 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparison of six derivatizing agents for the determination of nine synthetic cathinones using gas chromatography-mass spectrometry
Khalid A.
Alsenedi
 a and
Calum
Morrison
b
a and
Calum
Morrison
b
aForensic Medicine and Science, School of Medicine, Dentistry and Nursing, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 8QQ, United Kingdom. E-mail: khalidsenedi@gmail.com
bForensic Medicine and Science, School of Medicine, Dentistry and Nursing, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 8QQ, United Kingdom. E-mail: calum.morrison@glasgow.ac.uk
First published on 3rd May 2017
Abstract
Six acylation reagents have been compared for their derivatisation potential towards nine synthetic cathinones by gas chromatography-mass spectrometry (GC-MS). The evaluated reagents were pentafluoropropionic anhydride (PFPA), trifluoroacetic anhydride (TFA), chlorodifluoroacetic anhydride (CLF2AA), heptafluorobutyric anhydride (HFBA), acetic anhydride (AA) and propionic anhydride (PA). The synthetic cathinones included flephedrone (4-fluoromethcathinone or 4-FMC), mephedrone (4-methylmethcathinone or 4-MMC), pentedrone (also known as α-methylamino-valerophenone), methedrone (4-methoxy-N-methcathinone, p-methoxymethcathinone), methylone (3,4-methylenedioxy-N-methylcathinone or bk-MDMA), butylone (β-keto-N-methylbenzodioxolylbutanamine or bk-MBDB), ethylone (3,4-methylenedioxy-N-ethylcathinone MDEC or bk-MDEA), pyrovalerone (4-methyl-β-keto-prolintane) and 3,4-methylenedioxypyrovalerone (MDPV). The derivatizing agents were optimised for incubation time and temperature with some important validation parameters studied to evaluate derivatisation reactions. The anhydrides studied proved to be suitable for synthetic cathinones – all of them showing RSD and accuracy below 20%. PFPA and HFBA followed by TFA are the best choice of derivatising agents based on validation parameters. Five internal standards were evaluated with good results. Three way ANOVA, interference, fragmentation patterns and high peak area values at a concentration of 0.50 μg ml−1 were evaluated and discussed. AA and PA derivatives give high relative abundance for most drugs examined. HFBA gives more ions and multi-fragmentation patterns.
Introduction
Cathinone (β-Keto-amphetamine) is found in the leaves of the Catha edulis (Khat) plant.1 Synthetic cathinones have similar properties to other stimulants and hallucinogenic drugs including amphetamines and ring-substituted amphetamines. They have pharmacological effects, known as “cardiovascular and neurological side-effects”, and can cause deaths, many of which have occurred in Europe.2–5 Despite the misuse potential associated with these compounds, the legislation governing their use is not consistent worldwide. Additionally, due to chemical modifications, these new psychoactive substances (NPS) are rapidly altered to produce new variants to bypass drug legislations of a particular country6,7 with detection and identification of these drugs proving difficult because of a lack of reference standards.8A review by Zuba and colleagues discussed pathways and unknown structures of cathinones based on mass spectrometry9 with the isomers of substituted cathinones having been investigated using NMR spectroscopy by Kavanagh.10 More than 70 synthetic cathinones have been reported by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) with synthetic cathinones making up the second biggest portion of NPS identified in 2015.11,12
The GC analysis of cathinones generally requires the use of derivatising reagents. To select an appropriate derivatization reagent for GC analysis, the following criteria can be used as guidance:13
(a) The reagent should generate >95% of complete derivatives.
(b) During derivative formation, the reagent should not alter/rearrange the structure of the compound.
(c) Loss of sample should not occur during the reaction.
(d) Derivatives produced should not interact with the GC column.
(e) A stable derivative should be formed.
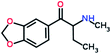
To achieve the above goals, acylation was selected for this study instead of other common derivatization methods (e.g. silylation and alkylation) since it is a popular derivatising technique widely used to increase the sensitivity, produce excellent fragmentation in mass spectra, improve the chromatographic peak shape and resolution as well as reduction in the polarity of analytes. PFPA, TFA, CLF2AA, HFBA, AA, and PA were the chosen acylation reagents in this study (see Fig. 1 for the chemical structures of reagents).
The first problem encountered with analysis of synthetic cathinones is that during GCMS method development several cathinones have only one or two important ions in the mass spectrum. Most of the remaining ions have smaller abundance (<10%) when compared with the highest abundance ion, consequently resulting in poor detection. Secondly, some cathinones have positional isomers, which produce ambiguous mass spectra. Thirdly, MDPV and pyrovalerone are not derivatised, and the analyst is dependent on a limited number of mass ions. Fourthly, some cathinones have overlap between the high abundance ions and those from internal standards. Taking these factors into account with additional legal implications, derivatisation techniques are necessary to produce many patterns and high abundance resolution of fragmentations aiding correct identification of these compounds.
In this study, the evaluation and comparison of these reagents for selected cathinones were investigated with the focus on the following:
∘ Effect of time and temperature on the reaction and optimising these conditions.
∘ Examination of the highest values of peak areas.
∘ Quality of fragmentation ions vs. reagents.
∘ Quality of mass spectrum based on its relative ion intensities.
∘ Total ion chromatograms from interference studies.
∘ Data treatment analysis by running ANOVA.14
∘ Choice of best-fit regression between internal standards.
Some validation parameters including the LOD, linearity, accuracy, RSD, and recovery were used.
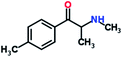
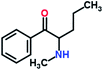
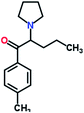
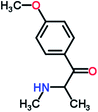
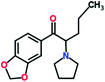
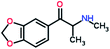
Mephedrone, flephedrone, pentedrone, methylone, ethylone, methedrone, MDPV, butylone, and pyrovalerone are the most frequently abused cathinones in the UK and in Europe and therefore selected as the target analytes15 (see Table 1).
Materials and methods
Materials
Reference standards of the nine synthetic cathinones (1 mg ml−1) – flephedrone, mephedrone, pentedrone, methedrone, methylone, butylone, ethylone, pyrovalerone, and MDPV; five internal standards (0.10 mg ml−1) – mephedrone-d3, methylone-d3, butylone-d3, ethylone-d5 and MDPV-d8 as their hydrochloride salts; seven derivatization agents – pentafluoro-propionic anhydride (PFPA) ≥ 99%, trifluoro-acetic anhydride (TFA) ≥ 99%, chloro di-fluoro acetic anhydride (CLF2AA) ≥ 98%, heptafluoro-butyric anhydride (HFBA) ≥ 99%, acetic anhydride (AA) ≥ 99%, propionic anhydride (PA) ≥ 99%, and butyric anhydride (BA) ≥ 98% were purchased from Sigma-Aldrich, Gillingham, UK. Ethyl acetate (EtOAc), methanol, ammonium hydroxide, sodium phosphate dibasic, sodium chloride, sodium phosphate monobasic, dichloromethane (DCM), isopropanol (IPA), ammonium hydroxide (NH4OH), and acetic acid were obtained from VWR International, East Grinstead, UK. Blank blood was supplied by the Scottish National Blood Transfusion Service based at Gartnaval Hospital, Glasgow. Phosphate buffer and sodium phosphate were purchased from Fisher Scientific, Loughborough, UK. Solid phase extraction columns (200 mg clean screen® part number ZSDAU20 manufactured by United Chemical Technologies) were purchased from Chromatography Direct, Runcorn, UK.Methods
Preparation of standards
Stock solutions (100 μg ml−1) of the nine drugs were prepared by dilution of the purchased standards via 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution in methanol. The working solution of each standard was prepared by dilution of stock solutions 100 μg ml−1via 1
10 dilution in methanol. The working solution of each standard was prepared by dilution of stock solutions 100 μg ml−1via 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 methanol to reach 10 μg ml−1. Working internal standards of the deuterated standards were similarly prepared taking into account the different concentrations of the supplied standards.
10 methanol to reach 10 μg ml−1. Working internal standards of the deuterated standards were similarly prepared taking into account the different concentrations of the supplied standards.
Optimisation of temperature and incubation time study
For optimisation of the reaction temperature and incubation time within the study, cathinones were derivatised at the same time in the following way: 50 μl of the 10 μg ml−1 standard drug mixture and 50 μl of 2 μg ml−1 of mixture of internal standards were added to samples. Then the mixture was evaporated to dryness at Room Temperature (RT) under a stream of nitrogen followed by derivatisation with 50 μl of PFPA and EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 50 μl of TFA and EtOAc (2
1); 50 μl of TFA and EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 50 μl CLF2AA and EtOAc (2
1); 50 μl CLF2AA and EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 65 μl of HFBA and EtOAc (3
1); 65 μl of HFBA and EtOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), AA and EtOAc (3
2), AA and EtOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and PA and pyridine (2
2), and PA and pyridine (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). All samples were capped and vortexed immediately for 15 seconds and then incubated for specific times (5–10–15–20–25–30–35–40 min) and at specific temperatures (RT, 40 °C, 55 °C, 70 °C). The samples were evaporated under a stream of nitrogen with the hot block set at RT, 40 °C and 50 °C thereafter reconstituted in 50 μl of ethyl acetate. The top layer of EtOAc was transferred to an auto-sampler vial for GC-MS analysis. The GC syringe was washed three times before injection in EtOAc. A volume of 1.0 μl was injected at 225 °C and GC-MS was run under the conditions outlined below.
1). All samples were capped and vortexed immediately for 15 seconds and then incubated for specific times (5–10–15–20–25–30–35–40 min) and at specific temperatures (RT, 40 °C, 55 °C, 70 °C). The samples were evaporated under a stream of nitrogen with the hot block set at RT, 40 °C and 50 °C thereafter reconstituted in 50 μl of ethyl acetate. The top layer of EtOAc was transferred to an auto-sampler vial for GC-MS analysis. The GC syringe was washed three times before injection in EtOAc. A volume of 1.0 μl was injected at 225 °C and GC-MS was run under the conditions outlined below.
Samples were prepared in triplicate on eight days at concentrations of 0.50 μg ml−1 and 0.10 μg ml−1 for internal standards. From day one to four, 72 samples (18 samples for each temperature) were added each day at RT, 40 °C, 55 °C and 70 °C respectively and set of the same time at 10, 20, 30, and 40 minutes for each derivatization reagent. From day five until day eight, samples were set in the same way as previous days; however, the times were changed to 5, 15, 25, and 35 minutes. Each temperature and each incubation time were analysed in triplicate. The samples were evaporated under a stream of nitrogen at RT for all reagents on days 1, 2, 3 and 4. The temperature on days 5, 6, 7 and 8 for PFPA, TFA and HFBA was RT, while 40 °C was used for AA and ClF2AA and 50 °C for PA.
The 72 samples (18 samples for each temperature) under nitrogen gas were also evaluated using a TurboVap® in the following way: triplicate samples were run in one day when the hot block was set at 50 °C and the period of incubation was 20 minutes under RT, 40, 55 and 70 °C.
A different procedure was carried out to examine the effect of pyridine as a solvent in BA and PA in the following way: 200 μl of 10 μg ml−1 from the mixtures of cathinones was added followed by evaporation at RT. The triplicates of 18 derivatized samples of BA and PA were closed and vortexed for 15 seconds and then incubated at 90 °C for 30 minutes and then in the evaporation step the samples were set at RT, 40 and 50 °C.
54 samples were set in the same way as mentioned above in one day to evaluate the reaction at RT, 55 °C, and 70 °C in 30 minutes (18 samples for each temperature). The evaporation step was set at RT. Again, each temperature was analysed in triplicate.
Optimisation procedure
The optimisation procedure for the incubation time and the temperature of the hot block were as follows: PFPA and TFA were set at RT and 40 °C, respectively, for 20 min; the hot block was set at RT; ClF2AA and HFBA were set at 55 °C for 25 min; the hot block was set at 40 °C; AA and PA were set at 70 °C for 20 min; and the hot block was set at 50 °C.The above procedures were used in this study to calculate peak areas, relative standard deviation (RSD), accuracy values and significant differences between the reaction temperature and time using ANOVA.
Study of linearity, LOD, recovery and internal standards
For linearity, the samples were prepared in triplicate and spiked with cathinones at seven concentrations (2, 1, 0.75, 0.50, 0.25, 0.10, and 0.05) μg ml−1, covering the range in which a common stimulant (amphetamine) is commonly encountered within toxicological samples.For the LOD, the samples were prepared in triplicate and spiked at seven concentrations (250, 100, 50, 25, 10, 5, and 1 ng ml−1).
SPE method used for recovery study
1 ml of whole blood of each sample was added and mixed with 1 ml of 0.10 M phosphate buffer (pH = 6), and all samples were then mixed and centrifuged. The extraction column was conditioned using 3 ml of methanol, followed by 3 ml deionized water and then 1 ml of 0.10 M phosphate buffer at pH 6 for washing the cartridges and removing unwanted substances. The samples were added and allowed to pass through the columns completely. Washing consisted of addition of 3 ml of deionized water, followed by 1 ml of 100 mM acetic acid and then 3 ml methanol followed by drying under full vacuum for 5 minutes. The samples were eluted with 3 ml of DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA
IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4OH (78
NH4OH (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and then evaporated under a stream of nitrogen at RT until dry. The dried extracts were then derivatised in the same way as mentioned above in the optimisation procedure.
2), and then evaporated under a stream of nitrogen at RT until dry. The dried extracts were then derivatised in the same way as mentioned above in the optimisation procedure.
For ISDs, the procedure outlined in the linearity study was used.
GC-MS conditions
Gas chromatography-mass spectrometry (GC-MS) was carried out using a 7890A GC/5975C MSD equipped with a split/splitless inlet and a DB-5ms (5% phenyl/95 methylsiloxane; 30 m × 0.25 mm, 0.25 μm film thickness) separation column (All Agilent Technologies, Waldbronn, Germany). Helium was used as a carrier gas (99.99% purity). Splitless injection at 225 °C was employed. The MS transfer line temperature was maintained at 250 °C. The MS was operated in the electron impact ionization mode (70 eV). The ion source was maintained at 200 °C. MS data acquisition was initiated at 7 minutes and was performed in selected ion monitoring (SIM) mode and scan mode. The column temperature program was initially started at 70 °C and then increased by 10 °C per minute to reach 280 °C with a final hold time of 23 minutes. The mass spectrometer was operated in full scan mode (m/z 40–500) to study ion and peak interference. Selected ion monitoring (SIM) mode was used to study the linearity, limit of detection (LOD), recoveries, and peak areas.Results and discussion
Fragment ions and relative ion intensities
The observed fragment ions and relative ion intensities for the different cathinone derivatives are summarised in Table 2. The target ions in bold were used to calculate accuracy and RSD values. The highest abundance ion values were used to calculate the peak area values.Temperature, reaction time, and three way ANOVA study
The optimum temperature and reaction time for each compound using each reagent are shown in Table 3.| Drug name/derv. | PFPA (RT) | TFA (RT) | CLF2AA (40 °C) | HFBA (40 °C) | AA (50 °C) | PA (50 °C) |
|---|---|---|---|---|---|---|
| Flephedrone | 20 min RT | 20 min 40 °C | 20 min 40 °C | 20 min 40 °C | 25 min 55 °C | 25 min 70 °C |
| Mephedrone | 10 min RT | 20 min 40 °C | 25 min 70 °C | 25 min 55 °C | 20 min 40 °C | 25 min 70 °C |
| Pentedrone | 20 min RT | 20 min 40 °C | 25 min 55 °C | 20 min 40 °C | 25 min 55 °C | 25 min 55 °C |
| Methedrone | 20 min 40 °C | 20 min 40 °C | 25 min 55 °C | 25 min 70 °C | 25 min 55 °C | 25 min 70 °C |
| Methylone | 35 min 70 °C | 20 min 40 °C | 25 min 55 °C | 20 min 40 °C | 25 min 55 °C | 25 min 70 °C |
| Butylone | 20 min 40 °C | 20 min 40 °C | 25 min 55 °C | 20 min 40 °C | 25 min 70 °C | 25 min 70 °C |
| Ethylone | 20 min 40 °C | 20 min 40 °C | 25 min 55 °C | 20 min 40 °C | 15 min 70 °C | 25 min 70 °C |
| Pyrovalerone | 35 min 70 °C | 25 min 70 °C | 25 min 70 °C | 25 min 55 °C | 25 min 70 °C | 25 min 70 °C |
| MDPV | 35 min 70 °C | 25 min 70 °C | 25 min 70 °C | 25 min 55 °C | 15 min 70 °C | 25 min 70 °C |
| Optimisation | 20 min RT | 20 min 40 °C | 25 min 55 °C | 20 min 55 °C | 25 min 70 °C | 25 min 70 °C |
The optimum time and temperature in Table 3 were chosen for the mixture of synthetic cathinones to develop a method that works for the drug substances in each reagent. Therefore, the combination of information from Tables 3 and 4 illustrates the optimal conditions for reagents and drugs. Using the PFPA derivative of flephedrone as an example, the reaction conditions of RT for 20 min duration were chosen from Table 3 in combination with the ANOVA results from Table 4.
| Drug name/derv. | PFPA | TFA | CLF2AA | HFBA | AA | PA |
|---|---|---|---|---|---|---|
| Flephedrone | Yes | No | No | No | No | Yes |
| Mephedrone | Yes | No | Yes | No | Yes | Yes |
| Pentedrone | No | No | Yes | No | No | Yes |
| Methedrone | No | No | No | Yes | Yes | Yes |
| Methylone | No | No | Yes | Yes | No | Yes |
| Butylone | No | No | Yes | Yes | No | Yes |
| Ethylone | No | No | No | Yes | No | Yes |
| Pyrovalerone | No | No | No | No | No | Yes |
| MDPV | No | No | No | No | No | Yes |
The optimal derivatization conditions for each compound were chosen according to the average of the highest values of peak areas at a concentration of 0.50 μg ml−1.
The peak area values of the target ions of cathinones were more evident using reaction conditions of 25 minutes at 70 °C with the exception of PFPA and TFA derivatives which showed excellent responses from RT derivatisation conditions. It should be noted that AA and PA are preferable for most of the cathinones when a high temperature of 70 °C is applied. The cathinones generally require high temperatures for most of the derivatisation reagents which may be due to properties including the boiling point of each reagent and its molecular weight. It may be concluded that the higher the boiling points of reagents the higher the temperatures for reactions. PA, AA, HFBA, CLF2AA, TFA and PFPA have the boiling points of 167 °C, 139.8 °C, 120 °C, 96–97 °C, 72.4 °C, and 69–70 °C, respectively. Mephedrone and flephedrone are more volatile compounds than other drugs because they have a lower molecular weight. However, the responses are improved when the reaction occurs at high temperatures when PFPA and TFA were excluded.
Due to the high boiling point (198 °C) of butyric anhydride the reaction is not successful at 70 °C. The excess reagent is not evaporated under nitrogen even when the temperature is higher than 70 °C for 20 minutes. Additionally, this reagent provided a poor response for all compounds except when applied at a high concentration (5 μg ml−1). For the above reasons this reagent was not investigated further.
The R programming language was used to perform a three-way ANOVA considering three factors (temperature and reaction time during incubation and the temperature of the hot block during the evaporation step) as independent variables. The dependent variables (54 different ANOVA = 9 drugs × 6 reagents) were the mean of peak area values at each specific time and temperature for each drug and for each derivatisation reagent alone (5184 tests of peak area values were produced; 5184 tests = 8 days × 72 samples per day × 9 drugs). In order to infer that there was a difference in the results it was expected to see at least one of the three independent variables to appear as statistically significant within the 5% level of confidence. If the probability factor (F) was higher than 5%, this means that the difference between peak area values, produced by altering the three variables noted above, was statistically significant. The data in Table 4 demonstrate that we should run the sample under a strict procedure or under specific conditions if there is significant difference (yes) in the derivatised drug. For example, the samples should follow the optimised procedure in the case of flephedrone and mephedrone derivatised by PFPA to get the best response; if not the peak area values will significantly change above the 95% confidence limit then, as the consequence will give a bad response. In the case of TFA derivatisation, the probabilities for all drugs to give the same values of peak areas even with changes in time or temperature within the 95% limit confidence are significantly the same. Therefore, many incubation times and temperatures are appropriate for this reagent. All substances derivatised with PA should follow the optimised procedure specifically the temperature of the hot block in the evaporation step. PA samples may need more than an hour to evaporate at RT.
The uncertainty studies may require answering the question: why do we have no significant differences?
It may be the effects of many factors such as losing the drug during the evaporation step or as a result of thermal decomposition of derivatised drugs in the injector port.16
Internal standards, RSD and accuracy, linearities, limit of detection, and recovery studies
The internal standard results are shown in Table 5. The purpose of this procedure was to evaluate the application of internal standards.| Compound with ISDs/derv. | PFPA (R2) | TFA (R2) | CLF2AA (R2) | HFBA (R2) | AA (R2) | PA (R2) |
|---|---|---|---|---|---|---|
| a ISD used to study validation parameters. b B.R is bad response = <0.900. | ||||||
| Flephedrone–ISD mephadrone d3 | 0.998a | 0.999a | B.R | 0.998a | 0.997a | 0.999a |
| Flephedrone–ISD methylone d3 | 0.996 | 0.999 | 0.999a | B.Rb | 0.990 | 0.991 |
| Flephedrone–ISD butylone d3 | 0.997 | 1.000 | 0.999 | 0.995 | 0.995 | B.R |
| Flephedrone–ISD ethylone d5 | 0.995 | 0.990 | 0.995 | B.R | B.R | B.R |
| Flephedrone–ISD MDPV d8 | 0.994 | 0.998 | B.R | 0.996 | 0.991 | 0.999 |
| Mephadrone–ISD mephadrone d3 | 0.999a | 0.999a | B.R | 0.999a | 0.997a | 1.000a |
| Mephadrone–ISD methylone d3 | 0.997 | 0.997 | 0.997a | B.R | 0.942 | 0.995 |
| Mephadrone–ISD butylone d3 | 0.997 | 0.996 | 1.000 | 0.994 | 0.959 | B.R |
| Mephadrone–ISD ethylone d5 | 0.995 | 0.996 | 0.998 | B.R | B.R | B.R |
| Mephadrone–ISD MDPV d8 | 0.994 | 0.989 | B.R | 0.994 | 0.941 | 0.988 |
| Pentedrone–ISD mephadrone d3 | 0.998a | 0.998a | B.R | 0.998a | 0.997a | 0.997a |
| Pentedrone–ISD methylone d3 | 0.995 | 0.995 | 0.997a | B.R | 0.955 | 0.978 |
| Pentedrone–ISD butylone d3 | 0.995 | 0.995 | 0.997 | 0.997 | 0.967 | B.R |
| Pentedrone–ISD ethylone d5 | 0.994 | 0.995 | 0.994 | B.R | B.R | B.R |
| Pentedrone–ISD MDPV d8 | 0.994 | 0.988 | B.R | 0.998 | 0.954 | 0.986 |
| Methadrone–ISD mephadrone d3 | 1.000a | 0.999a | B.R | 1.000a | 0.999a | 0.996 |
| Methadrone–ISD methylone d3 | 0.996 | 1.000 | 0.999a | 0.999 | 0.999 | 0.999a |
| Methadrone–ISD butylone d3 | 0.994 | 0.999 | 1.000 | 0.996 | 0.999 | B.R |
| Methadrone–ISD ethylone d5 | 0.998 | 0.999 | 0.998 | B.R | B.R | B.R |
| Methadrone–ISD MDPV d8 | 0.996 | 0.996 | B.R | B.R | 0.999 | 0.997 |
| Methylone–ISD mephadrone d3 | 0.998 | 0.999 | B.R | 0.998 | 0.999 | 0.995 |
| Methylone–ISD methylone d3 | 0.999a | 0.999a | 0.998a | 0.999a | 0.998a | 1.000a |
| Methylone–ISD butylone d3 | 0.999 | 0.999 | 1.000 | 0.998 | 0.999 | B.R |
| Methylone–ISD ethylone d5 | 0.999 | 0.998 | 0.999 | B.R | B.R | B.R |
| Methylone–ISD MDPV d8 | 0.993 | 0.998 | B.R | B.R | 0.997 | 0.997 |
| Butylone–ISD mephadrone d3 | 0.997 | 0.999 | B.R | 0.996 | 0.999 | 0.996 |
| Butylone–ISD methylone d3 | 0.999 | 1.000 | 0.995 | 0.999 | 0.997 | 1.000a |
| Butylone–ISD butylone d3 | 0.999a | 1.000a | 0.999a | 1.000a | 1.000a | B.R |
| Butylone–ISD ethylone d5 | 0.999 | 0.999 | 0.997 | B.R | B.R | B.R |
| Butylone–ISD MDPV d8 | 0.995 | 0.997 | B.R | B.R | 0.996 | 0.996 |
| Ethylone–ISD mephadrone d3 | 0.994 | 0.999 | B.R | B.R | 0.995 | 0.996 |
| Ethylone–ISD methylone d3 | 0.998 | 1.000 | 0.996 | 0.942 | 0.998 | 1.000a |
| Ethylone–ISD butylone d3 | 0.999 | 1.000 | 0.999 | 0.978a | 0.994a | B.R |
| Ethylone–ISD ethylone d5 | 0.999a | 0.999a | 0.999a | B.R | B.R | B.R |
| Ethylone–ISD MDPV d8 | 0.998 | 0.997 | B.R | B.R | 0.997 | 0.996 |
| Pyrovalerone–ISD mephadrone d3 | 0.992 | 0.994 | B.R | 0.998 | 0.995 | 0.986 |
| Pyrovalerone–ISD methylone d3 | 0.996 | 0.995 | 0.997 | 0.997 | 0.998 | 0.998 |
| Pyrovalerone–ISD butylone d3 | 0.997 | 0.995 | 0.996a | 0.996a | 0.994 | B.R |
| Pyrovalerone–ISD ethylone d5 | 0.997 | 0.994 | 0.992 | B.R | B.R | B.R |
| Pyrovalerone–ISD MDPV d8 | 0.997a | 0.999a | B.R | B.R | 0.998a | 0.994a |
| MDPV–ISD mephadrone d3 | 0.990 | 0.992 | B.R | 0.998 | 0.995 | 0.982 |
| MDPV–ISD methylone d3 | 0.996 | 0.993 | B.R | 0.993 | 0.995 | 0.996 |
| MDPV–ISD butylone d3 | 0.997 | 0.993 | 0.909a | 0.994a | 0.988 | B.R |
| MDPV–ISD ethylone d5 | 0.997 | 0.992 | B.R | B.R | B.R | B.R |
| MDPV–ISD MDPV d8 | 0.999a | 0.999a | B.R | B.R | 0.995a | 1.000a |
• Can we use one or two internal standards (ISDs) for all studied cathinones?
• Which ISD has the best-fit regression?
To answer the above points, we applied each of the five internal standards to all nine drugs. All internal standards worked well and gave more than 0.990 when PFPA and TFA were applied. The ISDs that gave a poor response were avoided in all experiments.
The RSD (%), accuracy, linearity, LOD and recovery data were only calculated according to optimal conditions.
The RSD (%) values were calculated from the procedure of optimal methods only (the mean of SD ÷ the mean of peak area ratio) × 100 at a concentration of 0.5 μg ml−1. According to the RSD values of peak areas at 0.5 μg ml−1, the best results were given by ClF2AA followed by PFPA then AA, HFBA, TFA and PA respectively.
The accuracy values were calculated from (the mean of calculation of concentration – true values ÷ true values) × 100 at a concentration of 0.5 μg ml−1. The best results were given by PFPA then HFBA, TFA, PA, CLF2AA and lastly AA. The anhydrides proved to be suitable for cathinone derivatization because none exceeded 20% for both RSD and accuracy, which is recommended in ref. 14 (see Table 6).
| Derv./drug name | PFPA | TFA | ClF2AA | HFBA | AA | PA | |
|---|---|---|---|---|---|---|---|
| Flephedrone | Mean* | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 283 283![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 223 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 178 178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147 147 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 881 881![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 598 598 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563 563![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 229 229 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 407 407![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 035 035 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 263 263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952 952 |
| RSD (%) | 4.07% | 3.41% | 10% | 14% | 1.13% | 5.5% | |
| Accuracy | 1.81% | −9.8% | −19% | −4.83% | 3.67% | 1.81% | |
| Mephedrone | Mean | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 467 467![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 657 657![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 698 698![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786 786 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 702 702![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 338 338 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 086![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 728 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523 523![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 269 269 |
| RSD (%) | 1.96% | 0.99% | 6.4% | 2.02% | 2.71% | 11% | |
| Accuracy | 4.79% | 3.47% | −12% | −0.36% | −9.0% | −12% | |
| Pentedrone | Mean | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 714 714![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 988 988 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 860 860![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552 552 |
368![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 017 017 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 582 582![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 720 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 145 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 452 452 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 099 099![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143 143 |
| RSD (%) | 1.51% | 2.37% | 2.20% | 4.33% | 2.59% | 12% | |
| Accuracy | 10% | 4.09% | −9.3% | 11% | −9.3% | 10% | |
| Methedrone | Mean | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 720 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 822 822![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 657 657![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 846 846 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 353![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 019 019 |
574![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 827 827 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 489 489![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 097 097 |
| RSD (%) | 4.49% | 2.89% | 4.43% | 7.7% | 2.59% | 0.18% | |
| Accuracy | 5.62% | 12% | −7.1% | 13% | 5.2% | −12% | |
| Methylone | Mean | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421 421 |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 973 973![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 042 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 487 487![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591 591![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 099 099![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 157 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 643 643 |
| RSD (%) | 1.46% | 1.76% | 0.45% | 0.98% | 1.76% | 0.06% | |
| Accuracy | −11% | −1.11% | −12% | −2.55% | −6.8% | 1.42% | |
| Butylone | Mean | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 835 835![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 783 783 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 881 881![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 945 945 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132 132![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 108 108 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 476![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375 375 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 139 139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855 855 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 680 |
| RSD (%) | 1.96% | 7.6% | 8.2% | 9.7% | 2.43% | 5.6% | |
| Accuracy | −3.28% | −13% | −16% | −7.5% | 8.5% | −3.28% | |
| Ethylone | Mean | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147 147 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 161 161![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 097 097 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026 026![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 282 282 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914 914![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 781 781 |
63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 541.29 541.29 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 680 |
| RSD (%) | 1.14% | 1.81% | 7.9% | 6.8% | 3.82% | 5.6% | |
| Accuracy | 2.09% | −12% | −9.0% | 14% | −17% | 0.44% | |
| Pyrovalerone | Mean | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 801 801![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 857 857 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 929 929![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 385 385 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626 626![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518 518 |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 895 895![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943 943 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658 658![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 976 976![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 504 504 |
| RSD (%) | 12% | 1.01% | 4.74% | 6.8% | 10% | 12% | |
| Accuracy | −13% | 15% | −19% | 14% | 3.43% | 13% | |
| MDPV | Mean | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 735 735![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 709 709![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 708 708 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519 519![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 016 016 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 421 421![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153 153 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 039 039 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523 523![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
| RSD (%) | 14% | 2.54% | 10% | 11% | 12% | 15% | |
| Accuracy | 9.5% | 7.5% | −16% | −19% | 0.39% | −5.8% | |
Linear correlation coefficients (R2) were calculated from the triplicate samples at seven concentrations (2, 1, 0.75, 0.5, 0.25, 0.1, and 0.05 μg ml−1). All R2 values were greater than 0.905. The best results were obtained with PFPA and HFBA; all values were higher than 0.998 followed by PA, AA, TFA, and then ClF2AA (pyrovalerone and MDPV were excluded).
The LOD was measured in SIM mode using methanol spiked with mixtures of cathinones in the range of 1 to 250 ng ml−1. The signal-to-noise (S/N) ratio was calculated from triplicate measurements at seven concentrations (250, 100, 50, 25, 10, 5, and 1 ng ml−1). The lowest concentration at which the S/N was greater than 3 was considered to be the LOD. PFPA, PA, TFA, HFBA, CLF2AA, and AA provided the best results respectively (see Table 7).
| Derv./drug name | Flephedrone | Mephedrone | Pentedrone | Methedrone | Methylone | Butylone | Ethylone | Pyrovalerone | MDPV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | (R2) | LOD | (R2) | LOD | (R2) | LOD | (R2) | LOD | (R2) | LOD | (R2) | LOD | (R2) | LOD | (R2) | LOD | (R2) | |
| PFPA | 1 | 0.998 | 1 | 0.999 | 1 | 0.997 | 1 | 0.999 | 5 | 0.999 | 5 | 0.999 | 5 | 0.998 | 50 | 0.956 | 50 | 0.944 |
| TFA | 5 | 0.995 | 5 | 0.999 | 5 | 0.998 | 5 | 0.999 | 5 | 0.999 | 5 | 0.997 | 10 | 0.999 | 50 | 0.912 | 50 | 0.985 |
| ClF2AA | 10 | 0.992 | 5 | 0.995 | 5 | 0.997 | 5 | 0.994 | 25 | 0.998 | 50 | 0.998 | 25 | 0.996 | 50 | 0.955 | 50 | 0.978 |
| HFBA | 1 | 0.998 | 1 | 0.999 | 1 | 0.998 | 5 | 0.999 | 10 | 1.000 | 5 | 0.999 | 100 | 0.935 | 100 | 0.912 | 100 | 0.905 |
| AA | 50 | 0.996 | 25 | 0.990 | 1 | 0.996 | 50 | 0.999 | 25 | 0.998 | 250 | 0.998 | 100 | 0.996 | 25 | 0.998 | 25 | 0.995 |
| PA | 10 | 0.997 | 10 | 0.981 | 5 | 0.997 | 10 | 1.000 | 5 | 0.999 | 5 | 0.999 | 5 | 0.998 | 5 | 0.996 | 5 | 0.994 |
The recovery study was completed to check that all drugs can be derivatised after extraction of whole blood. Relative recoveries were calculated using a concentration of 3 μg ml−1. The samples were extracted three times without internal standards present until addition prior to the evaporation step under nitrogen. At the same time three un-extracted standards were prepared at 3 μg ml−1 with internal standards. The recovery of each drug was calculated using the following equation: recovery% = (peak area ratio of extracted standards ÷ peak area ratio of un-extracted standards) × 100. The recovery results with precision are shown in Table 8.
| Drug name/derv. | PFPA | TFA | ClF2AA | HFBA | AA | |
|---|---|---|---|---|---|---|
| Flephedrone | Recovery | 69% | 69% | 100% | 59% | 81% |
| RSD (%) | 7.4% | 12% | 17% | 6.2% | 2.14% | |
| Mephedrone | Recovery | 107% | 104% | 94% | 64% | 121% |
| RSD (%) | 7.1% | 1.43% | 9.7% | 20% | 7.6% | |
| Pentedrone | Recovery | 70% | 112% | 92% | 43% | 68% |
| RSD (%) | 2.63% | 3.48% | 17% | 20% | 5.2% | |
| Methedrone | Recovery | 107% | 129% | 100% | 110% | 94% |
| RSD (%) | 9.6% | 7.9% | 7.5% | 19% | 10% | |
| Methylone | Recovery | 101% | 98% | 98% | 126% | 82% |
| RSD (%) | 0.75% | 2.37% | 3.59% | 16% | 2.35% | |
| Butylone | Recovery | 145% | 51% | 37% | 53% | 75% |
| RSD (%) | 5.3% | 0.84% | 56% | 1.80% | 18% | |
| Ethylone | Recovery | 229% | 117% | 97% | 14% | 119% |
| RSD (%) | 32% | 1.27% | 5.4% | 7.3% | 15% | |
| Pyrovalerone | Recovery | 77% | 19% | 64% | 52% | 187% |
| RSD (%) | 1.90% | 11% | 23% | 15% | 15% | |
| MDPV | Recovery | 58% | 122% | 63% | 134% | 106% |
| RSD (%) | 1.13% | 2.86% | 20% | 3.76% | 3.24% | |
Study of interference, fragmentation patterns and the highest peak area values
Mixtures of nine cathinones were examined to study the fragmentation pattern (Fig. 2) and interference (Fig. 3). No co-elution problems were observed except in two cases: butylone could not be separated effectively from ethylone if they were derivatized with AA and PA. However, ethylone has a unique ion allowing the compounds to be distinguished from each other. Butylone and ethylone have the same M.W.; the differences between the fragmentation patterns were discussed by Kerrigan.16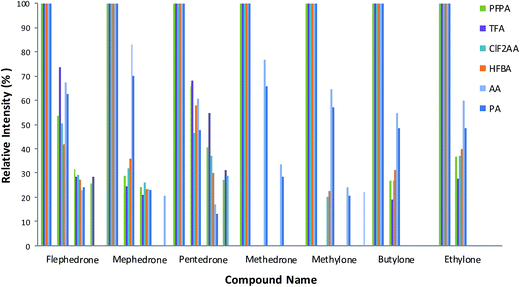 | ||
| Fig. 2 Fragmentation patterns for each substance applied to selected reagents. Less than 10% fragmentation ions were deleted. The optimised methods were used to show the fragmentation patterns. | ||
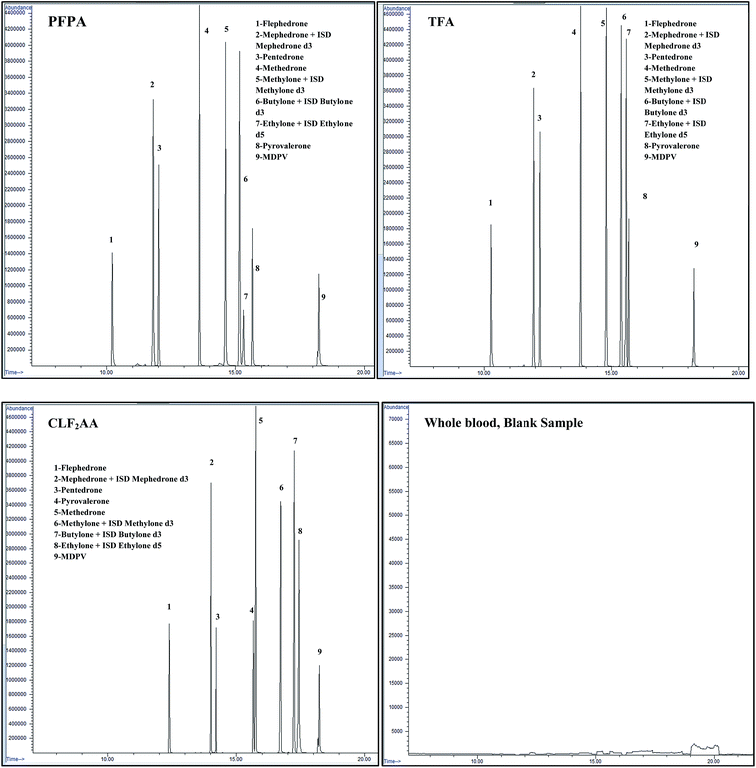 | ||
| Fig. 3 Chromatograms for three different acetylation derivatives of synthetic cathinones at a concentration of 0.50 μg ml−1 and blank blood sample. | ||
A number of fragmentation ions were studied for the drugs in each reagent. In general AA followed by PA then HFBA, PFPA, TFA and CLF2AA respectively give the maximum abundance ions based on ion intensities and greater fragmentation patterns than other reagents (see Fig. 2).
The highest abundance ion values were used to calculate the peak areas. These ions were chosen instead of target ions (quantification ions) because we need to compare the ions that give 100% of abundance in the background of fragmentation ions for six different reagents. Each one of the reagents has different ions and so we should apply all of them with the same relative ion intensity (100%), as illustrated in Fig. 4. All valid results for derivatised drugs after optimising conditions have good peak areas excluding AA for ethylone and methedrone as well CLF2AA for pentedrone.
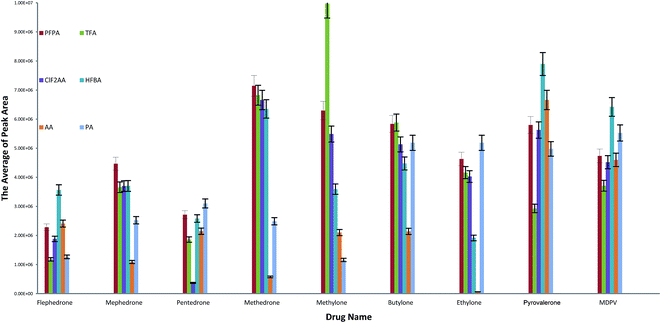 | ||
| Fig. 4 Mean of the highest peak area values in selected compounds and agents. The optimised methods were used in this calculation. | ||
Overview
Table 9 shows which reagent provides the best results under different factors. For example, if a screening method suggests the presence of flephedrone is positive, we should use TFA for quantification methods due to the following reasons:| Drug name | Ratio between all ions | No. of unique ions | No. of ions | LOD | Linearity | RSD | Accuracy | The highest peak area | Evaporation 25 °C after derv. | Evaporation 50 °C after derv. |
|---|---|---|---|---|---|---|---|---|---|---|
| Flephedrone | TFA | TFA, CLF2A | TFA, HFBA | PFPA, HFBA | All fit | Valid | Valid | HFBA/AA | Valid | Bad response |
| Mephedrone | AA | PFPA, HFBA, TFA, CLF2A | HFBA | PFPA, HFBA | All fit | Valid | Valid | PFPA/HFBA | Valid | Bad response |
| Pentedrone | TFA | HFBA, AA | PFPA | PFPA, HFBA, AA | All fit | Valid | Valid | PA/PFPA | Valid | Bad response |
| Methedrone | PA | HFBA, AA | HFBA | PFPA | All fit | Valid | Valid | PFPA/TFA | Valid | Bad response |
| Methylone | AA | HFBA, CLF2AA, AA, PA | HFBA | PFPA, TFA, PA | All fit | Valid | Valid | TFA/PFPA | Valid | Bad response |
| Butylone | AA | No one | HFBA, PA | All except CLF2AA and AA | All fit | Valid | Valid | TFA/PFPA | Valid | Valid |
| Ethylone | PFPA | All | PA | PFPA, PA | All fit | Valid | Valid | PA/PFPA | Valid | Valid |
| Pyrovalerone | Underivatized | Underivatized | Underivatized | PA | PA | PA | PA | HFBA/AA | Bad response | Valid |
| MDPV | Underivatized | Underivatized | Underivatized | PA | PA | PA | PA | HFBA/AA | Bad response | Valid |
• Good fragmentation patterns are evident.
• High quality fragmentation ions are present.
• High response is observed compared to the main ion or remaining ions.
• It has the largest number of unique ions and ions in total.
• It is valid in linearity, accuracy and precision.
The example above can be applied to all figures using a similar explanation.
Conclusion
The mass spectra of nine synthetic cathinones were compared to one another after derivatization with a number of different acylation reagents (PFPA, TFA, CLF2AA, HFBA, AA and PA). The optimisation of conditions for the incubation time and reaction temperature was discussed, and all anhydrides tested proved to be suitable for synthetic cathinones with RSD and accuracy below 20% under the optimised conditions for the reagents. The independent variables were assessed using a three-way ANOVA approach and demonstrated that the procedure should be strictly followed for combinations of drugs and reagents. In this paper, we have shown that one or two ISDs may be sufficient to provide good linearity in the mixture of cathinones chosen. The overview section shows some suggestions of which reagent can be chosen for each compound and applied to quantification or semi-quantification methods only. In general, PFPA and HFBA, followed by TFA, are the best choice of derivatising agents. Therefore, our future aim will be to fully validate a method using PFPA. MDPV and pyrovalerone are tertiary amines that were not derivatized with reagents tested. Therefore, we can conclude that several combinations of cathinones and derivatization reagents are suitable for GC-MS analysis.Acknowledgements
This work was gratefully supported by Forensic Medicine and Science, University of Glasgow, UK, and the Ministry of High Education of Saudi Arabia (I946).Notes and references
- S. Jones, et al., Cathinone increases body temperature, enhances locomotor activity, and induces striatal c-fos expression in the Siberian hamster, Neurosci. Lett., 2014, 559, 34–38 CrossRef CAS PubMed.
- M. Coppola and R. Mondola, Synthetic cathinones: chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food”, Toxicol. Lett., 2012, 211(2), 144–149 CrossRef CAS PubMed.
- A. Helander, et al., Identification of novel psychoactive drug use in Sweden based on laboratory analysis-initial experiences from the STRIDA project, Scand. J. Clin. Lab. Invest., 2013, 73(5), 400–406 CrossRef CAS PubMed.
- L. Karila, et al., Synthetic cathinones: a new public health problem, Curr. Neuropharmacol., 2015, 13(1), 12–20 CrossRef CAS PubMed.
- H. Torrance and G. Cooper, The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland, Forensic Sci. Int., 2010, 202(1), e62–e63 CrossRef CAS PubMed.
- L. Iversen, et al., Consideration of the Cathinones. Advisory Council on the Misuse of Drugs, 2010, http://https://www.gov.uk/government/publications/acmd-report-on-the-consideration-of-the-cathinones, accessed April 2017 Search PubMed.
- Drug Enforcement Administration, D.o.J., Government of United States of America. 4-Methylmethcathinone in Oregon, Microgram Bull., 2009, 42, 62 Search PubMed.
- R. P. Archer, R. Treble and K. Williams, Reference materials for new psychoactive substances, Drug Test. Anal., 2011, 3(7–8), 505–514 CrossRef CAS PubMed.
- D. Zuba, Identification of cathinones and other active components of ‘legal highs’ by mass spectrometric methods, TrAC, Trends Anal. Chem., 2012, 32, 15–30 CrossRef CAS.
- P. Kavanagh, et al., The analysis of substituted cathinones. Part 3. Synthesis and characterisation of 2,3-methylenedioxy substituted cathinones, Forensic Sci. Int., 2012, 216(1), 19–28 CrossRef CAS PubMed.
- O. D'Agnone, What have we learned and what can we do about NPS?, Drugs Alcohol Today, 2015, 15(1), 28–37 CrossRef.
- A. M. Dines, et al., Acute recreational drug and new psychoactive substance toxicity in Europe: 12 months data collection from the European Drug Emergencies Network (Euro-DEN), Clin. Toxicol., 2015, 53(9), 893–900 CrossRef PubMed.
- F. Orata, Derivatization Reactions and Reagents for Gas Chromatography Analysis, INTECH Open Access Publisher, Rijeka, 2012 Search PubMed.
- J. N. Miller and J. C. Miller, Statistics and Chemometrics for Analytical Chemistry, Pearson Education, 2005 Search PubMed.
- Addiction, E.M.C.f.D.a.D., European Drug Report 2014: Trends and Developments, 2015 Search PubMed.
- S. Kerrigan, et al., Thermal degradation of synthetic cathinones: implications for forensic toxicology, J. Anal. Toxicol., 2016, 40(1), 1–11 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |


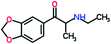
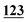
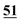
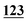
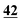
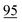
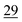
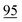
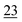
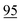
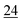
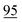
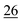
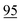
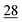
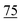
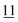
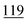
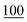
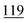
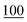
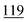
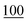
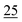
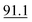
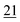
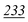
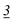
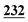
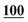
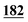
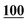
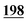
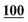
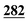
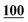
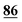
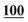
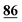
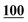
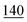
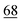
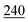
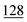
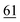
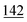
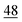
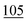
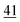
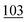
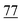
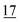
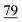
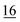
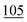
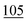
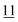
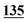
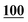
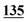
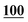
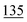
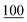
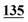
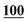
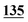
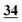
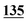
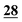
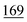
![[7 with combining low line]](https://www.rsc.org/images/entities/char_0037_0332.gif)
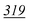
![[6 with combining low line]](https://www.rsc.org/images/entities/char_0036_0332.gif)
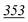
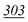
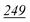
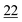
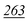
![[9 with combining low line]](https://www.rsc.org/images/entities/char_0039_0332.gif)
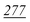
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif)
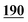
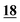
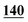
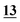
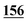
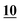
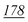
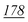
![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif)