Chemo- and regioselective click reactions through nickel-catalyzed azide–alkyne cycloaddition†
Abstract
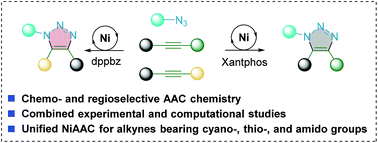
Metal-catalyzed cycloaddition is an expeditious synthetic route to functionalized heterocyclic frameworks. However, achieving reactivity-controlled metal-catalyzed azide–alkyne cycloadditions from competing internal alkynes has been challenging. Herein, we report a nickel-catalyzed [3 + 2] cycloaddition of unsymmetrical alkynes with organic azides to afford functionalized 1,2,3-triazoles with excellent regio- and chemoselectivity control. Terminal alkynes and cyanoalkynes afford 1,5-disubstituted triazoles and 1,4,5-trisubstituted triazoles bearing a 4-cyano substituent, respectively. Thioalkynes and ynamides exhibit inverse regioselectivity compared with terminal alkynes and cyanoalkynes, affording 1,4,5-trisubstituted triazoles with 5-thiol and 5-amide substituents, respectively. Density functional theory calculations are performed for the elucidation of the reaction mechanism. The computed mechanism suggests that a nickellacyclopropene intermediate is generated by the oxidative addition of the alkyne substrate to the Ni(0)–Xantphos catalyst, and the subsequent C–N coupling of this intermediate with an azide is responsible for the chemo- and regioselectivity.
- This article is part of the themed collections: Synthetic methodology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...