Palladium-catalyzed C–H activation of anisole with electron-deficient auxiliary ligands: a mechanistic investigation†
Abstract
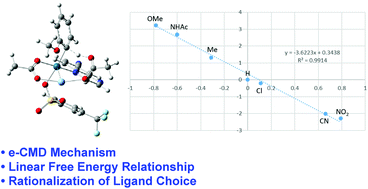
Palladium-catalyzed selective C–H activation–functionalization has shown its significance in organic transformations. Recently, Yu et al. reported a palladium–norbornene co-catalyzed meta-selective arylation of electron-rich arenes. Although the experimentally observed site-selectivity has been successfully explained by the computational work of Dongju Zhang and co-workers, some important experimental factors, such as the ligand choice and narrow substrate scope, remain unrationalized. In contrast to what has been suggested by Dongju Zhang, we proposed the palladium–silver dinuclear species as reactive intermediates in this work. The substituent effect was estimated to unravel the e-CMD nature of the rate-determining C–H activation step. Based on this realization, the experimentally observed substrate scope and ligand choice have also been rationalized.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...