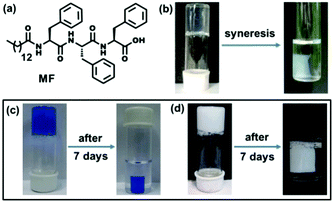
A tripeptide-based self-shrinking hydrogel for waste-water treatment: removal of toxic organic dyes and lead (Pb2+) ions†
Shibaji
Basak‡
a,
Nibedita
Nandi‡
a,
Subir
Paul
a,
Ian W.
Hamley
 b and
Arindam
Banerjee
b and
Arindam
Banerjee
 *a
*a
aDepartment of Biological Chemistry, Indian Association for the Cultivation of Science, Jadavpur, Kolkata, 700032, India. E-mail: bcab@iacs.res.in; Fax: (+91) 33-2473-2805
bDepartment of Chemistry, University of Reading, Whitenights, Reading, RG6, 6AD, UK
First published on 10th May 2017
Abstract
A triphenylalanine-based superhydrogel shows automatic syneresis (self-compressing properties) with time and this self-shrinking behavior has been successfully utilized to remove toxic lead ions and organic dyes from waste-water efficiently with the ability to re-use for a few times.
Supramolecular hydrogels1 composed of low molecular weight gelators (LMWGs) are a rapidly expanding area of recent research due to their wide range of applications including controlled release of biologically active compounds and drugs,2 cell culture and tissue engineering,3 nano-particle and nano-cluster synthesis,4 catalysis5 and other purposes. Peptides are particularly useful candidates for studying self-assembly and gelation as these molecules are bio-compatible and biodegradable in nature6 as well as incorporate a diversity of amino acids which lead to versatility in the control of peptide sequences. Among the peptide gels so far reported it was found that hydrophobic amino acids especially phenylalanine have an important role in the gelation process.7 However, the use of phenylalanine has a limitation as it reduces the solubility of the gelator molecule in an aqueous medium.8 The hydrophobicity of gelators often leads to ‘syneresis’,9 a phenomenon involving the expulsion of the gelling solvent from the gel phase and ultimately macroscopic contraction of the gel, which occurs within several hours. This phenomenon has been mostly observed for many sol–gel based polymeric systems.10 In contrast, there are only a few reports on stimuli-responsive syneresis in supramolecular hydro- or organogels driven by external stimuli including pH,11a,b metal ion,11c photo-irradiation,11d mechanical pressure11e or in the presence of external chemicals.11f So, there is a need to explore supramolecular hydrogels that show shrinking properties without any external stimulus. In the course of our investigation to study the self-assembly and gelation behavior of self-assembling peptide-based molecules, a tripeptide-based amphiphile has been discovered to form a hydrogel at pH 7.4 and it has been found to exhibit a remarkable self-shrinking property with time (Fig. 1b).
Water pollution caused by toxic organic dyes and leaching of heavy metal ions including lead (Pb), cadmium (Cd), mercury (Hg) and others from industrial waste is a great threat to modern society.12 The dye effluents have a negative impact on the immune system and reproductive systems, as well as they exhibit potential geno-toxicity and cardio-toxicity.12 On the other hand, lead has a deleterious effect on the human health including renal, reproductive, and central nervous and hematopoietic systems through the enhancement of oxidative stress.13 There is thus a prime interest for the development and analysis of environmentally friendly materials to protect the environment by removing toxic pollutants from contaminated water. There are some methods to remove pollutants from waste-water using functionalized CNTs, photocatalysis, reverse osmosis and others.14 In this connection, supramolecular hydrogels are wonderful materials due to their highly porous fibrous network structure and large surface areas in the gel matrix to trap the toxic pollutants.15 There are a few examples of polymer based and graphene containing hydrogels for the removal of lead ions from waste-water.16 However, none of these above-mentioned hydrogels exhibit self-shrinkage properties. Herein, we report a novel triphenylalanine-based hydrogel that can offer an easy and effective way to remove water-soluble pollutants to have clean and safe water upon instant syneresis. In this study, the hydrogel has a good capacity to entrap toxic Pb2+ ions as well as toxic organic dyes (Methylene blue and Brilliant blue) from waste-water (Fig. 1c and d). The scavenging capacity of removing toxins from contaminated water is excellent as an appreciable amount (1995 mg) of Pb2+ ions can be removed by per gram of the gelator from waste-water. To the best of our knowledge, it is the highest Pb2+ ion absorption capacity by a hydrogel reported to date.16a Moreover, only a small amount of the gelator is required (<1 mg mL−1) to prepare this superhydrogel and it is recyclable several times to efficiently remove toxic substances from waste-water.
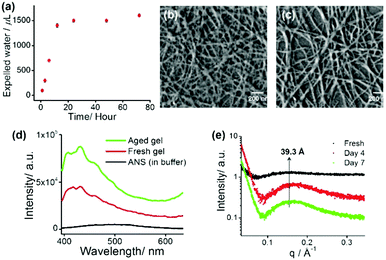
The tripeptide-based amphiphilic gelator MF (Fig. 1a) was observed to self-assemble in phosphate buffer of pH 7.4. The gel is stable in the pH range of 7.0–8.5 and it is also thermo-reversible in nature. The gelator molecule MF has three phenylalanine (Phe) moieties bearing a hydrophobic π-core, thus making the molecule highly hydrophobic in nature. Therefore, it is hard to dissolve the gelator in an aqueous medium and it requires 15–20 minutes of heating on a hot plate followed by cooling at room temperature (25 °C) to obtain a transparent hydrogel. The minimum gelation concentration (MGC) was found to be 1.2 mM (0.08% wt/v) to form a self-supporting gel. The gel melting temperature (Tgel) at MGC was found to be 56 °C. The temperature vs. concentration plot (Fig. S4, ESI†) of the freshly prepared hydrogel shows very high thermal stability. Surprisingly, a more interesting feature of volume phase transition – ‘syneresis’ – was observed for this hydrogel. The hydrogel started to shrink immediately after its formation followed by the expulsion of water from the gel matrix. The freshly prepared gel was transparent in nature, while the aged gel gradually became opaque upon shrinking (Fig. S5, ESI†). The shrinking capacity of the hydrogel was quantitatively evaluated by measuring the volume of the expelled water from the shrunken gel as well as volume of the shrunken gel itself. It was observed that the volume of the expelled water gradually increases with time and after 7 days the volume of the hydrogel contracts to about 75% of its original volume by releasing about 80% clear water from 2 mL of hydrogel (1.5 mM) (Fig. 2a). The shrinkage behaviour of this hydrogel is thermal, pH and pressure dependent in nature (Fig. S6, ESI†).
The syneresis of the hydrogel may be due to the extreme hydrophobic nature of the nanofibres obtained from the self-assembling gelator molecules containing three hydrophobic Phe residues. An attempt has been made to explore the mechanism of the shrinkage behaviour by using a dye (ANS) binding assay fluorimetrically (Fig. 2d). A time dependent blue shift and the steady increase in fluorescence of ANS upon binding11e with the gel fibres clearly suggest that the hydrophobic nature of the gel system increases with time and this ultimately causes more and more proximity of the gel fibres within the gel matrix by expelling water molecules from the system.
Field emission scanning electron microscopy (FE-SEM) was carried out for the xerogels obtained from freshly prepared gel and the 7 day aged gel. Fig. 2b and c show that both of these xerogels appeared to contain well-developed numerous intertwined nano-fibres (average width 25–45 nm) in their self-assembled states. A comparison between these two images (freshly prepared and 7 day aged gel) indicates that the morphology of the fibrous network does not notably change upon syneresis.11e
To get insight into the mechanical strength and flow behaviour of the hydrogels, rheological experiments were performed for the fresh hydrogel, and 4 day and 7 day aged gels. The frequency sweep experiments (Fig. S7a, ESI†) suggest that the storage moduli of these hydrogels are very similar and in the order of 103–104 Pa. The oscillatory stress sweep experiment (Fig. S7b, ESI†) also shows that the stiffness of the freshly prepared gel is more than those of the 4 day and 7 day aged gels and it is evident that syneresis does have a negative effect on the mechanical strength of the gel and the exact reason for that is yet to be explored.
The slow shrinkage of the hydrogel inspired us to look into the detailed packing pattern of the gelator molecules within the hydrogel. In a small angle X-ray scattering (SAXS) experiment, a broad peak is observed at 39.3 Å, which becomes more pronounced with time as indicated in Fig. 2e. This length (39.3 Å) is greater than the molecular length of a single gelator molecule (calculated to be 28 Å), but shorter than double the calculated molecular length indicating an interdigitated arrangement of the molecules in the gel phase. In the wide angle powder X-ray diffraction (WXRD) pattern of the xerogels (fresh and aged), the appearance of peaks at 2θ = 19.2° (d = 4.5 Å) and 2θ = 22.5° (d = 3.7 Å) respectively suggest a β-sheet like structure and π–π stacking of the phenyl rings in the hydrogel states (Fig. S8, ESI†). In the FTIR study (Fig. S9, ESI†), the peaks at 3416 cm−1 and 3292 cm−1 are associated with non-hydrogen bonded and hydrogen bonded amide N–H stretching respectively and the peak at 1640 cm−1 is due to the amide carbonyl group stretching. This clearly indicates the presence of an intermolecular hydrogen bonded sheet-like extended backbone structure in the gel states (Fig. S9, ESI†).
The shrinkage property can be used as an easy method to remove toxic metal ions such as lead. An aliquot 10 μL of 66.2 mM solution of lead nitrate Pb(NO3)2 was added to a 2 mL hot buffer solution of amphiphile MF at 1.5 mM concentration and the whole solution was sonicated for a few seconds. A white precipitate was formed that dispersed homogeneously into the whole solution. After cooling the solution to room temperature a translucent hydrogel was formed. Interestingly, this white-coloured opaque hydrogel containing Pb2+ ions started to shrink within an hour of its formation by expelling clear water (without Pb2+ ions). The separation of the white hydrogel leaving behind colourless water indicates that Pb2+ ions got entrapped into the self-compressed hydrogel network (Fig. 1d). The effect of the anions was also tested by taking lead perchlorate (Pb(ClO4)2) as a Pb2+ ion source. However, no significant anion effect was found as Pb(ClO4)2 also got separated from the solution (Fig. S10, ESI†). The whole study can be represented as a schematic diagram as in Fig. S11 (ESI†).
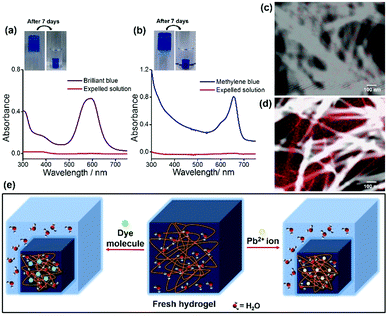
Atomic absorption spectroscopy (AAS) was performed to determine the efficiency of the hydrogel for Pb2+ ion separation. An amount of 20 μL of the shrinkage gel and 20 μL of the expelled water were taken respectively to prepare 20 mL solutions to measure the Pb2+ ion concentration (ppm). These solutions were stirred for 4 h at room temperature, followed by filtration. The concentration of the Pb2+ ions in the initial solution was determined through AAS using a standard calibration curve. However, the results are striking as the freshly made hydrogel contains a Pb2+ ion concentration of 2.9285 ppm whereas the Pb2+ ion concentration in the expelled water part is 0.0469 ppm. This indicates that the Pb2+ ion concentration is negligible in the water phase compared to the aged and shrunken hydrogel as the concentration of Pb2+ ions in the aqueous phase is insignificant. This performance is better than those of previous reports16a as the efficiency and extent of Pb2+ ion removal from water by using our gelator is improved (98.4%). To the best of our knowledge, this is the first report of a peptide-based supramolecular superhydrogel that shows a fascinating self-compressing property through the expulsion of water. It also demonstrates the potential usability of this hydrogelator in waste-water treatment by removing toxic heavy metal ions (Pb2+) and environmentally harmful organic dyes, and Fig. 3e represents a schematic diagram for the entire phenomenon.
For the dye removal study, 10 μL of 1 mM solution of Brilliant blue and Methylene blue was added to a 1.5 mM 2 mL hydrogel in phosphate buffer respectively. Upon cooling, hydrogels were formed and the dye molecules were trapped in the hydrogel matrix (Fig. 1c). Within 1 h these hydrogels started to shrink by expelling clear water (Fig. 1c). The UV-visible spectra (Fig. 3a and b) show that a negligible amount of the dye molecules are present in the expelled water part compared to the initial concentration of dye molecules present in the hydrogels. The highest loading capacities for both dyes are given in Table S1 (ESI†). The dye removal capacity was found to be remarkably high for both of these tested dyes (99.8%). Moreover, the removal of all the toxic substances (Pb2+ ions and organic dyes) was also tested in a mixture of these three substances and all these substances are removed leaving behind clear water. This indicates that this tripeptide-based gelator molecule has great potential for future waste-water treatment. Moreover, the removal of toxic substances (Pb2+ ions and organic dyes) together in a mixture was also tested and all the substances are separated and sequestered (Fig. S12, ESI†).
We were curious to know whether Pb2+ ions interact with the gelator peptide or not and to examine this, shrunken and aged gels containing Pb2+ ions were taken out from the vial and were placed in a saturated solution of EDTA-Na2. Interestingly, it was found that as time elapsed, the white coloured gel was gradually transformed into a transparent gel through its periphery (Fig. S13, ESI†). This observation can be due to the leakage of Pb2+ ions from the gel matrix upon complexation with EDTA. So, it can be envisaged that Pb2+ ions may be physically absorbed or entrapped in the three-dimensional porous network structure of the hydrogel without any complexation with the gelator molecule and that is why Pb2+ ions can be easily removed with a strong complexation with EDTA without rupturing the gel structure. To explore the distribution of Pb2+ ions in the gel network, elemental mapping of Pb (element) was performed using FE-SEM and TEM studies before and after the shrinkage of the Pb2+ ion containing gel. Fig. S14 (ESI†) and Fig. 3c and d reveal that Pb2+ ions are randomly distributed within the gel matrix in a nonspecific manner as these elements are found both on nanofibres as well as within the pores of the entangled nanofibres of the gel matrix. The FTIR data also support this result as there is no significant change in the FT-IR spectral pattern before and after the addition of Pb2+ ions into the gel (Fig. S15, ESI†). The XPS data (Fig. S16, ESI†) reveal the possibility of the entrapment of Pb2+ ions within the gel network as hydroxide of Pb2+ ions17 during syneresis.
To check reusability, the dye and metal ion-containing shrunken gels were treated with a saturated potassium hydrogen sulphate solution and it was then extracted with ethyl acetate following the standard methods by pH variation.15e It was found that the highly water soluble pollutants (dye and metal ions) remain in the aqueous phase and the gelator molecules were extracted through the organic phase. As shown in Fig. S17 (ESI†) by using the above stated method, we can use the gelator molecule for a maximum of four cycles with only a nominal loss of the gelator compound.
In summary, a tripeptide-based potent hydrogelator has been discovered, which forms a super-hydrogel at a very low concentration. This hydrogel exhibits a remarkable time-dependent self-shrinking property by expelling water molecules. Moreover, this hydrogel has been successfully utilized for efficient removal of pollutants including toxic heavy metal ions (Pb2+) and toxic organic dyes from waste-water. The relatively cheap starting materials to synthesize the gelator, the low amount required to form a gel (<1 mg to make 1 mL gel), the higher absorption capacity and reusability of the gel hold future promise for using this soft material in waste-water management.
N. N. and S. P. gratefully acknowledge CSIR, New Delhi, India, for financial assistance. A. Banerjee and I. W. Hamley gratefully acknowledge DST-UKIERI bilateral project (project no. DST/INT/UK/P-64/2014). We acknowledge Shilaj Roy, Department of Chemistry, IIT Guwahati, Assam, and JEOL JEM-2100 TEM facility for elemental mapping analysis. We thank Prof. Asim Bhaumik, Department of Materials Science, IACS, India, for AAS analysis.
Notes and references
- (a) J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC; (b) R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519 CrossRef CAS PubMed; (c) M. Häring and D. D. Díaz, Chem. Commun., 2016, 52, 13068 RSC; (d) L. Meazza, J. A. Foster, K. Fucke, P. Metrangolo, G. Resnati and J. W. Steed, Nat. Chem., 2013, 5, 42 CrossRef CAS PubMed; (e) X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165 CrossRef CAS PubMed; (f) N. Nandi, S. Basak, S. Kirkham, I. W. Hamley and A. Banerjee, Langmuir, 2016, 32, 13226 CrossRef CAS PubMed.
- (a) Y. Yamada and J. P. Schneider, Biomacromolecules, 2016, 17, 2634 CrossRef CAS PubMed; (b) S. R. Jadhav, B.-S. Chiou, D. F. Wood, G. DeGrande-Hoffman, G. M. Glenn and G. John, Soft Matter, 2011, 7, 864 RSC; (c) K. Basu, A. Baral, S. Basak, A. Dehsorkhi, J. Nanda, D. Bhunia, S. Ghosh, V. Castelletto, I. W. Hamley and A. Banerjee, Chem. Commun., 2016, 52, 5045 RSC; (d) J. E. P. Sun, B. Stewart, A. Litan, S. J. Lee, J. P. Schneider, S. A. Langhans and D. J. Pochan, Biomater. Sci., 2016, 4, 839 RSC; (e) R. M. Gouveia, R. R. Jones, I. W. Hamley and C. J. Connon, Biomater. Sci., 2014, 2, 1222 RSC; (f) V. Castelletto, A. Kaur, I. W. Hamley, R. H. Barnes, K.-A. Karatzas, D. Hermida-Merino, S. Swioklo, C. J. Connon, J. Stasiak, M. Reza and J. Ruokolainen, RSC Adv., 2017, 7, 8366 RSC.
- (a) W. Liyanage, K. Vats, A. Rajbhandary, D. S. W. Benoit and B. L. Nilsson, Chem. Commun., 2015, 51, 11260 RSC; (b) L. Szkolar, J.-B. Guilbaud, A. F. Miller, J. E. Gough and A. Saiani, J. Pept. Sci., 2014, 20, 578 CrossRef CAS PubMed.
- (a) K. P. Divya, M. Miroshnikov, D. Dutta, P. K. Vemula, P. M. Ajayan and G. John, Acc. Chem. Res., 2016, 49, 1671 CrossRef CAS PubMed; (b) B. Adhikari and A. Banerjee, Chem. – Eur. J., 2010, 16, 13698 CrossRef CAS PubMed; (c) E. Pazos, E. Sleep, C. M. R. Pérez, S. S. Lee, F. Tantakitti and S. I. Stupp, J. Am. Chem. Soc., 2016, 138, 5507 CrossRef CAS PubMed; (d) M. Maity, V. S. Sajisha and U. Maitra, RSC Adv., 2015, 5, 90712 RSC; (e) S. Bhattacharya and S. K. Samanta, Chem. Rev., 2016, 116, 11967 CrossRef CAS PubMed.
- M. Tena-Solsona, J. Nanda, S. Díaz-Oltra, A. Chotera, G. Ashkenasy and B. Escuder, Chem. – Eur. J., 2016, 22, 6687 CrossRef CAS PubMed.
- D. Yuan, X. Du, J. Shi, N. Zhou, J. Zhou and B. Xu, Angew. Chem., Int. Ed., 2015, 54, 5705 CrossRef CAS PubMed.
- (a) W. Liyanage and B. L. Nilsson, Langmuir, 2016, 32, 787 CrossRef CAS PubMed; (b) M. Pellach, S. Mondal, L. J. W. Shimon, L. Adler-Abramovich, L. Buzhansky and E. Gazit, Chem. Mater., 2016, 28, 4341 CrossRef CAS.
- P. W. J. M. Frederix, G. G. Scott, Y. M. Abul-Haija, D. Kalafatovic, C. G. Pappas, N. Javid, N. T. Hunt, R. V. Ulijn and T. Tuttle, Nat. Chem., 2015, 7, 30 CrossRef CAS PubMed.
- L. L. Hench and J. K. West, Chem. Rev., 1990, 90, 33 CrossRef CAS.
- (a) N. Zhou, X. Cao, X. Du, H. Wang, M. Wang, S. Liu, K. Nguyen, K. Schmidt-Rohr, Q. Xu, G. Liang and B. Xu, Angew. Chem., Int. Ed., 2017, 56, 2623 CrossRef CAS PubMed; (b) S. J. Kim, G. M. Spinks, S. Prosser, P. G. Whitten, G. G. Wallace and S. I. Kim, Nat. Mater., 2006, 5, 48 CrossRef CAS PubMed.
- (a) S.-L. Zhou, S. Matsumoto, H.-D. Tian, H. Yamane, A. Ojida, S. Kiyonaka and I. Hamachi, Chem. – Eur. J., 2005, 11, 1130 CrossRef CAS PubMed; (b) D. J. Adams, L. M. Mullen, M. Berta, L. Chen and W. J. Frith, Soft Matter, 2010, 6, 1971 RSC; (c) L. Qin, P. Duan, F. Xie, L. Zhang and M. Liu, Chem. Commun., 2013, 49, 10823 RSC; (d) F. Xie, L. Qin and M. Liu, Chem. Commun., 2016, 52, 930 RSC; (e) M. P. Conte, N. Singh, I. R. Sasselli, B. Escuder and R. V. Ulijn, Chem. Commun., 2016, 52, 13889 RSC; (f) L. Qin, F. Xie, X. Jin and M. Liu, Chem. – Eur. J., 2015, 21, 11300 CrossRef CAS PubMed.
- Ratna and B. S. Padhi, Int. J. Environ. Sci., 2012, 3, 940 CAS.
- G. Flora, D. Gupta and A. Tiwari, Interdiscip. Toxicol., 2012, 5, 47 CAS.
- R. M. Brooks, M. Bahadory, F. Tovia and H. Rostami, Int. J. Soil, Sediment Water, 2010, 3, 1 CAS.
- (a) B. O. Okesola and D. K. Smith, Chem. Soc. Rev., 2016, 45, 4226 RSC; (b) S. Ray, A. K. Das and A. Banerjee, Chem. Mater., 2007, 19, 1633 CrossRef CAS; (c) B. Adhikari, G. Palui and A. Banerjee, Soft Matter, 2009, 5, 3452 RSC; (d) B. O. Okesola and D. K. Smith, Chem. Commun., 2013, 49, 11164 RSC; (e) N. Nandi, A. Baral, K. Basu, S. Roy and A. Banerjee, Pept. Sci., 2017, 108, e22915 CrossRef PubMed.
- (a) F. Li, X. Wang, T. Yuan and R. Sun, J. Mater. Chem. A, 2016, 4, 11888 RSC; (b) L. Ling, W.-J. Liu, S. Zhang and H. Jiang, J. Mater. Chem. A, 2016, 4, 10336 RSC.
- W.-J. Liu, F.-X. Zeng, H. Jiang and X.-S. Zhang, Chem. Eng. J., 2011, 170, 21 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, instrumentation, synthetic procedures, NMR, HRMS, FE-SEM, TEM, FTIR, XRD, XPS, SAXS, spectroscopic and rheological studies. See DOI: 10.1039/c7cc01774j |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |