Antarctica, Environ. Pollut., 2006, 140, 371–382 CrossRef CAS PubMed.
- N. Borghesi, S. Corsolini and S. Focardi, Levels of polybrominated diphenyl ethers (PBDEs) and organochlorine pollutants in two species of Antarctic fish (Chionodraco hamatus and Trematomus bernacchii), Chemosphere, 2008, 73, 155–160 CrossRef CAS PubMed.
- A. Cincinelli, T. Martellini, T. K. Pozo, P. Kukučka, O. Audy and S. Corsolini, Trematomus bernacchii as an indicator of POP temporal trend in the Antarctic seawaters, Environ. Pollut., 2016, 217, 19–25 CrossRef CAS PubMed.
- NIC, World's largest ever recorded iceberg continues to break apart near Antarctica, News Release JW-15-01, National Ice Center (NIC), Washington, DC, USA, 16 September 2014, http://www.natice.noaa.gov/doc/NATICE_Press%20Release_Ant%20Iceberg_9_16_2014_released.pdf Search PubMed.
- S. Corsolini, T. Romeo, N. Ademollo, S. Greco and S. Focardi, POPs in key species of marine Antarctic ecosystem, Microchem. J., 2002, 73, 187–193 CrossRef CAS.
- S. Corsolini, Industrial contaminants in Antarctic biota, J. Chromatogr. A, 2009, 1216, 598–612 CrossRef CAS PubMed.
- A. Strobel, P. Schmid, H. Segner, P. Burkhardt-Holm and M. Zennegg, Persistent organic pollutants in tissues of white-blooded Antarctic fish Champsocephalus gunnari and Chaenocephalus aceratus, Chemosphere, 2016, 161, 555–562 CrossRef CAS PubMed.
- A. Cabrerizo, J. Dachs, D. Barceló and K. C. Jones, Climatic and biogeochemical controls on the remobilization and reservoirs of persistent organic pollutants in Antarctica, Environ. Sci. Technol., 2013, 47, 4299–4306 CrossRef CAS PubMed.
- N. B. Lana, P. Berton, A. Covaci, N. F. Ciocco, E. Barrera-Oro, A. Atencio and J. C. Altamirano, Fingerprint of persistent organic pollutants in tissues of Antarctic notothenioid fish, Sci. Total Environ., 2014, 499, 89–98 CrossRef CAS PubMed.
- K. Vorkamp, J. H. Christensen, M. Glasius and F. Riget, Persistent halogenated compounds in black guillemots (Cepphus grylle) from Greenland – levels, compound patterns and spatial trends, Mar. Pollut. Bull., 2004, 48, 111–121 CrossRef CAS.
- B. M. Braune, Temporal trends of organochlorines and mercury in seabird eggs from the Canadian Arctic, 1975-2003, Environ. Pollut., 2007, 148, 599–613 CrossRef CAS PubMed.
- J. Verreault, G. W. Gabrielsen and J. O. Bustnes, The Svabard glaucous gull as bioindicator species in the European Arctic: insight from 35 years of contaminants research, in Reviews of Environmental Contamination and Toxicology, ed. D. M. Whitacre, H. N. Nigg and D. R. Doerge, Springer Science+Business Media, LLC, 2010, vol. 205, pp. 77–106, ISBN 978-1-4419-5622-4, DOI:10.1007/978-1-4419-5623-1_2.
- V. Kalia, S. S. Schuur, K. A. Hobson, H. H. Chang, L. A. Waller, S. R. Hare and M. O. Gribble, Relationship between the Pacific Decadal Oscillation (PDO) and persistent organic pollutants in sympatric Alaskan seabird (Uria aalge and U. lomvia) eggs between 1990 and 2010, Chemosphere, 2021, 262, 127520 CrossRef CAS PubMed.
- D. G. Ainley, D. N. Nettleship, H. R. Carter and A. E. Storey, Common murre (Uria aalge), in Birds of the World, ed. S. M. Billerman, Cornell Lab of Ornithology, 2020, DOI:10.2173/bow.commur.01.
- A. J. Gaston and J. M. Hipfner, Thick-billed murre (Uria lomvia), in Birds of the World, ed. S. M. Billerman, Cornell Lab of Ornithology, 2020, DOI:10.2173/bow.thbmur.01.
- B. M. Braune, A. J. Gaston, K. A. Hobson, H. G. Gilchrist and M. L. Mallory, Changes in trophic position affect rates of contaminant decline at two seabird colonies in the Canadian Arctic, Ecotoxicol. Environ. Saf., 2015, 115, 7–13 CrossRef CAS PubMed.
- K. L. Foster, B. M. Braune, A. J. Gaston and M. L. Mallory, Climate influence on legacy organochlorine pollutants in Arctic seabirds, Environ. Sci. Technol., 2019, 53, 2518–2528 CrossRef CAS PubMed.
- J. O. Bustnes, G. W. Gabrielsen and J. Verreault, Climate variability and temporal trends of persistent organic pollutants in the Arctic: a study of glaucous gulls, Environ. Sci. Technol., 2010, 44, 3155–3161 CrossRef CAS PubMed.
- H. N. Geisz, R. M. Dickhut, M. A. Cochran, W. R. Fraser and H. W. Ducklow, Melting glaciers: a probable source of DDT to the Antarctic marine ecosystem, Environ. Sci. Technol., 2008, 42, 3958–3962 CrossRef CAS PubMed.
- A. L. Chiuchiolo, R. M. Dickhut, M. A. Cochran and H. W. Ducklow, Persistent organic pollutants at the base of the Antarctic marine food web, Environ. Sci. Technol., 2004, 38, 3551–3557 CrossRef CAS PubMed.
- A. J. Cook, A. J. Fox, D. G. Vaughan and J. G. Ferrigno, Retreating glacier fronts on the Antarctic Peninsula over the past half-century, Science, 2005, 308, 541–544 CrossRef CAS PubMed.
- L. Sun, X. Yin, C. Pan and Y. Wang, A 50-years record of dichloro-diphenyl-trichloroethanes and hexachlorocyclohexanes in lake sediments and penguin droppings on King George Island, maritime Antarctic, J. Environ. Sci., 2005, 17, 899–905 CAS.
- A. Gaden, S. H. Ferguson, L. Harwood, H. Melling, J. Alikamik and G. A. Stern, Western Canadian Arctic ringed seal organic contaminant trends in relation to sea ice break-up, Environ. Sci. Technol., 2012, 46, 4427–4433 CrossRef CAS PubMed.
- M. Houde, X. Wang, T. L. L. Colson, P. Gagnon, S. H. Ferguson, M. G. Ikonomou, C. Dubetz, R. F. Addison and D. C. G. Muir, Trends of persistent organic pollutants in ringed seals (Phoca hispida) from the Canadian Arctic, Sci. Total Environ., 2019, 665, 1135–1146 CrossRef CAS PubMed.
- F. Rigét, K. Vorkamp, K. A. Hobson, D. C. Muir and R. Dietz, Temporal trends of selected POPs and the potential influence of climate variability in a Greenland ringed seal population, Environ. Sci.: Processes Impacts, 2013, 15, 1706–1716 RSC.
- R. Bossi, C. A. Skjøth and H. Skov, Three years (2008-2010) of measurements of atmospheric concentrations of organochlorine pesticides (OCPs) at Station Nord, North-East Greenland, Environ. Sci.: Processes Impacts, 2013, 15, 2213–2219 RSC.
- M. Noël, L. L. Loseto and G. Stern, Legacy contaminants in the eastern Beaufort Sea beluga whales (Delphinapterus leucas): are temporal trends reflecting regulations?, Arctic Science, 2018, 4, 373–387 Search PubMed.
- T. A. Smythe, L. L. Loseto, A. Bignert, B. Rosenberg, W. Budakowski, T. Halldorson, K. Pleskach and G. T. Tomy, Temporal trends of brominated and fluorinated contaminants in Canadian Arctic beluga (Delphinapterus leucas), Arctic Science, 2018, 4, 388–404 Search PubMed.
- M. A. McKinney, E. Peacock and R. J. Letcher, Sea ice-associated diet change increases the levels of chlorinated and brominated contaminants in polar bears, Environ. Sci. Technol., 2009, 43, 4334–4339 CrossRef CAS PubMed.
- M. A. McKinney, S. J. Iverson, A. T. Fisk, C. Sonne, F. F. Rigét, R. J. Letcher, M. T. Arts, E. W. Born, A. Rosing-Asvid and R. Dietz, Global change effects on the long-term feeding ecology and contaminant exposures of East Greenland polar bears, Global Change Biol., 2013, 19, 2360–2372 CrossRef PubMed.
- R. Dietz, F. F. Rigét, I. Eulaers, J.-P. Desforges, K. Vorkamp, R. Bossi, J. Søndergaard, P. Ambus, M. McKinney, R. J. Letcher and C. Sonne, Unexpected increases of persistent organic pollutants and mercury levels in East Greenland polar bears (UNEXPECTED), Technical Report No. 214, Aarhus University, DCE – Danish Centre for Environment and Energy, 2021, p. 44, http://dce2.au.dk/pub/TR214.pdf Search PubMed.
- S. Tartu, S. Bourgeon, J. Aars, M. Andersen, A. Polder, G. W. Thiemann, J. M. Welker and H. Routti, Sea ice-associated decline in body condition leads to increased concentrations of lipophilic pollutants in polar bears (Ursus maritimus) from Svalbard, Norway, Sci. Total Environ., 2017, 576, 409–419 CrossRef CAS PubMed.
- M. O. Hammill and T. G. Smith, The role of predation in the ecology of the ringed seal in Barrow Strait, Northwest Territories, Canada, Mar. Mammal Sci., 1991, 7, 123–135 CrossRef.
- S. Ferguson, I. Stirling and P. D. McLoughlin, Climate change and ringed seal (Phoca hispida) recruitment in western Hudson Bay, Mar. Mammal Sci., 2005, 21, 121–135 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2em00134a |
| This journal is © The Royal Society of Chemistry 2022 |