Insights into the past and future of Janus-di-N-heterocyclic carbenes
Macarena
Poyatos
 and
Eduardo
Peris
and
Eduardo
Peris
 *
*
Institute of Advanced Materials (INAM), Universitat Jaume I, Av. Vicente Sos Baynat s/n, Castellón, E-1271, Spain. E-mail: eperis@uji.es; Web: http://www.twitter.com/Peris_qomcat
First published on 19th July 2021
Abstract
Janus di-N-heterocyclic carbene (NHC) ligands are a subclass of poly-NHCs that feature coordination to two transition metals in a facially opposed manner. The combination of the structural features of Janus type ligands, with the properties conferred by the NHC ligands, has conferred Janus-di-NHCs with privileged attributes for their use in diverse areas of research, such as homogeneous catalysis, materials chemistry and supramolecular chemistry. In molecular chemistry, Janus di-NHCs constitute one of the most useful chemical platforms for constructing dimetallic structures, and this includes both homo- and hetero-dimetallic compounds. This review aims to cover the most relevant advances in the use of Janus-di-NHCs during the last 15 years, by classifying them according to their specific structural features.
A. Introduction
During the past three decades, N-heterocyclic carbene ligands (NHCs) have provided a new dimension to the design of new organometallic complexes with applications that already transcend their well-recognized success in homogeneous catalysis,1 and now cover fields such as supramolecular chemistry,2 materials chemistry3 or biomedicine.4 The development of new modular scaffolds for linking transition metals is crucial for expanding the boundaries of these scientific areas. In fact, most of the success of NHCs resides in the limitless access to a variety of topologies with properties that can be adapted to the particular requirements needed for achieving specific applications. This adaptability, or tunability, of NHCs made us recently refer to them as SMART ligands,1a as a way to highlight their ability to adapt to new times and needs for novel reactivities.Among NHCs, poly-NHCs have attracted a great share of attention because they allow the preparation of metal complexes with a large variety of architectures,5 which may result from bischelating, pincer, tripodal or bridging coordination modes. Di-NHC ligands featuring geometrically isolated carbenes bound by rigid systems constitute a very interesting subclass of poly-NHCs because they can give access to structurally dynamic homopolymers,6 metallosupramolecular architectures,2 or dimetallic catalysts that may show enhanced properties compared to their monometallic analogues.7 Di-NHC ligands that can feature coordination to two transition metals in a facially opposed manner are referred to as Janus-di-NHCs, due to their analogy to the roman god Janus, usually depicted as having two faces looking in opposite directions, one looking to the future and the other looking to the past (Fig. 1). Although the term Janus in molecular chemistry was first used to refer to ‘Janus molecules’ (or Janus-head molecules) in 1990,8 it was not until 2002 that it was specifically used to refer to facially opposed ditopic ligands.9
The purpose of this review is to describe all types of Janus di-NHCs ligands, by considering their distinctive constitutional features, their coordination abilities and their most relevant applications. Considering their different geometrical and constitutional features, we have classified the types of Janus-di-NHCs as follows: (i) Janus-di-NHCs formed by a single heterocyclic ring, or N-heterocylic-di-carbenes (NHDCs), (ii) di-carbenes with polycyclic aromatic hydrocarbon spacers, (iii) di-carbenes connected by ‘single’ C–C bonds, (iv) di-carbenes linked by fused non-aromatic cycles, and (v) di-carbenes fused to metalloporphyrins.
The review will also cover some rigid ditopic di-NHC ligands that do not strictly facilitate a facially opposed coordination, but do facilitate the coordination of two metals in a ‘quasi’-directionally opposed manner. The structural features of these special types of Janus ligands will be conveniently discussed as they appear along the text.
B. Janus di-NHCs based on a single heterocyclic ring: N-heterocyclic di-carbenes (NHDCs)
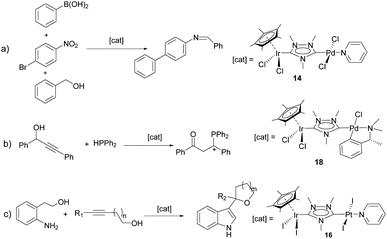
N-Heterocyclic-di-carbenes (NHDCs) are di-NHCs based on a single N-heterocyclic ring. The first described Janus di-NHC ligand was 1,2,4-triazol-3,5-diylidene published by Bertrand and co-workers in 1997.10 This NHDC (which we eventually called ditz) was first coordinated to silver yielding a one-dimensional polymeric compound that was fully characterized, including its single crystal X-ray diffraction structure. In 2007, we were able to obtain discrete dimetallic complexes of rhodium and iridium based on ditz (2a and 2b in Scheme 1) and even described the preparation of the first hetero-dimetallic di-NHC complex by the sequential deprotonation of the starting trimethyl triazolium dication salt (1) and subsequent coordination to iridium(I) and rhodium(I) (Scheme 1).11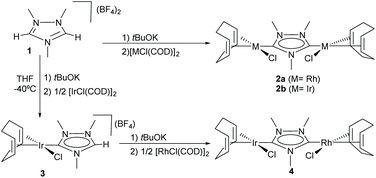 | ||
| Scheme 1 First discrete homo- and heterodimetallic complexes based on a triazole-di-ylidene (ditz) ligand. | ||
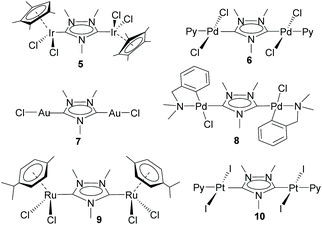
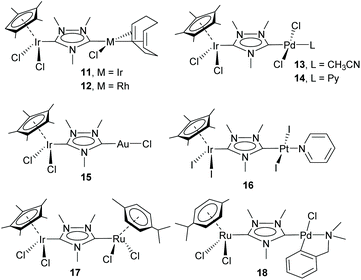
This study opened the door to the preparation of a large series of homo- and heterodimetallic complexes based on the ditz ligand, with metals of the platinum group and gold. In this regard, during the following years, the ditz ligand showed great coordination versatility, and allowed the preparation of homo-dimetallic complexes of iridium,11,12 rhodium,11 ruthenium,13 palladium,14 platinum15 and gold,16 some of which are depicted in Scheme 2. This di-NHC ligand established a metal-to-metal separation of ca. 7 Å. The hetero-dimetallic compounds obtained included complexes of Ir/Rh,11,12 Ir/Pd,17 Ir/Pt,15 Ir/Au16 and Ru/Pd,18 as those shown in Scheme 3. It was thought that the study of these complexes as homogeneous catalysts would provide insights into the possible catalytic cooperativity between the two metals comprised in the dimetallic unit.
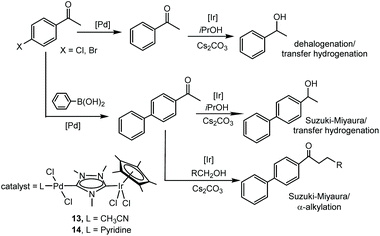
Probably, one of the most interesting features of the heterodimetallic complexes shown in Scheme 3, is their ability to catalyse tandem processes by mediating two fundamentally different reactions, a feature that was described in detail in a previous review article.7 Here, we will refer briefly to some relevant examples. For instance, the Ir/Pd complexes 13 and 14 were very active in the dehalogenation/transfer hydrogenation of haloacetophenones, in the Suzuki–Miyaura coupling/transfer hydrogenation to afford biphenylated secondary alcohols, and in the Suzuki–Miyaura coupling/α-alkylation of haloacetophenones to yield biphenylated-alkylated ketones, as shown in Scheme 4.17a Interestingly, equimolecular mixtures of the homodimetallic complexes 5 and 6, were much less efficient in all these three reactions, therefore strongly suggesting the positive cooperation of the Ir and Pd catalytic partners in the heterometallic complexes.
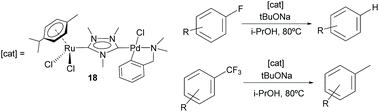
Additionally, the Ru/Pd complex 18 was very efficient in the hydrodefluorination of a wide variety of fluoroarenes and trifluorotoluenes, affording quantitative yields in very short reaction times, and very mild reaction conditions (iPrOH, 80 °C), as shown in Scheme 5.18b In this reaction, the palladium unit cleaves the C–F bond, while the ruthenium part of the catalyst facilitates the reductive step of the reaction, by transfer hydrogenation using a secondary alcohol and a base. Complex 18 is one of the most efficient catalysts for this type of reaction.19 Again, the activity shown by an equimolecular mixture of the homodimetallic complexes (8 and 9), was far less efficient than that shown by 18, thus adding support to the idea of the catalytic cooperativity between the two vicinal metals in 18.18b
Other interesting examples of tandem catalytic reactions facilitated by the heterodimetallic complexes shown in Scheme 3 are the synthesis of diphenyl imines by the direct reaction between phenyl boronic acid, nitroarenes and primary alcohols catalyzed by 14 (Scheme 6a),17b the isomerization followed by the asymmetric hydrophosphination of 1,3-diphenylpropargyl alcohol catalysed by 18 (Scheme 6b),17c and the oxidative cyclization of an amino alcohol to form a molecule of indole, which is subsequently added to the unsaturated bond of an enol ether formed by the cycloisomerization of an alkynyl alcohol (Scheme 6c), a process mediated by Ir/Pt complex 16.15
The analysis of the electronic properties of the triazole-di-ylidene ligand ditz, was performed independently using cyclic voltammetry (CV) and DFT studies. The CV studies revealed that the metal-to-metal electronic communication on dimetallic complexes bridged by the ditz ligand is weak,18a although stronger than the couplings displayed by dimetallic complexes linked by other larger Janus-di-NHCs.3d,20 The electron-donating properties of the ligand were determined by DFT studies by considering that one of the edges of the ligand is coordinated to Ni(CO)3, while the other end is coordinated to different transition metals. The study showed that the Tolman electronic parameter (TEP) of ditz is strongly dependent on the nature of the metal bound to the ligand, and may vary as much as Δ(TEP) = 7.5 cm−1, thus exemplifying a way to quantify the metal-to-metal coupling for ditz-bound heterometallic complexes.21
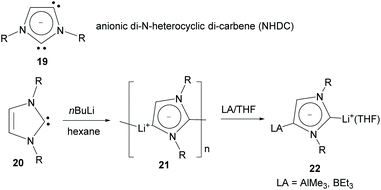
Also using a five-member heterocyclic ring, in 2006 Arnold and co-workers reported the preparation of potassium/lanthanide complexes bridged by an anionic imidazolyl-di-ylidene (NHDC, 19 in Scheme 7) exhibiting normal and abnormal coordination modes.22 In 2010, Robinson and co-workers reported the preparation of lithium–NHDC polymeric compound 21 from the reaction of imidazolylidene 20 with nBuLi in hexane at room temperature.23 The dicarbene character of 21 was unambiguously demonstrated by the formation of group 13 Lewis acid adducts, such as 22.
These two seminal studies describing the use of imidazole-based NHDCs were followed by a series of studies in which a series of anionic dicarbene complexes of main group elements were described, and a series of review articles were published on this topic.24 With regard to transition metals, by using the same anionic imidazol-di-ylidene scaffold, a series of dimetallic complexes of Zn(II),25 Au,25b and Pd26 were described (Scheme 8).
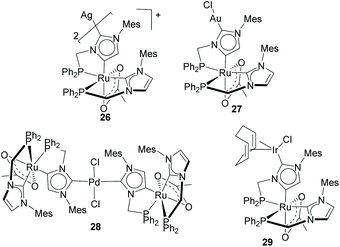
Baratta and Kühn used the same type of imidazole-based NHDCs for the preparation of several heterodimetallic complexes combining Ru(II) with Ag(I),27 Au(I),27 Pd(II)28 and Ir(I),29 as the ones depicted in Scheme 9. All these complexes were obtained starting from a cationic Ru(II) complex bearing a chelating phosphine–aNHC ligand. The C2–H deprotonation of one of the aNHC moieties facilitates the coordination of the second metal unit. Ru/Pd complex 28 was tested in the tandem Suzuki–Miyaura/transfer hydrogenation of bromoacetophenones and boronic acids to form biphenyl alcohols, where it proved to be more active than the combination of the related monometallic species.28 The same authors also performed detailed electrochemical studies and showed how the Ru(II)/Ru(III) redox potential is sensitive to changes produced on the nature of the metal unit bound to the normal edge of the NHDC ligand, thus suggesting that the metals are electronically coupled along the π-system of the heterocyclic ligand.29
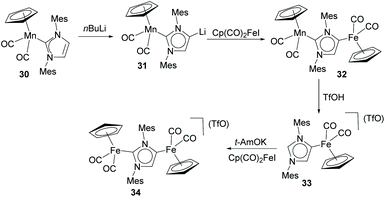
In a more recent contribution, Lugan, César and Vayaev, described the preparation of a series of dimetallic complexes of Fe and Mn/Fe, from the stepwise metalation of IMes (IMes = 1,3-bis(2,4,6-trimethylphenyl)-2H-imidazol-2-ylidene), as shown in Scheme 10.30 The characterization of the Mn/Li heterodimetallic complex 31 had been described by the same authors in a previous study.31 The authors demonstrated that the electron-donating properties of both the normal and abnormal edges of the NHDC are increased by the metalation at the C4 and C2 positions. This observation is explained as a consequence of the simultaneous increase of the σ-donation and decrease of the π-back donation of the two carbenic sides, although the effect is more pronounced for the abnormal carbenic part. The authors also tested dimetallic Fe complex 34 in the hydrosilylation of ketones, where it proved to be more active than the related monometallic complexes of Fe bound to normal and abnormal (33) IMes.30
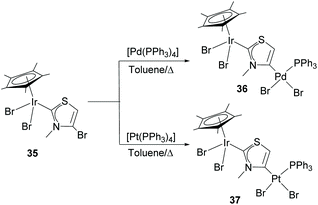
In a very recent report, Hahn and co-workers described the preparation of heterobimetallic complexes through the 2,4-metallation of a bromo-substituted thiazolium salt. The complexes were prepared by the initial reaction of the bromo-thiazolium salt with Ag2O followed by a reaction with [IrCp*Br2]2 to give monometallic complex 35.32 The presence of the C–Br bond at the NHC ligand of 35 allowed subsequent C–Br oxidative addition to [M(PPh3)4] (M = Pd or Pt) to give hetero-dimetallic complexes 36 and 37 bound by what can be regarded as an anionic thiazole-di-ylidene ligand, as shown in Scheme 11.
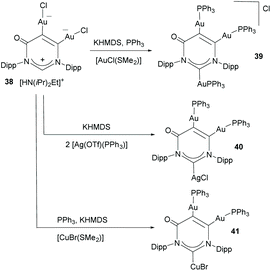
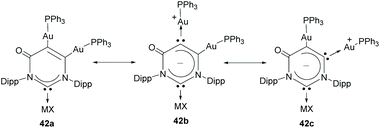
Janus di-NHCs based on six-membered heterocycles have also been described in the recent literature. In 2018, Zhang and co-workers described a tritopic carbanionic NHC bound to coinage metals.33 The complexes were obtained by deprotonation of a ditopic NHC digold complex (38), as shown in Scheme 12. The carbene character of the donor groups in this tritopic NHDC was established on the grounds of structural, spectroscopic and computational analysis. In this regard, the authors demonstrated that from the three possible mesomeric structures shown in Scheme 13, those showing the di-NHC character of the complex (42b and 42c) are the major contributing resonance species.33
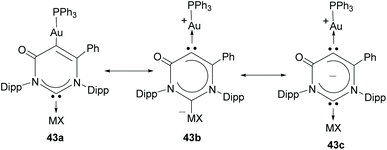
In a more recent contribution, the same authors described a six-membered Janus ditopic NHC that was used for the preparation of a digold complex, and a heterometallic complex of Au/Ag.34 Although this type of Janus ligand can be viewed formally as a di-carbene (see mesomeric structure 43c in Scheme 14), the authors demonstrated that mesomeric structure 43a, featuring the mono-carbene nature of the ligand, is the major contributing resonance structure; therefore this ditopic six-membered NHC is not really a part of the group of Janus-di-NHCs, but is closely related to them.
C. Janus di-NHCs with fused polycyclic aromatic hydrocarbon spacers
Di-NHCs bound by fused polyconjugated hydrocarbons constitute the largest group of Janus di-NHC ligands. Fused polycyclic aromatic hydrocarbons confer rigidity to the di-NHC ligand, and open up for electronic delocalization between each carbene moiety, and consequently, between the metals bound to the two edges of the di-NHC ligand. The first of such a type of Janus-di-NHC described, and undoubtedly the one that has been used more widely, is the benzo-fused-di-NHC motif 44 that was described by Bielawski and co-workers in 2005 (Scheme 15).35 This di-carbene scaffold was first used for the synthesis of organometallic polymers of Pd, Pt and Ni, as shown in Scheme 15.35,36 These seminal studies constituted elegant examples of the rational preparation of main-chain organometallic polymers, in which transition metals and linear organic ligands constitute the primary components of the backbone of the polymers,37 a structural feature that can be translated into interesting physical, electronic, optical and catalytic properties. An interesting feature of polymer 45 (M = Pd, X = Br), is that it was utilized by Karimi and co-workers as a reusable self-supported catalyst for the Suzuki–Miyaura coupling of even unactivated aryl chlorides and fluorides in water.38 The same authors were able to make this type of palladium polymer water-soluble by replacing the N-wingtips with triethylene glycol chains. This maintained its catalytic activity in the Suzuki–Miyaura coupling while increasing its recyclability properties.39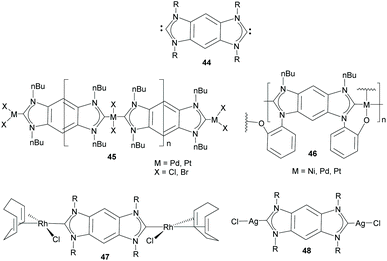 | ||
| Scheme 15 Benzo-bis-imidazolylidene and some related main chain organometallic polymers and discrete dimetallic complexes. | ||
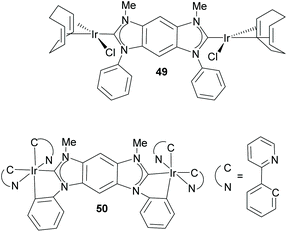
Bielawski and co-workers also described the first discrete homobimetallic complexes based on benzo-fused-di-NHC 44. These were di-rhodium and di-silver complexes 47 and 48 shown in Scheme 15.40 The ligand established a metal-to-metal separation of 10.5 Å. The electronic communication between the metals bound to 44 was also investigated by Bielawski and co-workers using the di-iridium complexes 49 and 50 (Scheme 16).20b The electrochemical studies on 49 demonstrated that the oxidation of one of the Ir(I) units had an influence on the oxidation of the other iridium center (ΔE1/2 ≈ 60 mV). However, this electrochemical coupling was not observed for the di-iridium(III) complex 50, probably due to a less effective NHC–M orbital overlap in 50 than in 49. The electronic communication between the metals in 50 was also studied by means of its luminescence properties, which showed that the emission profile of 50 was superimposable with that of its monometallic analogue. This made the authors conclude that there was no communication between the metals bound by the di-NHC ligand.20b
Albrecht and co-workers also made detailed studies in order to determine the ability of benzo-bis-imidazolylidene ligand 44 to transfer electronic information between metal centers.41 Their studies were first performed using di-Fe(II) and di-Ru(II) complexes 51 and 52, depicted in Scheme 17. The cyclic voltammetry (CV) studies showed that, while the di-Fe(II) complex (51) showed two oxidations that are separated by ΔE1/2 = 80 mV, the di-Ru complex (52) did not show any measurable coupling between the two oxidation processes. The weak (for 51) and negligible (for 52) intermetallic coupling was initially attributed to a combination of the limited conjugation between the imidazolylidenes and the central arene fragment, and the weak orbital dMetal–πNHC overlap due to an unfavorable arrangement of the ligand plane and the metal coordination system that reduces the π-character of the M–CNHC bond.20a,41 Although these results seemed to indicate that the benzo-bis-imidazolylidene ligand is unsuitable for facilitating intermetallic communication, in 2013 the same research group demonstrated that by using rigidly planar chelating wingtip groups, this di-NHC ligand became effective for imparting electronic coupling between the two pseudo-octahedral ruthenium centers in 53 and 54 (Scheme 17).42 CV studies on these complexes revealed couplings of 244 mV and 134 mV, for 53 and 54, respectively. This was explained to be a consequence of the alignment of the metal 4d and ligand π orbitals to facilitate an effective dMetal–πNHC overlap, thus demonstrating that the electronic communication is determined by the relative orientation of the metal center with respect to the ligand, and not due to the inherent properties of the di-NHC ligand.42
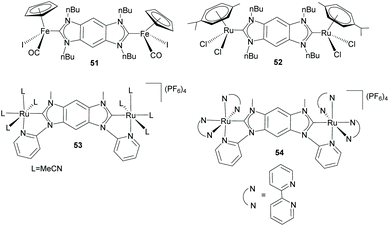 | ||
| Scheme 17 Benzo-bis-imidazolylidene complexes of Fe and Ru used for studying the metal-to-metal electronic communication. | ||
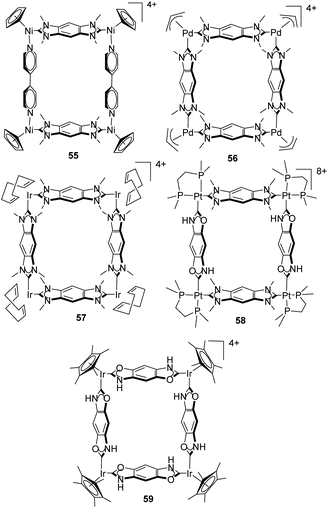
The benzo-bis-NHC ligand 44 has been demonstrated to be particularly useful for the construction of metallosupramolecular assemblies. Hahn and co-workers pioneered the introduction of organometallic chemistry in the field of metallosupramolecular chemistry by describing a series of metallosquares and metallorectangles of Pt,43 Ni,44 Ir,45 Pd45b and Rh,45a some of which are displayed in Scheme 18. The structures of the palladium and iridium tetrametallic assemblies 56 and 57, are based on four benzo-bis-imidazolylidene linkers, while nickel metallorectangle 55 combines two benzo-bis-imidazolylidenes with two 4,4′-dipyridines. Platinum metallosquare 58 is supported by two benzo-di-imidazolylidenes and two NH,O-substituted benzo-fused di-carbenes. Finally, metallosquare 59 is supported by four di(NH,O–NHC) ligands, which actually constitute another interesting example of Janus benzo-fused di-NHCs. The preparation of these NH,O–NHCs is performed using β-hydroxyphenyl isocyanides using suitable metal templates, a method developed by Hahn and co-workers.46
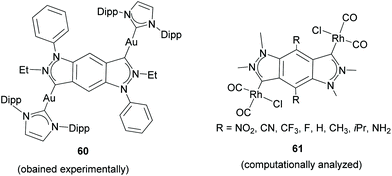
Related to these types of Janus di-NHCs, in 2016 the group of Breher described a benzo-fused di-pyrazolylidene and its coordination to gold (60, in Scheme 19).47 The authors performed detailed experimental and computational studies (on Rh-based model complexes 61) in order to determine the ability of the ligand to mediate electronic metal-to-metal communication, and concluded that this coupling is a direct function of the number of metal–ligand contacts, in line with the findings published by Albrecht and co-workers for Janus-bis-imidazolylidene 44.42 Although the benzo-bis-NHC ligand in 60 and 61 does not establish the coordination to the two metals strictly in a ‘directionally opposed’ manner, we consider it a close relative of the Janus-di-NHC family.
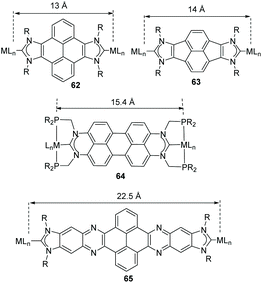
Together with the benzo-fused-di-NHCs mentioned above, a series of di-carbenes connected by polycyclic aromatic hydrocarbons (PAHs) were prepared and coordinated to a number of transition metals. This series of rigid and poly-conjugated Janus di-NHCs constitute a useful library of ligands that provide a suitable tool for modulating the metal-to-metal separation from the 7 Å established by the ditz ligand, to the 22.5 Å for quinoxalinophenanthrophenazine-connected di-NHC 65 (Scheme 20).
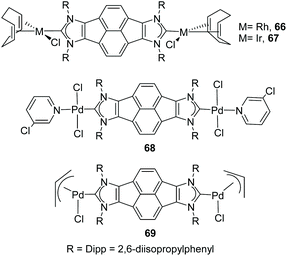
The first described PAH-connected di-NHC ligand among those shown in Scheme 20 was the pyracene-bis-imidazolylidene ligand in 63, which was obtained by us in collaboration with Alcarazo in 2012.20c This Janus-di-NHC was coordinated to Ir(I) and Rh(I) to afford complexes 66 and 67, which can be seen in Scheme 21. The CV analysis of iridium complex 67, revealed that the complex displays two Ir(I)/Ir(II) oxidation bands that are separated by 70 mV, therefore indicating that the two metals are weakly electronically connected. In 2013, we used the same ligand for the preparation of palladium complexes 68 and 69, which were tested as catalysts for the acylation of aryl halides with hydrocinnamaldehyde, and in the Suzuki–Miyaura coupling of aryl halides and aryl boronic acids.48 The catalytic results showed that the presence of the second metal in the structure of the complexes is beneficial in terms of the catalytic outcomes, because both dimetallic catalysts displayed higher activities than related monometallic analogues.
Among all the PAH-connected Janus di-NHCs shown in Scheme 20, the di-NHC in 62 is the one that has given the largest number of complexes, with applications as catalysts, as luminescent materials and as supramolecular receptors. The first complexes obtained with this ligand were rhodium and iridium complexes 70 and 71 (Scheme 22) and their derived carbonylated complounds.49 The CV analysis of these complexes revealed that the metals are essentially decoupled (ΔE1/2 = 30 mV). The same ligand was also used for the preparation of dimetallic complexes with interesting catalytic applications. For example, the di-ruthenium(II) complex 72, was very active in the arylation of arylpyridines with aryl halides, and in the hydroarylation of alkenes.50 In fact, the sequential combination of these two reactions allowed the one-pot preparation of mixed arylated/alkylated arylpyridines (Scheme 22b), thus affording an effective method for the preparation of unsymmetrically substituted pyridines. Di-iridium(III) complex 73, showed very good catalytic activity in the β-alkylation of 1-phenyl methanol (Scheme 22c), and its activity was superior to that shown by an Ir(III) monometallic complex with virtually identical stereoelectronic properties, therefore suggesting that the two metals in 73 cooperate in the catalytic process. Dimetallic complex 73 was also found to be active in the H/D exchange of organic substrates such as THF or benzo[h]quinolone, even in the absence of external additives.51
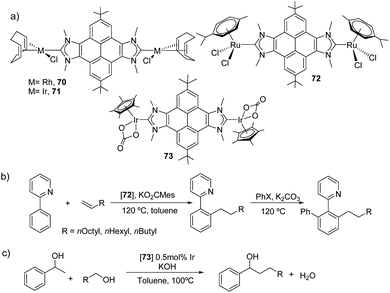 | ||
| Scheme 22 Three complexes with a pyrene-connected Janus di-NHC, and two examples of catalytic applications. | ||
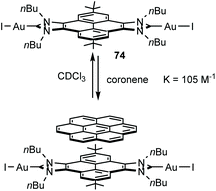
A very interesting feature of di-gold(I) complex 74 (Scheme 23), is that its catalytic activity is greatly influenced by the addition of π-stacking additives, such as pyrene or coronene.52 The analysis of the binding between 74 and coronene showed that the two compounds associate with a constant of 105 M−1 at room temperature in CDCl3. The dimetallic complex was tested in the hydroamination of phenylacetylenes, where it was observed that the addition of coronene to the reaction medium produced an enhancement in the activity of about 20–30% with respect to the reactions carried out in the absence of coronene.52 These results were interpreted as an example of the influence of supramolecular interactions in the activity of a homogeneous catalyst when using ligands decorated with rigid polyaromatic moieties, in line with some recent findings by our group.1b
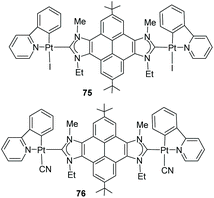
In 2015, we described a series of unsymmetrically N-substituted pyrene–bisimidazolium salts and their coordination to Pt(II) in order to evaluate their luminescence properties. It was observed that the emission of all bis-azole-based compounds was independent of their substitution patterns.53 The bis-azolium salts showed pyrene-centered emissions in the range of 370–420 nm, and quantum yields between Φ = 0.20–0.55. For platinum complexes 75 and 76 (Scheme 24) the quantum yield is significantly reduced (Φ = 0.025), because the coordination to Pt favors the relaxation via nonradiative decay processes.54
More recently, we described a family of dimetallic complexes in which a pyrene-based di-NHC connects two palladium centers that complete their coordination sphere with two (trans) bromide ligands and a benzimidazolylidene ligand. Hence, these complexes bear two types of NHCs differing in the size of the aromatic annulated backbone55
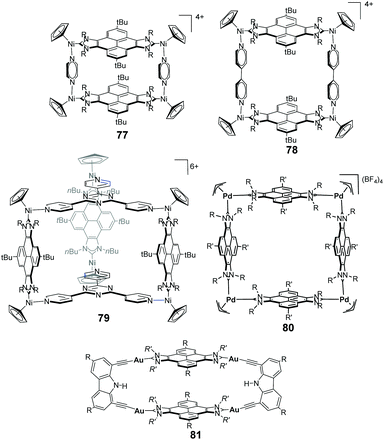
The pyrene-di-NHC ligand turned out to be a very useful synthon in the preparation of metallosupramolecular structures with metals such as nickel,56 palladium57 and gold58 (Scheme 25). By using this Janus di-NHC ligand, two nickel-conjoined metallorectangles were prepared, whose dimensions could be varied by either using pyrazine or 4,4′-bipyridine (77 and 78 in Scheme 25).56b Both assemblies were used as hosts for polycyclic aromatic hydrocarbons (PAHs). The different dimensions of these two molecular rectangles furnished them with significant differences in their host:guest chemistry properties. The rectangle with pyrazine (77) was able to host only one molecule of the polyaromatic guest, while the one with bipyridine (78) was capable of hosting up to two guest molecules.
Hexa-nickel trigonal prism 79, was obtained by combining the pyrene–di-NHC ligand with tri-pyridyl–pyrazine.56a This three-dimensional cage was used for the encapsulation of fullerenes C60 and C70, showing larger affinity for the latter one. The encapsulation of the fullerenes was entropically driven, due to the desolvation of both the host and guest.
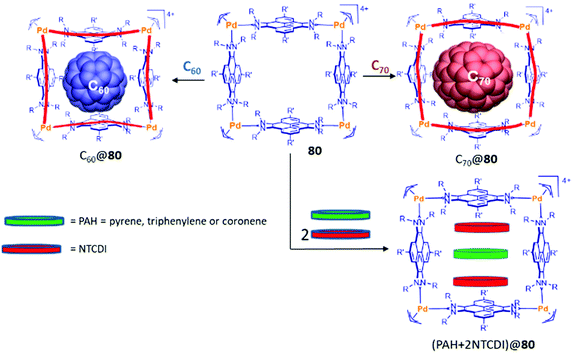
Tetra-palladium(II) metallo-square 80 showed interesting encapsulating properties that are clearly determined by the presence of the four flat pyrene moieties. This cage was able to encapsulate fullerenes C60 and C70, and its shape and size is able to adapt to the size of the encapsulated fullerene.57c (Fig. 2).
An interesting feature of the host:guest adducts C60@80 and C70@80 is that they both behave as stable and efficient single oxygen photosensitizers.57b Their photosensitizing properties are explained due to the spin-converting properties of fullerenes. This property made C60@80 and C70@80 as excellent photocatalysts for the peroxidation of cyclic and acyclic alkenes.57b
The distance of 13 Å between opposite pyrene panels in 80 made this metallorectangle an optimum host for encapsulating three polyaromatic guests. In fact, it was observed that this metallosquare is capable of encapsulating one molecule of an electron-rich polycyclic aromatic hydrocarbon (pyrene, triphenylene or coronene) and two molecules of the electron-deficient N,N′-dimethyl-naphthalenetetracarboxylic diimide (NTCDI), as shown in Fig. 2.57a The guest-induced distortions of this molecular host were recently unveiled using ion mobility mass spectrometry,57d showing that the cage is able to expand its cross collision section (CCS) by 39 Å2 from its empty form to the host:guest adduct with the three guests in (NTCDI)2(PAH)@80 (Fig. 1).
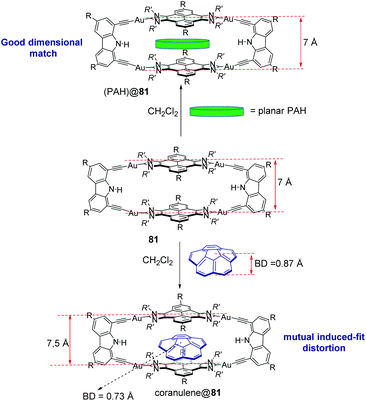
Tetragold metallorectangle 81 shows very large association constants with planar PAHs in CH2Cl2. The binding affinities were found to increase exponentially with the number of π-electrons of the guest, ranging from 1.39 < log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K < 6.64, for naphthalene through coronene.58 These large binding affinities are a consequence of the effective face-to-face overlap between the surfaces of the guests and the pyrene panels of the host. In addition, the two cofacial pyrene panels are separated by a distance of 7 Å (Fig. 3), therefore providing an excellent dimensional matching that facilitates the encapsulation which takes place at a minimum energy cost, due to the lack of structural distortions suffered by the host and guests.58 Metallorectangle 81 is also able to encapsulate corannulene, although the binding affinity is significantly smaller than that shown by the related planar PAHs. This is explained as a consequence of the energy cost derived from the distortions experienced by both the host and guest upon encapsulation (the host is expanded while the corannulene molecule is significantly compressed, as depicted in Fig. 3), together with the lack of effective π–π-stacking interactions over the convex surface of the guest in corannulene@81.58 In a more recent study, metallorectangle 81 was used as a host for a series of polycyclic aromatic hydrocarbons substituted with functions capable of hydrogen bonding.59 It was expected that these substituents improved the binding affinities by establishing hydrogen bonding interactions with the N–H group of the carbazolyl linker. The study revealed that, while hydrogen bonding interactions were not observed, the presence of the substituents produced larger association constants due to peripheral dispersion interactions, therefore highlighting the fact that London interactions may contribute significantly to the binding affinities of host–guest chemistry complexes.59
K < 6.64, for naphthalene through coronene.58 These large binding affinities are a consequence of the effective face-to-face overlap between the surfaces of the guests and the pyrene panels of the host. In addition, the two cofacial pyrene panels are separated by a distance of 7 Å (Fig. 3), therefore providing an excellent dimensional matching that facilitates the encapsulation which takes place at a minimum energy cost, due to the lack of structural distortions suffered by the host and guests.58 Metallorectangle 81 is also able to encapsulate corannulene, although the binding affinity is significantly smaller than that shown by the related planar PAHs. This is explained as a consequence of the energy cost derived from the distortions experienced by both the host and guest upon encapsulation (the host is expanded while the corannulene molecule is significantly compressed, as depicted in Fig. 3), together with the lack of effective π–π-stacking interactions over the convex surface of the guest in corannulene@81.58 In a more recent study, metallorectangle 81 was used as a host for a series of polycyclic aromatic hydrocarbons substituted with functions capable of hydrogen bonding.59 It was expected that these substituents improved the binding affinities by establishing hydrogen bonding interactions with the N–H group of the carbazolyl linker. The study revealed that, while hydrogen bonding interactions were not observed, the presence of the substituents produced larger association constants due to peripheral dispersion interactions, therefore highlighting the fact that London interactions may contribute significantly to the binding affinities of host–guest chemistry complexes.59
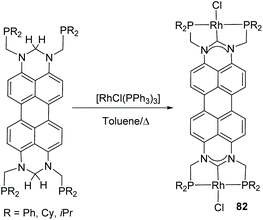
The perylene-connected Janus di-NHC ligand in 64 (Scheme 20), was described by Gade and co-workers in 2016.60 This facially opposed di-NHC constitutes an interesting example of a Janus-di-NHC ligand based on six-membered N-heterocyclic carbenes. The ligand was coordinated to Rh(I) directly by reaction of the isopropylphosphinomethyl-functionalized protioligand and [RhCl(PPh3)3], by a double geminal C–H activation that led to ditopic PCP pincer di-rhodium(I) complex 82 (Scheme 26). The authors performed studies on the photophysical properties of the protioligands and dirhodium complexes, and found that the latter were highly emissive (72% < Φ < 86%), whereas the fluorescence of the dirhodium complexes is almost quenched.60
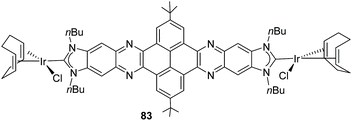
The quinoxalinophenanthrophenazine-connected di-NHC in 65 (Scheme 20) reported by us in 2015![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 is the largest PAH-connected Janus di-NHC described thus far. It establishes a metal-to-metal separation of 22.5 Å. This nanosized ligand was coordinated to iridium(I), affording complex 83, which is depicted in Scheme 27. The computational analysis of the complex, based on the theoretical determination of the changes produced in the Tolman electronic parameter (TEP) upon changes in the coordination of the ligand, revealed that the metals are essentially electronically decoupled.61
61 is the largest PAH-connected Janus di-NHC described thus far. It establishes a metal-to-metal separation of 22.5 Å. This nanosized ligand was coordinated to iridium(I), affording complex 83, which is depicted in Scheme 27. The computational analysis of the complex, based on the theoretical determination of the changes produced in the Tolman electronic parameter (TEP) upon changes in the coordination of the ligand, revealed that the metals are essentially electronically decoupled.61
D. Janus di-NHCs connected by ‘single’ C–C bonds
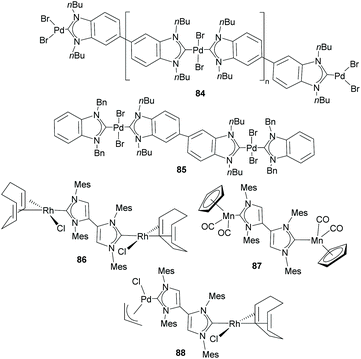
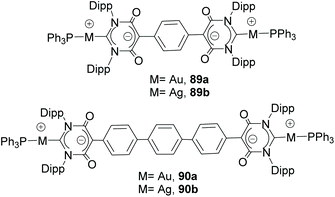
Together with the types of Janus di-NHCs described above, Janus di-NHCs can be constructed using polyaromatic spacers connected by single C–C bonds. The first example of this type of Janus di-NHC was described by Bielawski and co-workers in 2005,35 when they described a series of palladium-based discrete and polymeric compounds supported by a di-benzoimidazolylidene ligand. In these compounds, the di-NHC ligand is connected via a single C–C bond between the C5 positions of its imidazolylidene constituents (84 and 85, in Scheme 28). More recently, Valyaev and Lugan described a related Janus-di-imidazolylidene connected by the C4 positions, and were able to obtain the related homodimetallic and heterometallic complexes of dirhodium(I) (86), di-manganese(I) (87) and rhodium(I)/palladium(II) (88), respectively.62 An interesting feature of this type of di-NHC ligand is that the two NHC moieties are coplanar, so that the π-systems are aligned and therefore the ligand allows metal-to-metal coupling, although it is weak. This electronic coupling was quantified by CV studies, which revealed two oxidation waves separated by ΔE = 80 and 91 mV for 86 and 87, respectively.Another interesting family of Janus-di-NHCs was designed by the group of Tapu, who in 2014 described a dianionic bis(maloNHC) and its coordination to silver and gold (89) to form the respective zwitterionic complexes (Scheme 29).63 More recently, the same group described a related Janus dianionic bis(maloNHC), in which the two NHCs are connected by a p-terphenylene fragment.64 This di-NHC ligand was coordinated to gold and silver (90), and established a metal-to-metal separation of 24.2 Å, the longest distance reported so far for a Janus di-NHC. Both, 89 and 90 are the extended ditopic versions of the previously reported (mono) malo-NHC ligand described by Cesar and co-workers in 2008.65
E. Janus di-NHCs linked by fused non-aromatic cycles
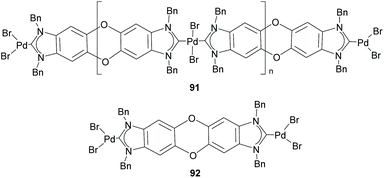
Among the discrete and polymeric complexes obtained by Bielawski and co-workers in 2005, 91 and 92 (Scheme 30), contain an interesting example of a Janus di-NHC in which the two imidazolylidenes are connected by a dibenzo–dioxin spacer.35 In 2010, the same team of researchers, in collaboration with Sessler and co-workers, described a quinobis(imidazolylidene) ligand that was coordinated to rhodium and iridium (93).66 The presence of the p-quinone spacer endows this Janus di-NHC ligand with interesting redox switchable properties. In fact, the one-electron reduction of the quinone moiety increases significantly the electron density of the metals, as observed by the decrease of the CO stretching frequencies of carbonyl complexes 94, as depicted in Scheme 31.66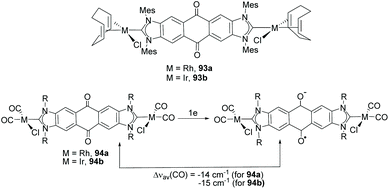 | ||
| Scheme 31 Complexes of rhodium and iridium with a redox-switchable quinobis(imidazolylidene) ligand, and effect of the one-electron reduction on the ν(CO) of the carbonylated species. | ||
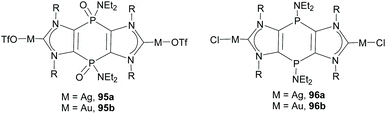
More recently, Nyulászi, Streubel and co-workers reported the synthesis of a phosphanido-bridged di-NHC ligand that was coordinated to silver and gold. An interesting feature of this type of ligand is that it can have the phosphorous centers in two oxidation states (III and V).67 This allowed the preparation of complexes with tricyclic PV/V and PIII/III-bridged bis-imidazolylidenes, similar to complexes 95 and 96, shown in Scheme 32.67b
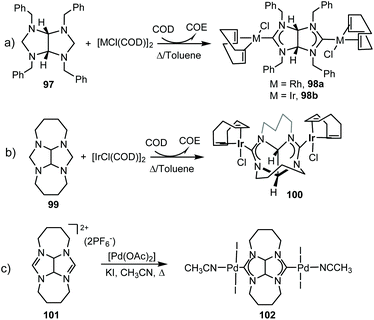
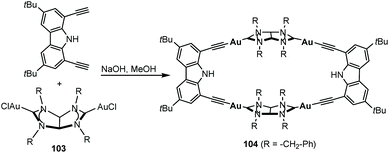
In 2011, we described the preparation of a series of di-iridium(I) and di-rhodium(I) complexes 98 and 100 with a back-to-back bis-imidazolinylidine ligand (Scheme 33a and b).68 These di-NHC complexes were obtained by directly treating the neutral bis-imidazolidines (97 or 99) with [MCl(COD)]2 (M = Rh, Ir). The coordination of the di-NHC ligand is produced by double C–H activation of the NCH2N group of the neutral N-heterocycle, with the concomitant reduction of 1,5-cyclooctadiene (COD) to cyclooctane (COE), as depicted in Scheme 33.68b The access to metals other than rhodium or iridium required the preparation of the corresponding bis-imidazolidinium salts, such as 101, which could be deprotonated with a base to facilitate the coordination of the in situ generated di-NHC to metals such as palladium (102, Scheme 33c),68b and gold (103, Scheme 34).69 A very interesting feature of this back-to-back bis-imidazolinylidene ligand, is that its rigid angular shape constitutes a very useful scaffold for the preparation of organometallic-based supramolecular assemblies, such as tetragold cyclophane 104, displayed in Scheme 34.69 Although the ligands in 98, 100, 102–104 do not strictly contain two NHC moieties in a directionally opposed manner (the angle between the two axes of coordination of the di-NHC ligand is in the range of 120–130°), we consider this ligand to be closely related to the family of Janus di-NHCs, and for this reason they are included in this section.
F. Janus di-NHCs fused to metalloporphyrins
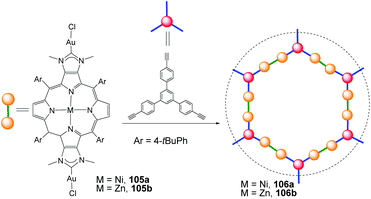
Over the last ten years, Richeter and co-workers have described a series of NHC ligands annulated to porphyrins.70 These types of porphyrin-functionalized NHC are interesting because they may show proton-responsive properties,70c or they can modulate their electronic properties by changing the central metal of the porphyrin.70b In an extension of their research, in 2020 Richeter and co-workers described a Janus bis-NHC ligand fused to a series of porphyrins.71 These were used for preparing discrete di-Au(I) complexes (105) and also two-dimensional main-chain organometallic polymers (106), as shown in Scheme 35. An interesting feature of one of the polymeric materials obtained (106b), is that it behaves as a heterogeneous photocatalyst for the production of singlet oxygen (1O2) upon irradiation with visible light.G. Conclusions and outlook
This revision intends to illustrate how Janus di-NHCs were initially regarded as mere laboratory curiosities and now have become useful tools with a multitude of applications across many different research fields. Janus di-NHCs constitute one of the most useful chemical platforms for constructing dimetallic structures. It was Malcolm Chisholm who said that ‘anything a metal can do, two can do too –and it's more fun’,72 to emphasize the many chemical and physical phenomena that can only be achieved with the participation of more than one metal atom. One of the most appealing aspects of Janus di-NHCs is the manner in which their properties can be modulated to match various specific applications. During the last 15 years we have witnessed how Janus di-NHCs have been utilized in diverse areas such as homogeneous catalysis, materials chemistry and supramolecular chemistry.In the field of catalysis, the coordination versatility of NHCs has allowed the application of Janus-di-NHCs in a large number of organic transformations, among which, the application of hetero-di-metallic complexes for tandem processes has helped to develop greatly the number of complex sequential transformations that now can be performed in one-pot processes.7 Also important is the number of Janus di-NHC that incorporate heteroatoms in their structures and that may have the potential for being applied for redox-switchable processes, a feature that has numerous applications in homogeneous catalysis.
Janus di-NHCs have boosted the development of the field of polymers, by providing convenient routes for the preparation of main chain organometallic polymers,3c,37b which integrate the physical and electronic properties of organic polymers with the electronic, optical and catalytic properties of organometallic complexes.
Janus di-NHCs have allowed the growth of supramolecular organometallic complexes (SOCs),2a with chemical and physical properties that are fundamentally different from those shown by traditional Werner-type supramolecular coordination complexes (SCCs). One of the major characteristics of the coordination self-assembly for the formation of Werner-type SCCs is kinetic reversibility, meaning that these assemblies are able to self-correct, and therefore the most stable thermodynamic products are the ones that are finally formed. The synthesis of SOCs formed by Janus di-NHCs is determined by the high stability of the NHC–M bond, and therefore coordination self-assembly yields the kinetic products. This also means that NHC-based supramolecular assemblies are remarkably stable, and can remain in solution for days without signs of decomposition. Although a number of di-NHCs have been used for the preparation of SOCs, the large variety of Janus di-NHCs that are now available make us think that this area of research will develop rapidly in the incoming years.
As for the future, some remaining challenges are still waiting to be approached. For example, despite great efforts being made to construct Janus-di-NHCs that facilitate electronic communication between metals, this has not been accomplished so far. Facilitating effective electronic communication between the metals present in dimetallic structures built with Janus-di-NHCs may open a gate for the preparation of new materials with improved electro-photophysical properties. Efforts are also needed towards preparing Janus-di-NHCs that are able to bind transition metals in the chelate/pincer form. This should be especially interesting for facilitating the appropriate orientation between the NHC and metal orbitals for maximizing dMetal–πNHC overlap, so that electronic communication between metals through a π-delocalized system can be improved. Chelate or pincer-based Janus di-NHCs should also be particularly interesting for preparing more stable dimetallic structures that render – for example – catalysts that are able to withstand harsher reaction conditions.
Another very interesting potential application of Janus-d-NHCs is the stabilization of nanoparticles. Over the last few years many groups have used NHCs for stabilizing nanoparticles with interesting catalytic and photophysical applications but to the best of our knowledge, Janus-di-topic NHCs have not been used so far. In addition, NHCs have been used recently for stabilizing and improving the properties of surfaces and monolayers of gold73 and silicon.74 Preparing discrete or polymeric multi-layered systems connected by Janus-di-NHCs may bring the opportunity to obtain enhanced materials with very interesting electro-photophysical properties. Such materials would be composed of a set of monolayers with separations fixed by the length of the Janus-di-NHCs, and such separations could be modulated by – for example – using the library of Janus-di-NHCs with fused-polyaromatic linkers displayed in section C in this article. Janus di-NHCs are expected to be very useful for steering interfacial processes between layers. We expect that researchers in these fields are willing to approach this challenge, as we anticipate extremely interesting results.
It is our hope that this review article encourages future research to take up new synthetic challenges that widen the scope of applications of Janus di-NHCs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the Ministerio de Ciencia y Universidades (PGC2018-093382-B-I00) and the Universitat Jaume I (UJI-B2020-01 and UJI-B2018-46).Notes and references
- (a) E. Peris, Chem. Rev., 2018, 118, 9988–10031 CrossRef CAS PubMed; (b) E. Peris, Chem. Commun., 2016, 52, 5777–5787 RSC; (c) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485–496 CrossRef CAS PubMed; (d) S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef CAS; (e) P. de Fremont, N. Marion and S. P. Nolan, Coord. Chem. Rev., 2009, 253, 862–892 CrossRef CAS; (f) N. Marion, S. Diez-Gonzalez and I. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 2988–3000 CrossRef CAS; (g) W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1291–1309 Search PubMed.
- (a) S. Ibáñez, M. Poyatos and E. Peris, Acc. Chem. Res., 2020, 53, 1401–1413 CrossRef PubMed; (b) M. M. Gan, J. Q. Liu, L. Zhan, Y. Y. Wang, F. E. Hahn and Y. F. Han, Chem. Rev., 2018, 118, 9587–9641 CrossRef CAS; (c) N. Sinha and F. E. Hahn, Acc. Chem. Res., 2017, 50, 2167–2184 CrossRef CAS; (d) A. Pöthig and A. Casini, Theranostics, 2019, 9, 3150–3169 CrossRef PubMed.
- (a) C. A. Smith, M. R. Narouz, P. A. Lummis, I. Singh, A. Nazemi, C. H. Li and C. M. Crudden, Chem. Rev., 2019, 119, 4986–5056 CrossRef CAS PubMed; (b) B. C. Norris and C. W. Bielawski, Macromolecules, 2010, 43, 3591–3593 CrossRef CAS; (c) A. B. Powell, C. W. Bielawski and A. H. Cowley, Comments Inorg. Chem., 2010, 31, 75–82 CrossRef CAS; (d) O. Schuster, L. Mercs and M. Albrecht, Chimia, 2010, 64, 184–187 CrossRef CAS.
- (a) S. Nayak and S. L. Gaonkar, ChemMedChem, 2021, 16, 1360–1390 CrossRef CAS PubMed; (b) Z. B. Yang, G. Z. Jiang, Z. R. Xu, S. Zhao and W. K. Liu, Coord. Chem. Rev., 2020, 423, 213492 CrossRef CAS; (c) S. A. Patil, A. P. Hoagland, S. A. Patil and A. Bugarin, Future Med. Chem., 2020, 12, 2239–2275 CrossRef CAS; (d) I. Ott, in Medicinal Chemistry, ed. P. J. Sadler and R. VanEldik, 2020, pp. 121–148 Search PubMed; (e) W. K. Liu and R. Gust, Coord. Chem. Rev., 2016, 329, 191–213 CrossRef CAS; (f) S. A. Patil, S. A. Patil, R. Patil, R. S. Keri, S. Budagumpi, G. R. Balakrishna and M. Tacke, Future Med. Chem., 2015, 7, 1305–1333 CrossRef CAS PubMed.
- M. Poyatos, J. A. Mata and E. Peris, Chem. Rev., 2009, 109, 3677–3707 CrossRef CAS PubMed.
- J. W. Kamplain and C. W. Bielawski, Chem. Commun., 2006, 1727–1729 RSC.
- J. A. Mata, F. E. Hahn and E. Peris, Chem. Sci., 2014, 5, 1723–1732 RSC.
- M. Veith, Chem. Rev., 1990, 90, 3–16 CrossRef CAS.
- F. Baier, Z. F. Fei, H. Gornitzka, A. Murso, S. Neufeld, M. Pfeiffer, I. Rudenauer, A. Steiner, T. Stey and D. Stalke, J. Organomet. Chem., 2002, 661, 111–127 CrossRef CAS.
- (a) O. Guerret, S. Sole, H. Gornitzka, G. Trinquier and G. Bertrand, J. Organomet. Chem., 2000, 600, 112–117 CrossRef CAS; (b) O. Guerret, S. Sole, H. Gornitzka, M. Teichert, G. Trinquier and G. Bertrand, J. Am. Chem. Soc., 1997, 119, 6668–6669 CrossRef CAS.
- E. Mas-Marza, J. A. Mata and E. Peris, Angew. Chem., Int. Ed., 2007, 46, 3729–3731 CrossRef CAS PubMed.
- A. Zanardi, R. Corberan, J. A. Mata and E. Peris, Organometallics, 2008, 27, 3570–3576 CrossRef CAS.
- M. Viciano, M. Sanau and E. Peris, Organometallics, 2007, 26, 6050–6054 CrossRef CAS.
- (a) A. Zanardi, J. A. Mata and E. Peris, Organometallics, 2009, 28, 4335–4339 CrossRef CAS; (b) A. Zanardi, J. A. Mata and E. Peris, Organometallics, 2009, 28, 1480–1483 CrossRef CAS.
- A. Zanardi, J. A. Mata and E. Peris, Chem. – Eur. J., 2010, 16, 13109–13115 CrossRef CAS PubMed.
- S. Sabater, J. A. Mata and E. Peris, Chem. – Eur. J., 2012, 18, 6380–6385 CrossRef CAS PubMed.
- (a) A. Zanardi, J. A. Mata and E. Peris, J. Am. Chem. Soc., 2009, 131, 14531–14537 CrossRef CAS PubMed; (b) A. Zanardi, J. A. Mata and E. Peris, Chem. – Eur. J., 2010, 16, 10502–10506 CrossRef CAS; (c) S. Sabater, J. A. Mata and E. Peris, Eur. J. Inorg. Chem., 2013, 4764–4769 CrossRef CAS.
- (a) S. Sabater, J. A. Mata and E. Peris, Organometallics, 2012, 31, 6450–6456 CrossRef CAS; (b) S. Sabater, J. A. Mata and E. Peris, Nat. Commun., 2013, 4, 2553 CrossRef PubMed.
- M. K. Whittlesey and E. Peris, ACS Catal., 2014, 4, 3152–3159 CrossRef CAS.
- (a) L. Mercs, A. Neels and M. Albrecht, Dalton Trans., 2008, 5570–5576 RSC; (b) A. G. Tennyson, E. L. Rosen, M. S. Collins, V. M. Lynch and C. W. Bielawski, Inorg. Chem., 2009, 48, 6924–6933 CrossRef CAS PubMed; (c) A. Prades, E. Peris and M. Alcarazo, Organometallics, 2012, 31, 4623–4626 CrossRef CAS.
- D. G. Gusev and E. Peris, Dalton Trans., 2013, 42, 7359–7364 RSC.
- P. L. Arnold and S. T. Liddle, Organometallics, 2006, 25, 1485–1491 CrossRef CAS.
- Y. Wang, Y. Xie, M. Y. Abraham, P. Wei, H. F. Schaefer, P. V. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2010, 132, 14370–14372 CrossRef CAS PubMed.
- (a) R. S. Ghadwal, Dalton Trans., 2016, 45, 16081–16095 RSC; (b) A. Nasr, A. Winkler and M. Tamm, Coord. Chem. Rev., 2016, 316, 68–124 CrossRef CAS.
- (a) Y. Z. Wang, Y. M. Xie, M. Y. Abraham, R. J. Gilliard, P. R. Wei, C. F. Campana, H. F. Schaefer, P. V. Schleyer and G. H. Robinson, Angew. Chem., Int. Ed., 2012, 51, 10173–10176 CrossRef CAS; (b) D. R. Armstrong, S. E. Baillie, V. L. Blair, N. G. Chabloz, J. Diez, J. Garcia-Alvarez, A. R. Kennedy, S. D. Robertson and E. Hevia, Chem. Sci., 2013, 4, 4259–4266 RSC.
- (a) U. L. Scheele, S. Dechert and F. Meyer, Chem. – Eur. J., 2008, 14, 5112–5115 CrossRef CAS PubMed; (b) A. Kruger, E. Kluser, H. Muller-Bunz, A. Neels and M. Albrecht, Eur. J. Inorg. Chem., 2012, 1394–1402 CrossRef.
- M. J. Bitzer, A. Pothig, C. Jandl, F. E. Kuhn and W. Baratta, Dalton Trans., 2015, 44, 11686–11689 RSC.
- M. J. Bitzer, F. E. Kuhn and W. Baratta, J. Catal., 2016, 338, 222–226 CrossRef CAS.
- L. Pardatscher, M. J. Bitzer, C. Jandl, J. W. Kuck, R. M. Reich, F. E. Kuhn and W. Baratta, Dalton Trans., 2019, 48, 79–89 RSC.
- A. A. Grineva, O. A. Filippov, Y. Canac, J. B. Sortais, S. E. Nefedov, N. Lugan, V. Cesar and D. A. Valyaev, Inorg. Chem., 2021, 60, 4015–4025 CrossRef CAS PubMed.
- A. A. Grineva, O. A. Filippov, S. E. Nefedov, N. Lugan, V. Cesar and D. A. Valyaev, Organometallics, 2019, 38, 2330–2337 CrossRef CAS.
- S. Termühlen, L. F. B. Wilm, P. D. Dutschke, A. Hepp and F. E. Hahn, Organometallics, 2021, 40, 1565–1570 CrossRef.
- F. Zhang, X. M. Cao, J. W. Wang, J. J. Jiao, Y. M. Huang, M. Shi, P. Braunstein and J. Zhang, Chem. Commun., 2018, 54, 5736–5739 RSC.
- Z. J. Hu, X. F. Ma, J. W. Wang, H. Wang, X. Y. Han, M. Shi and J. Zhang, Organometallics, 2019, 38, 2132–2137 CrossRef CAS.
- A. J. Boydston, K. A. Williams and C. W. Bielawski, J. Am. Chem. Soc., 2005, 127, 12496–12497 CrossRef CAS PubMed.
- A. J. Boydston, J. D. Rice, M. D. Sanderson, O. L. Dykhno and C. W. Bielawski, Organometallics, 2006, 25, 6087–6098 CrossRef CAS.
- (a) A. J. Boydston and C. W. Bielawski, Dalton Trans., 2006, 4073–4077 RSC; (b) K. A. Williams, A. J. Boydston and C. W. Bielawski, Chem. Soc. Rev., 2007, 36, 729–744 RSC.
- (a) B. Karimi and P. F. Akhavan, Chem. Commun., 2009, 3750–3752 RSC; (b) B. Karimi and P. F. Akhavan, Inorg. Chem., 2011, 50, 6063–6072 CrossRef CAS PubMed.
- B. Karimi and P. F. Akhavan, Chem. Commun., 2011, 47, 7686–7688 RSC.
- D. M. Khramov, A. J. Boydston and C. W. Bielawski, Angew. Chem., Int. Ed., 2006, 45, 6186–6189 CrossRef CAS PubMed.
- L. Mercs, G. Labat, A. Neels, A. Ehlers and M. Albrecht, Organometallics, 2006, 25, 5648–5656 CrossRef CAS.
- M. Nussbaum, O. Schuster and M. Albrecht, Chem. – Eur. J., 2013, 19, 17517–17527 CrossRef CAS.
- M. Schmidtendorf, T. Pape and F. E. Hahn, Angew. Chem., Int. Ed., 2012, 51, 2195–2198 CrossRef CAS PubMed.
- (a) F. E. Hahn, C. Radloff, T. Pape and A. Hepp, Organometallics, 2008, 27, 6408–6410 CrossRef CAS; (b) C. Radloff, F. E. Hahn, T. Pape and R. Fröhlich, Dalton Trans., 2009, 7215–7222 RSC.
- (a) M. Schmidtendorf, C. S. to Brinke and F. E. Hahn, J. Organomet. Chem., 2014, 751, 620–627 CrossRef CAS; (b) A. Sinha, F. Roelfes, A. Hepp and E. F. Hahn, Chem. – Eur. J., 2017, 23, 5939–5942 CrossRef PubMed; (c) F. M. Conrady, R. Fröhlich, C. Schulte to Brinke, T. Pape and F. E. Hahn, J. Am. Chem. Soc., 2011, 133, 11496–11499 CrossRef CAS PubMed.
- M. Tamm and F. E. Hahn, Coord. Chem. Rev., 1999, 182, 175–209 CrossRef.
- E. Deck, K. Reiter, W. Klopper and F. Breher, Z. Anorg. Allg. Chem., 2016, 642, 1320–1328 CrossRef CAS.
- G. Guisado-Barrios, J. Hiller and E. Peris, Chem. – Eur. J., 2013, 19, 10405–10411 CrossRef CAS PubMed.
- S. Gonell, M. Poyatos and E. Peris, Chem. – Eur. J., 2014, 20, 9716–9724 CrossRef CAS PubMed.
- S. Gonell and E. Peris, ACS Catal., 2014, 4, 2811–2817 CrossRef CAS.
- S. Ibáñez, M. Poyatos and E. Peris, Dalton Trans., 2016, 45, 14154–14159 RSC.
- D. Nuevo, M. Poyatos and E. Peris, Organometallics, 2018, 37, 3407–3411 CrossRef CAS.
- S. Ibáñez, A. Guerrero, M. Poyatos and E. Peris, Chem. – Eur. J., 2015, 21, 10566–10575 CrossRef.
- J. J. Hu, S. Q. Bai, H. H. Yeh, D. J. Young, Y. Chi and T. S. A. Hor, Dalton Trans., 2011, 40, 4402–4406 RSC.
- S. Gonell, E. Peris and M. Poyatos, Eur. J. Inorg. Chem., 2019, 3776–3781 CrossRef CAS.
- (a) V. Martinez-Agramunt, D. G. Gusev and E. Peris, Chem. – Eur. J., 2018, 24, 14802–14807 CrossRef CAS PubMed; (b) V. Martinez-Agramunt, S. Ruiz-Botella and E. Peris, Chem. – Eur. J., 2017, 23, 6675–6681 CrossRef CAS PubMed.
- (a) V. Martinez-Agramunt and E. Peris, Chem. Commun., 2019, 55, 14972–14975 RSC; (b) V. Martinez-Agramunt and E. Peris, Inorg. Chem., 2019, 58, 11836–11842 CrossRef CAS PubMed; (c) V. Martinez-Agramunt, T. Eder, H. Darmandeh, G. Guisado-Barrios and E. Peris, Angew. Chem., Int. Ed., 2019, 58, 5682–5686 CrossRef CAS PubMed; (d) C. Vicent, V. Martinez-Agramunt, V. Gandhi, C. Larriba-Andaluz and E. Peris, Angew. Chem., Int. Ed., 2021, 60, 15412–15417 CrossRef CAS PubMed.
- S. Ibañez and E. Peris, Angew. Chem., Int. Ed., 2020, 59, 6860–6865 CrossRef PubMed.
- S. Ibanez, D. G. Gusev and E. Peris, Organometallics, 2020, 39, 4078–4084 CrossRef CAS.
- S. Langbein, H. Wadepohl and L. H. Gade, Organometallics, 2016, 35, 809–815 CrossRef CAS.
- H. Valdes, M. Poyatos and E. Peris, Organometallics, 2015, 34, 1725–1729 CrossRef CAS.
- A. A. Grineva, D. A. Valyaev, V. Cesar, O. A. Filippov, V. N. Khrustalev, S. E. Nefedov and N. Lugan, Angew. Chem., Int. Ed., 2018, 57, 7986–7991 CrossRef CAS PubMed.
- D. Tapu, Z. McCarty and C. McMillen, Chem. Commun., 2014, 50, 4725–4728 RSC.
- A. Carter, A. Mason, M. A. Baker, D. G. Bettler, A. Changas, C. D. McMillen and D. Tapu, Organometallics, 2017, 36, 1867–1872 CrossRef CAS.
- V. Cesar, N. Lugan and G. Lavigne, J. Am. Chem. Soc., 2008, 130, 11286–11287 CrossRef CAS PubMed.
- A. G. Tennyson, R. J. Ono, T. W. Hudnall, D. M. Khramov, J. A. V. Er, J. W. Kamplain, V. M. Lynch, J. L. Sessler and C. W. Bielawski, Chem. – Eur. J., 2010, 16, 304–315 CrossRef CAS PubMed.
- (a) N. R. Naz, G. Schnakenburg, Z. Kelemen, D. Gal, L. Nyulászi, R. T. Boere and R. Streubel, Dalton Trans., 2021, 50, 689–695 RSC; (b) N. R. Naz, G. Schnakenburg, A. Mikehazi, Z. Kelemen, L. Nyulászi, R. T. Boere and R. Streubel, Chem. Commun., 2020, 56, 2646–2649 RSC.
- (a) A. Prades, M. Poyatos, J. A. Mata and E. Peris, Angew. Chem., Int. Ed., 2011, 50, 7666–7669 CrossRef CAS PubMed; (b) H. Valdés, M. Poyatos and E. Peris, Organometallics, 2013, 32, 6445–6451 CrossRef.
- A. Gutierrez-Blanco, S. Ibáñez, F. E. Hahn, M. Poyatos and E. Peris, Organometallics, 2019, 38, 4565–4569 CrossRef CAS.
- (a) J.-F. Longevial, A. Langlois, A. Buisson, C. H. Devillers, S. Clement, A. van der Lee, P. D. Harvey and S. Richeter, Organometallics, 2016, 35, 663–672 CrossRef CAS; (b) J.-F. Lefebvre, M. Lo, J.-P. Gisselbrecht, O. Coulembier, S. Clement and S. Richeter, Chem. – Eur. J., 2013, 19, 15652–15660 CrossRef CAS PubMed; (c) J.-F. Lefebvre, M. Lo, D. Leclercq and S. Richeter, Chem. Commun., 2011, 47, 2976–2978 RSC.
- J. F. Longevial, M. Lo, A. Lebrun, D. Laurencin, S. Clement and S. Richeter, Dalton Trans., 2020, 49, 7005–7014 RSC.
- J. F. Berry and C. M. Thomas, Dalton Trans., 2017, 46, 5472–5473 RSC.
- (a) J. D. Ren, M. Freitag, C. Schwermann, A. Bakker, S. Amirjalayer, A. Ruhling, H. Y. Gao, N. L. Doltsinis, F. Glorius and H. Fuchs, Nano Lett., 2020, 20, 5922–5928 CrossRef CAS PubMed; (b) D. T. Nguyen, M. Freitag, C. Gutheil, K. Sotthewes, B. J. Tyler, M. Bockmann, M. Das, F. Schluter, N. L. Doltsinis, H. F. Arlinghaus, B. J. Ravoo and F. Glorius, Angew. Chem., Int. Ed., 2020, 59, 13651–13656 CrossRef CAS PubMed; (c) S. Amirjalayer, A. Bakker, M. Freitag, F. Glorius and H. Fuchs, Angew. Chem., Int. Ed., 2020, 59, 21230–21235 CrossRef CAS PubMed; (d) M. R. Narouz, K. M. Osten, P. J. Unsworth, R. W. Y. Man, K. Salorinne, S. Takano, R. Tomihara, S. Kaappa, S. Malola, C. T. Dinh, J. D. Padmos, K. Ayoo, P. J. Garrett, M. Nambo, J. H. Horton, E. H. Sargent, H. Hakkinen, T. Tsukuda and C. M. Crudden, Nat. Chem., 2019, 11, 419–425 CrossRef CAS PubMed; (e) D. T. Nguyen, M. Freitag, M. Korsgen, S. Lamping, A. Ruhling, A. H. Schafer, M. H. Siekman, H. F. Arlinghaus, W. G. van der Wiel, F. Glorius and B. J. Ravoo, Angew. Chem., Int. Ed., 2018, 57, 11465–11469 CrossRef CAS PubMed; (f) A. F. Lv, M. Freitag, K. M. Chepiga, A. H. Schafer, F. Glorius and L. F. Chi, Angew. Chem., Int. Ed., 2018, 57, 4792–4796 CrossRef CAS PubMed; (g) A. Bakker, A. Timmer, E. Kolodzeiski, M. Freitag, H. Y. Gao, H. Monig, S. Amirjalayer, F. Glorius and H. Fuchs, J. Am. Chem. Soc., 2018, 140, 11889–11892 CrossRef CAS PubMed; (h) C. M. Crudden, J. H. Horton, M. R. Narouz, Z. J. Li, C. A. Smith, K. Munro, C. J. Baddeley, C. R. Larrea, B. Drevniok, B. Thanabalasingam, A. B. McLean, O. V. Zenkina, I. I. Ebralidze, Z. She, H. B. Kraatz, N. J. Mosey, L. N. Saunders and A. Yagi, Nat. Commun., 2016, 7, 12654 CrossRef CAS PubMed; (i) C. M. Crudden, J. H. Horton, I. I. Ebralidze, O. V. Zenkina, A. B. McLean, B. Drevniok, Z. She, H. B. Kraatz, N. J. Mosey, T. Seki, E. C. Keske, J. D. Leake, A. Rousina-Webb and G. Wu, Nat. Chem., 2014, 6, 409–414 CrossRef CAS.
- M. Franz, S. Chandola, M. Koy, R. Zielinski, H. Aldahhak, M. Das, M. Freitag, U. Gerstmann, D. Liebig, A. K. Hoffmann, M. Rosin, W. G. Schmidt, C. Hogan, F. Glorius, N. Esser and M. Dahne, Nat. Chem., 2021 DOI:10.1038/s41557-41021-00721-41552.
| This journal is © The Royal Society of Chemistry 2021 |