Origin of the ligand effect in the cobalt catalyzed regioselective hydroboration of 1,3-diene†
Abstract
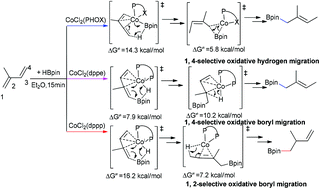
Hydroboration of 1,3-dienes can provide useful intermediates with multiple functionalities. However, achieving high regioselectivity is still a challenge. Recent experimental research studies indicate that this challenge could be overcome by the ligand effect. We made DFT calculations to elucidate the origin of ligand controlled regioselectivity in cobalt catalyzed hydroboration of 2-substituted 1,3-diene. The following conclusions have been reached: when using PHOX ((2-oxazolinyl)-phenyldiphenylphosphine) as the ligand, the favorable 1,4-selective oxidative hydrogen migration pathway was suggested to start with the rate-determining step of 1,4-selective oxidative hydrogen migration followed by reductive boryl migration. The unique 1,4-selectivity is proposed to be a result of the less steric hindrance between the substrate and the ligand PHOX. When dppp (1,3-bis-(diphenylphosphino)propane) is used as the ligand, the favorable pathway is proposed to be a 1,2-selective oxidative boryl migration pathway which involves 1,2-selective oxidative boryl migration and reductive hydrogen migration. Interestingly, another smaller-bite angle bisphosphine ligand dppe (1,2-bis(diphenylphosphino)ethane) favors the 1,4-selective oxidative boryl migration pathway. DFT calculations revealed that the preferred oxidative boryl migration pathway with both dppp and dppe is attributed to their electron-rich properties which accelerate the oxidative boryl migration step. The larger bite angle of dppp than that of dppe leads to bulkier steric hindrance and promotes 1,2-selective reductive hydrogen migration. On the other hand, for dppe with a smaller bite angle, the steric effect in the reductive hydrogen migration step is not dominant and 1,4-selective reductive hydrogen migration is favored. It is expected that the analysis of the ligand effect on the regioselectivity would enable further catalyst design.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...